Bioresorbable scaffolds: should we stay simple or go complex?
From its inception, scaffolding the coronary artery with bioresorbable scaffolds (BRS) has been projected as the fourth revolution in interventional cardiology, primarily designed to overcome drug-eluting stents (DES) limitations (1). This technology aims at proving some presumptive advantages over DES such as: restoration of coronary physiology and vascular adaptive responses; to obtain late lumen gain and plaque regression; and to decrease late and very-late events (1).
The most studied BRS is the everolimus-eluting Absorb GT1TM scaffold (Abbott Vascular, Santa Clara, California), which received CE marking in 2011 and have been approved by the US Food and Drug Administration in 2016 (2). Main characteristics of the Absorb includes: a 150 µm thick bioresorbable poly(L-lactide) scaffold, and a conformal bioresorbable poly(D,L-lactide) coating (1).
As expected for a developing technology, the former randomized trials presented very restrictive inclusion and exclusion criteria. Meanwhile, “real-world” registries were designed to include at least the majority eligible patients for Absorb implantation. For these reasons, although the majority and best quality evidence comes from simple scenarios—stable coronary artery disease and simple lesions—the use of Absorb on complex scenarios—acute coronary syndromes and complex lesions—comes from registries and small randomized clinical trials (RCT) (3,4).
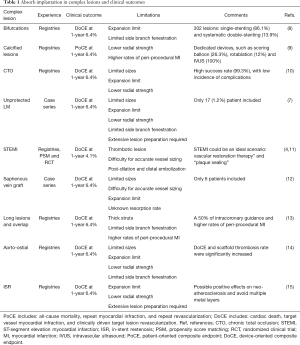
In the issue 26 of EuroIntervention (5) of 2016, Kraak et al., explore the 2-year clinical outcomes BRS in complex coronary artery disease patients. The authors analyzed data proceeding from the “AMC Single Centre Real World percutaneous coronary intervention Registry”. This is a prospective registry evaluating the clinical outcomes of all patients who underwent percutaneous coronary intervention (PCI) with Absorb between August 2012 and August 2013 in the Amsterdam Medical Center. The population in the “AMC Single Centre Real World PCI Registry” was composed of a 20% of diabetic patients mainly with stable angina (47%). Treated lesions reflected a significantly higher complexity compared to those treated in the pivotal RCT, including a 21% of scaffold overlapping, 15% of bifurcations, 11% of calcified lesions, and 8% of chronic total occlusions (CTO). The 2-year follow-up outcomes were acceptable [target vessel failure (TVF): 14% and scaffold thrombosis: 3.0%] and similar to the reported by other contemporaneous registries (6,7). The Table 1 shows the outcomes of similar registries according to the type of complex lesion. The authors categorized the sample according to the median of the SYNTAX score (SXscore) and the ABSORB II trial inclusion and exclusion criteria. Primary endpoint was TVF defined as a composite of the device-oriented endpoints of cardiac mortality, target vessel myocardial infarction (TV-MI), and target vessel revascularization (TVR). These outcomes and scaffold thrombosis were defined according to the Academic Research Consortium (16). An independent clinical events committee adjudicated all clinical events. No corelab was used for the assessment of the SYNTAX score.
Full table
A total of 135 patients (159 lesions) were enrolled, the median follow-up was 774 days (range, 742–829 days). At 2-year follow-up, TVF rate was 14.4% (cardiac death: 0.7%, TV-MI 5.3%, and TVR 13.6%). The 2-year definite ST rate was 3.0%, all of them within the first 6-month. The median SXscore was 11.5 (Q1–Q3: 6–17.5). Interestingly, when the sample was stratified by the SYNTAX score median. SXscore: <11.5 (SXlow) vs. ≥11.5 (SXhigh), there was a significantly lower rate of TVF in the SXlow when compared to the SXhigh group (6.5% vs. 21.8%, P=0.015). Moreover, when the sample was stratified according to the inclusion and exclusion criteria of the ABSORB II trial (17), there was a significantly lower rate of TVF in the in the patients fitting the ABSORB II criteria when compared to the in the patients not fitting the ABSORB II criteria (2.3% vs. 20.3%, P=0.007).
There are several points that readers should consider in order to put in perspective these results. The small sample size and methodological limitations of a registry are not negligible issues. Although, the objective of the analysis was to evaluate the outcomes in a patient population comparable with daily clinical practice, it should be highlighted that a median SYNTAX score of 11.5 do not reflect the complexity of currently treated coronary artery disease—median of 15.0 in some all-comer stent trials—at least in high-volume catheterization laboratories (18). Moreover, if we take in consideration the proportion of included patients vs. the implanted devices during the inclusion period, it represents approximately an 11%. Indeed, there was a selection bias for the Absorb-suitable patients, despite this, it also should be underlined that the median SYNTAX score was similar to those reported in other Absorb registries (7). All these issues, added to the low rate event, should make us take the conclusions as hypothesis generator.
At 2-year follow-up, outcomes were acceptable in patients with a SYNTAX score <11.5. When the sample was divided in two according to the median SYNTAX score, patients with a high SYNTAX score have significantly impaired 2-year clinical outcomes when compared to patients with low SYNTAX score. A priory, these findings are expected as in DES era complex lesion were associated with higher rate of stent failure. Furthermore, the high crossing profile and ticker struts diameter are disadvantage of Absorb that could increase this risk (1). Finally, we should mention that despite SYNTAX score has been used to predict clinical outcomes, it was designed to predict cardiac mortality from a three-vessel disease population; which is not the sweet-spot for use of Absorb (19). A SYNTAX score cutoff of 11.5—not low nor intermediate—has no validated prognosis value (20). As there is no a specific score, is acceptable to use it taking in consideration the commented limitations.
Implantation technique
Regarding Absorb technology, implantation technique has emerged as a fundamental issue to avoid scaffold failure. A recent report has proposed that suboptimal implantation technique may be associated with this increased risk of scaffold thrombosis (21). For this reason, a European expert consensus has proposed an Absorb-specific implantation protocol, emphasizing the importance of proper lesion preparation, accurate vessel sizing, and almost mandatory post-dilation (22).
In the commented report, pre-dilation was highly performed (98%), post-dilation was performed only in 55% of the cases, and the use of intracoronary imaging was not reported. The report of other implantation variable such as: type and size of the used balloons, reference vessel diameter (RVD), and post-dilation pressure; would have been useful. Given the importance of the implantation technique and the fact that it was not completely reported in this analysis, we believe that it cannot be rule out that the implantation technique could acts as a confounding variable between groups (low SYNTAX vs. high SYNTAX score or patients fitting the Absorb II criteria vs. those not fitting). Although evidence is limited, it is at least reasonable, that implantation technique could have greater impact on complex lesions (23).
Intracoronary imaging guidance
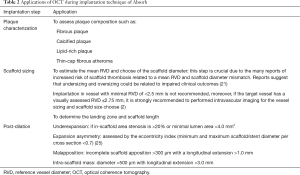
The use of intracoronary imaging techniques, especially, optical coherence tomography (OCT) can be extremely useful for the optimization of scaffold deployment. Although the evidence that supports its use is limited, specifically in complex lesions, OCT guidance for scaffold implantation can improve post-procedural in-scaffold measurements (23). Despite this, even in the context of RCT, intravascular imaging techniques are use in less than a quarter of the procedures (24). In the commented analysis the report of the use of intracoronary imaging would have been helpful because it could have an impact on clinical outcomes. Some applications of OCT during implantation technique of Absorb are shown in Table 2.
Full table
Future perspectives
The AIDA trial (NCT01858077) and Compare Absorb trial (NCT02486068), are two RCT comparing the performance of Absorb vs. Cobalt-Chromium Everolimus-eluting Stent (CoCr-EES) in all-comers contemporary population. Both trials aim to include >2,000 patients with broader inclusion criteria. However, some complex scenarios still excluded because they are considered off-label indications (2).
The OPTICO-BVS trial (NCT02683356) will assess the hypothesis if a strategy of OCT-guided PCI using BVS is superior to angiography-guided PCI. The protocol will test if OCT guidance can improve MLA as assessed by OCT at 6-month follow-up.
Despite the critical importance of implantation technique, evidence supporting the actual Absorb-specific implantations protocol is limited to expert opinion and registry analysis (21,22). This is one of the unmet clinical needs regarding Absorb technology and should be topic of further research.
Final thoughts
Kraak et al. has made an interesting contribution to the Absorb literature, reaffirming that clinical outcomes at 2-year follow-up in a “real-world” clinical practice are acceptable. They raise the concern about possible impaired outcomes in patient with a SYNTAX score ≥11.5 and not fitting the ABSORB-II criteria.
Interventionist should be aware of the unique characteristic of this device, especially the higher strut-thickness and expansion limits. We recommend that Absorb implantation in complex lesions should be performed by experienced operators—in both complex lesions and Absorb technology—threshold level for use of intracoronary imaging should be low, and implantation technique must be perfect. Off-label indications as: in-stent or in-scaffold restenosis, aorto-ostial lesions, saphenous vein grafts lesions, left main lesions, and bifurcations lesions requiring 2-stent technique; should be avoided due to impaired clinical outcomes or very limited experience (2,12,14).
Undoubtedly, the experience of treatment of complex lesion comes entirely from metallic stents and it has been translated to the Absorb technology, but the differences in devices characteristics and implantation technique should not be forgotten. The key question in this new device era is: should we stay simple or go complex? Probably in selected cases and performed by expert hands it is feasible and safe, but until RCT do not support these indications, it should not be taken as a worldwide standard daily clinical practice.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Giacchi G, Ortega-Paz L, Brugaletta S, et al. Bioresorbable vascular scaffolds in clinical practice: state-of-the-art. Panminerva Med 2016;58:130-42. [PubMed]
- Steinvil A, Rogers T, Torguson R, et al. Overview of the 2016 U.S. Food and Drug Administration Circulatory System Devices Advisory Panel Meeting on the Absorb Bioresorbable Vascular Scaffold System. JACC Cardiovasc Interv 2016;9:1757-64. [Crossref] [PubMed]
- Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol 2015;65:791-801. [Crossref] [PubMed]
- Sabaté M, Windecker S, Iñiguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J 2016;37:229-40. [Crossref] [PubMed]
- Kraak RP, Grundeken MJ, Hassell ME, et al. Two-year clinical outcomes of Absorb bioresorbable vascular scaffold implantation in complex coronary artery disease patients stratified by SYNTAX score and ABSORB II study enrolment criteria. EuroIntervention 2016;12:e557-65. [Crossref] [PubMed]
- Felix CM, Fam JM, Diletti R, et al. Mid- to Long-Term Clinical Outcomes of Patients Treated With the Everolimus-Eluting Bioresorbable Vascular Scaffold: The BVS Expand Registry. JACC Cardiovasc Interv 2016;9:1652-63. [Crossref] [PubMed]
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015;10:1144-53. [Crossref] [PubMed]
- Naganuma T, Colombo A, Lesiak M, et al. Bioresorbable vascular scaffold use for coronary bifurcation lesions: A substudy from GHOST EU registry. Catheter Cardiovasc Interv 2017;89:47-56. [Crossref] [PubMed]
- Panoulas VF, Miyazaki T, Sato K, et al. Procedural outcomes of patients with calcified lesions treated with bioresorbable vascular scaffolds. EuroIntervention 2016;11:1355-62. [Crossref] [PubMed]
- Azzalini L, Giustino G, Ojeda S, et al. Procedural and Long-Term Outcomes of Bioresorbable Scaffolds Versus Drug-Eluting Stents in Chronic Total Occlusions: The BONITO Registry (Bioresorbable Scaffolds Versus Drug-Eluting Stents in Chronic Total Occlusions). Circ Cardiovasc Interv 2016;9:e004284. [Crossref] [PubMed]
- Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv 2015;8:189-97. [Crossref] [PubMed]
- Roleder T, Wanha W, Smolka G, et al. Bioresorbable vascular scaffolds in saphenous vein grafts (data from OCTOPUS registry). Postepy Kardiol Interwencyjnej 2015;11:323-6. [Crossref] [PubMed]
- Ortega-Paz L, Capodanno D, Giacchi G, et al. Impact of overlapping on 1-year clinical outcomes in patients undergoing everolimus-eluting bioresorbable scaffolds implantation in routine clinical practice: Insights from the European multicenter GHOST-EU registry. Catheter Cardiovasc Interv 2017;89:812-8. [Crossref] [PubMed]
- Gori T, Wiebe J, Capodanno D, et al. Early and midterm outcomes of bioresorbable vascular scaffolds for ostial coronary lesions: insights from the GHOST-EU registry. EuroIntervention 2016;12:e550-6. [Crossref] [PubMed]
- Jamshidi P, Nyffenegger T, Sabti Z, et al. A novel approach to treat in-stent restenosis: 6- and 12-month results using the everolimus-eluting bioresorbable vascular scaffold. EuroIntervention 2016;11:1479-86. [Crossref] [PubMed]
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344-51. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015;385:43-54. [Crossref] [PubMed]
- Iijima R, Nagashima Y, Sato K, et al. SYNTAX score predicts major bleeding following drug-eluting stent implantation in an all-comers population. Rev Esp Cardiol (Engl Ed) 2015;68:54-62. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Puricel S, Cuculi F, Weissner M, et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol 2016;67:921-31. [Crossref] [PubMed]
- Tamburino C, Latib A, van Geuns RJ, et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: a European perspective. EuroIntervention 2015;11:45-52. [Crossref] [PubMed]
- Mattesini A, Secco GG, Dall'Ara G, et al. ABSORB biodegradable stents versus second-generation metal stents: a comparison study of 100 complex lesions treated under OCT guidance. JACC Cardiovasc Interv 2014;7:741-50. [Crossref] [PubMed]
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016;387:1277-89. [Crossref] [PubMed]
- Suwannasom P, Sotomi Y, Ishibashi Y, et al. The Impact of Post-Procedural Asymmetry, Expansion, and Eccentricity of Bioresorbable Everolimus-Eluting Scaffold and Metallic Everolimus-Eluting Stent on Clinical Outcomes in the ABSORB II Trial. JACC Cardiovasc Interv 2016;9:1231-42. [Crossref] [PubMed]


