Regression of coronary atherosclerosis with infusions of the high-density lipoprotein mimetic CER-001 in patients with more extensive plaque burden
Introduction
Considerable attentions have focused on atheroprotective properties of high-density lipoproteins (HDLs) to further reduce atherosclerotic cardiovascular diseases (1-6). Infusional agent of HDL represents an attractive therapeutic approach to enhance reverse lipid transport capacity by temporarily increasing the number of pre-beta HDL particles in the circulation (7). CER-001 is an engineered negatively-charged lipoprotein particle mimicking pre-beta HDL, consisting of a combination of recombinant human Apo A-I and two phospholipids (97% sphingomyelin, 3% dipalmitoyl phosphatidylglycerol). It has been reported to promote cholesterol efflux and anti-inflammatory response (8,9). The Can HDL Infusions Significantly Quicken Atherosclerosis Regression (CHI-SQUARE) study evaluated the impact of infusing CER-001 in patients with acute coronary syndrome (ACS) using serial coronary intravascular ultrasound (IVUS) imaging (10). Although primary endpoint did not show any significant differences between placebo and CER-001, there was a favourable trend toward plaque regression under infusing 3 mg/kg, the lowest of three doses, of CER-001 (10). This observation may underscore identification of factors including optimal dose of CER-001 which induces plaque regression.
In a previous trial investigating a HDL mimetic containing apoA-IMilano, baseline atheroma burden was a significant determinant of the degree of regression under this therapy (11,12). Based on these observations, we hypothesize that the degree of baseline coronary atheroma burden as well as dose of CER-001 may influence its clinical efficacy. Therefore, this post-hoc blinded analysis of CHI-SQUARE study investigated (I) the influence of baseline coronary atheroma burden on the efficacy of CER-001 and (II) its optimal dose associated with significant regression of coronary atheroma.
Methods
CHI-SQUARE study protocol
The details of the CHI-SQUARE protocol have been described in detail previously (10) (NCT01201837). In brief, 507 patients with ACS, defined as unstable angina, non-ST or ST segment elevation myocardial infarction were enrolled in the study. Subjects were treated with six weekly volume-matched infusions of either placebo or CER-001 at a dose of 3, 6 or 12 mg/kg. Coronary IVUS imaging was performed at baseline and at the end of the study (9 weeks) in 417 patients. An independent and blinded analysis performed by our core laboratory in anatomically matched segments identified 369 subjects with evaluable images that were included in the current analysis (placebo: n=93, CER-001 3 mg/kg: n=88, 6 mg/kg: n=100, 12 mg/kg: n=88). The remaining 48 subjects were deemed not suitable for plaque analysis (suboptimal image quality: n=22, calcification: n=15, wrong procedure for IVUS imaging: n=11). The study was approved by each participating center’s institutional review board and all patients provided written, informed consent prior to entering the study.
Image acquisition and analysis
The detail of ultrasonic image acquisition and analysis has been described in detail previously (10-12). Briefly, following anticoagulation therapy and administration of intracoronary nitroglycerin, a high frequency (40–45 MHz) ultrasound transducer (Volcano Corp or Boston Scientific) was placed as distally as possible within the target coronary artery. The angiographical inclusion criteria for the target coronary artery imaged by IVUS was one major coronary artery which did not have more than 50% luminal narrowing with a minimum length of 40 mm. The target coronary artery must not have undergone percutaneous intervention in the past or at the time of the baseline study.
Imaging was acquired while continuously withdrawing the catheter through the artery back to the aorta at a constant rate of 0.5 mm/s by a motorized pullback at both time-points. During this pullback, images were obtained at 30 frames per second. Images were digitized, and analysis of each segment was selected by using proximal and distal side branches as reference points to enable subsequent analysis of the same segment at follow-up. Subsequently, every 60th image was analyzed, representing cross-sections spaced exactly 1.0 mm apart. Cross-sectional IVUS images were analyzed by using customized software (Image J version 1.42; National Institutes of Health, Bethesda, Maryland). IVUS measurements were performed in accordance with the standards of the American College of Cardiology and the European Society of Cardiology (13). For each 1-mm apart cross-sectional image, the leading edges of the luminal and external elastic membrane (EEM) borders were traced by manual planimetry. Following these measurement, the aforementioned software automatically calculated maximum atheroma thickness in each cross-sectional image. Atheroma area was calculated as EEM area minus luminal area. As reported previously, observer variability to measure EEM and lumen areas are negligible (14).
Based on the Simpson rule, the primary end point, percent atheroma volume (PAV), was calculated as follows:
PAV (%) = [Σ(EEMarea − LUMENarea)/Σ EEMarea] ×100
EEMarea is the cross-sectional area of the EEM, and lumenarea is the cross-sectional area of the lumen. The change in PAV was calculated as the PAV at 9 weeks minus the PAV at baseline.
A secondary efficacy end point, normalized total atheroma volume (TAVnormalized), was calculated as follows:
TAVnormalized (mm3) = [Σ(EEMarea − LUMENarea)/Number of slices in pullback] × Median number of slices in study population
The change in TAVnormalized was calculated as the TAVnormalized at 9 weeks minus the TAVnormalized at baseline.
Any progression was defined as change in PAV >0%.
Volumes occupied by the lumen and EEM were similarly calculated by summation of their respective areas in each measured image and subsequently normalized to account for differences in segment length between subjects as follows:
Lumen volume (mm3) = [Σ(LUMENarea)/Number of slices in pullback] × Median number of slices in study population
EEM volume (mm3) = [Σ(EEMarea)/Number of slices in pullback] × Median number of slices in study population
Statistical analysis
Continuous variables are expressed as mean ± SD or median and categorical variables as percentage. The Chi-square test was used to test for differences in categorical variables between groups and continuous data were compared using unpaired t-tests, or Mann-Whitney log rank tests when the variable was not normally distributed.
Simple linear regression was used for assessing the correlation between clinical variables and change in PAV under CER-001 infusions. Local polynomial regression curve fitting technique was used for plotting the relationships between baseline PAV and change in PAV in 276 subjects receiving CER-001. Receiver operating characteristics curve analysis using the Youden index was performed to identify the cut-off value of baseline PAV associated with any regression of atheroma burden (change in PAV <0%). Serial change in atheroma volume in patients stratified according to the cut-off value of baseline PAV was compared by using analysis of covariance adjusting for clinical characteristics (age, gender, body mass index, hypertension and hypercholesterolemia), medication use (statin, high-intensity statin and angiotensin converting enzyme inhibitor) and the degree of risk factor control (change in glycated hemoglobin, high-sensitivity c-reactive protein and diastolic blood pressure). A high-intensity statin was defined as the dose of atorvastatin and rosuvastatin ≥40 and 20 mg, respectively. In sensitivity analyses, change in PAV was examined using analysis of covariance, with the baseline value as a covariate.
Based on the optimal cut-off value of baseline PAV from the above analysis, overall subjects in CHI-SQUARE study (n=369) were stratified into two groups. In each group, the effect of CER-001 at different doses (3, 6 or 12 mg/kg) on change in PAV was compared to placebo group by using analysis of covariance. A value of P<0.05 was considered significant. All statistical analyses were performed using SPSS statistics version 22.0 (IBM, Chicago, IL, USA).
Results
Relationship of baseline PAV with atheroma regression in subjects receiving CER-001
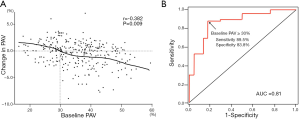
Table 1 summarizes the relationship between clinical variables and change in PAV in 276 patients receiving CER-001 infusions (Table 1). Significant correlations were observed between baseline PAV, absolute change in glycated hemoglobin, and the change in PAV. These correlations remained significant on multivariate analysis [baseline PAV; β coefficient, −0.08, 95% confidence interval (CI), −0.11 to −0.05, P=0.006, absolute change in glycated hemoglobin: β coefficient, −0.39; 95% CI, −0.56 to −0.22, P=0.03] (Table 1). The relationship of baseline PAV with change in PAV was illustrated in Figure 1A. Greater baseline PAV was associated with more atheroma regression in response to infusions of CER-001 (r=−0.382, P=0.009). In particular, baseline PAV below 29.8% corresponded to no net regression of coronary atheroma, whereas atheroma regressed in patients with baseline PAV above 29.8%. Receiver operating characteristics curve analysis determined that a baseline PAV ≥30.0% associated with PAV regression following CER-001 infusions (Figure 1B, AUC =0.81, sensitivity =89.5%, specificity =83.8%).
Full table
Clinical demographics and risk factor control in subjects receiving CER-001
Based on the above observation, the current study firstly compared clinical demographics and IVUS data in 276 patients receiving CER-001 infusions stratified according to baseline PAV <30% (n=74) and ≥30% (n=202). Baseline clinical characteristics are summarized in Table 2. Patients with baseline PAV ≥30% were more likely to have hypercholesterolemia (93.6% vs. 85.1%, P=0.03). They were less likely to be obese (29.4±5.6 vs. 30.8±6.0 kg/m2) and were more likely to have a history of hypertension (91.0% vs. 82.4%) and receive a high-intensity statin (36.6% vs. 24.3%) and angiotensin converting enzyme inhibitor (45.5% vs. 33.7%) although all of these comparisons did not meet statistical significance (P=0.07, 0.06, 0.06 and 0.07, respectively). The use of other anti-atherosclerotic medical therapies, assigned dose of CER-001 and the prevalence of unstable angina, ST segment and non-ST segment elevation myocardial infarction were comparable between the groups (Table 2). Risk factor control under infusions of CER-001 is summarized in Table 3. There were no significant differences in risk factor profiles at baseline. Patients with baseline PAV ≥30% exhibited a higher level of diastolic blood pressure at follow up (76.6±10.4 vs. 73.3±9.1 mmHg, P=0.01).
Full table
Full table
Baseline atheroma burden and vessel dimensions in subjects receiving CER-001
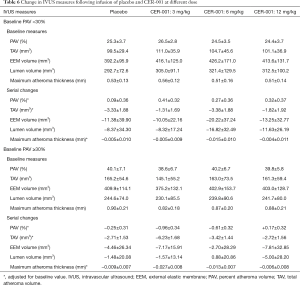
Table 4 shows baseline IVUS measures in the two baseline PAV groups treated with CER-001. Predictably, all measures of plaque burden were greater at baseline in patients with baseline PAV ≥30% (PAV: 39.6%±6.4% vs. 25.3%±3.4%, P<0.001; TAVnormalized: 157.3±64.3 vs. 106.1±39.2 mm3, P<0.001; maximum atheroma thickness: 0.8±0.2 vs. 0.5±0.1 mm, P<0.001). EEM volume was similar in the two groups (395.0±139.7 vs. 418.5±140.9 mm3, P=0.21), whereas patients with baseline PAV ≥30% demonstrated a smaller lumen volume (237.6±85.5 vs. 312.3±105.8 mm3, P<0.001), consistent with more extensive disease.

Full table
Serial changes in atheroma burden and vessel dimensions in subjects receiving CER-001
Changes in atheroma burden and vascular dimensions in patients treated with CER-001 are summarized in Table 5. Under the therapy, patients with baseline PAV ≥30% exhibited a more favorable effect on change in PAV (−0.45%±2.65% vs. +0.34%±1.69%, P=0.01). After adjusting for differences in baseline PAV and clinical demographics, greater regression of PAV was still observed (−0.28%±0.49% vs. −0.07%±0.42%, P=0.03). Additionally, patients with baseline PAV ≥30% were less likely to demonstrate greater reduction of TAV (−3.91±12.77 vs. −2.21±9.31 mm3, P=0.009) and any degree of PAV progression (45.4% vs. 67.5%, P=0.001). The decrease in EEM and lumen volume was smaller in patients with baseline PAV ≥30% (EEM volume: −5.6±27.0 vs. −14.1±30.6 mm3, P=0.02; lumen volume: −1.74±21.8 vs. −11.96±25.3 mm3, P=0.001).

Full table
Sensitivity analysis
Change in PAV was compared between patients receiving CER-001 who were stratified according to another cut-off value of baseline PAV; 29.8% from Figure 1A (corresponding to no net regression) and 35.8% (median value), respectively. Patients with baseline PAV above 29.8% showed a trend toward more regression of PAV (−0.27%±0.45% vs. −0.10%±0.43%), but this did not meet statistical significance (P=0.08). There was no significant difference in change in PAV between subjects with baseline PAV above vs. below 35.8% (−0.22%±2.79% vs. −0.04%±2.05%, P=0.19).
The degree of atheroma regression in patients receiving different doses of CER-001 versus placebo
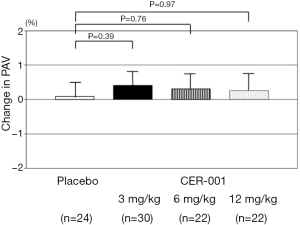
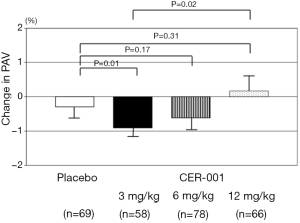
In the overall 369 subjects from CHI-SQUARE study, the effect of CER-001 at different doses (3, 6 or 12 mg/kg) on change in PAV was evaluated and compared to placebo in patients with a baseline PAV <30% or ≥30%, respectively (Figures 2 and 3). In patients with baseline PAV <30%, any dose of CER-001 did not exhibit significant changes in PAV compared to placebo (placebo: +0.09%±0.36%; CER-001 3 mg/kg: +0.41%±0.32%, P=0.39 vs. placebo; 6 mg/kg: +0.27%±0.36%, P=0.76 vs. placebo; 12 mg/kg: +0.32%±0.37%, P=0.97 vs. placebo, Figure 2). In patients with baseline PAV ≥30%, significant regression of PAV was observed in patients receiving CER-001 at 3 mg/kg compared to placebo (−0.96%±0.34% vs. −0.25%±0.31%, P=0.01), whereas there was a non-significant trend in change in PAV between patients receiving placebo vs. CER-001 6 mg/kg (−0.61%±0.32%, P=0.17 vs. placebo) and no difference at 12 mg/kg (+0.17%±0.32%, P=0.31 vs. placebo) (Figure 3). IVUS measures at baseline and its serial change were summarized in Table 6.
Full table
Discussion
HDL infusion therapy has received considerable interest as a novel therapeutic approach to potentially modulate atherosclerotic plaque and reduce cardiovascular events. CER-001 is an engineered pre-beta HDL mimetic agent, with the ability to enhance atheroprotective functionality of HDL (8). While the primary analysis finding of CHI-SQUARE did not show the benefit of this agent to coronary atherosclerosis compared to placebo (10), the current independent and blinded analysis, performed in anatomically matched segments, demonstrated the greatest regression of coronary atherosclerosis in patients with baseline extensive atherosclerosis receiving the 3 mg/kg CER-001 dose, and lesser regression or no regression at 6 and 12 mg/kg doses. These findings underscore baseline plaque burden as a modifiable phenotype and identify patients who are more likely to benefit from use of such therapies in the setting of ACS.
The finding that greater baseline plaque burden is associated with a greater propensity for its regression with CER-001 is supported by a large body of literature. A similar finding was observed with infusions of reconstituted HDL containing apoA-IMilano in which the greatest degree of regression along the course of an artery was observed at the segment containing the greatest plaque burden (11,12). The current finding extends this observation to the patient level and would also support recent reports that baseline plaque burden is a strong predictor of response to statin therapy (15,16).
Pathological and imaging studies have demonstrated the coronary tree of ACS patients contains greater amounts of lipidic and inflammatory material (17), which may underscore reports that therapies tend to have a greater therapeutic effect in such patients (18,19). The presence of more plaque in specific patients portending a greater chance of regression may also suggest that this pattern of disease is likely to contain even greater amounts of lipid and inflammation, an observation supported by emerging intravascular imaging modalities (20-22).
The current study provides additional insights into a potentially effective dose of CER-001 required to induce atheroma regression in the setting of ACS. In patients with baseline PAV ≥30%, infusion of CER-001 at 3 mg/kg induced a marked and significant regression of coronary atheroma compared to placebo, whereas the 6 mg/kg demonstrated only a trend to regression, and the highest dose of 12 mg/kg did not exhibit any benefit. It is of interest to note that infusions of apoA-IMilano also demonstrated less regression at the higher dose, an observation leading to some speculation that there could be a saturation of lipid mobilization leading to a “reverse” dose response with such infusions (11). Early experience with CER-001 has produced findings which may further support this concept of a U-shaped dose-response curve. When administered to apoE knockout mice, CER-001 across the dosing range of 2–5 mg/kg reduced cholesterol content within the carotid artery wall, while further dosing increases had no greater effect (23). Of particular interest, administration of higher CER-001 doses in mouse models ultimately led to substantial reductions in expression of the cholesterol transporter, ATP binding cassette A1 (ABCA1) (23). This work has suggested that the ABCA1 down-regulation induced by higher doses of CER-001 is associated with a reduction in the ABCA1-specific cholesterol efflux (23). Therefore, the maximally efficient CER-001-mediated cholesterol removal from atherosclerotic plaque can be achieved by minimizing dose-dependent down-regulation of ABCA1 expression. Further investigation will be required what degree lipid composition and particle charge may influence anti-atherosclerotic properties at higher doses.
The findings of this analysis also have potential implications for the use of imaging to triage patients to more intensive therapy. The observation that those with greater plaque burden were more likely to regress with CER-001 infusions complements observations that the presence of greater plaque burden identifies patients who are still likely to have a cardiovascular event, despite treatment with high intensity statin therapy (24). While there is accumulating literature to suggest that such findings tends to promote greater use of medical therapies, the field requires clinical trials to determine whether use of imaging to triage patients to more intensive therapy is a cost effective approach to reducing cardiovascular risk.
Recent failure of agents raising HDL-C in prospective randomized trials have led to a shift in thinking to the importance of promoting HDL quality as opposed to quantity of circulating cholesterol in the HDL fraction (25). HDL infusions rapidly increase circulating pre-beta-like HDL particle number, enhance efflux capacity for cellular cholesterol and may positively impact other HDL properties that have been previously reported (7). These findings are hypothesis generating, and inform the design of future clinical trials that will evaluate CER-001 as a potential therapy. As the current analysis describes CER-001 efficacy at a 3 mg/kg dose in patients with more atheroma burden, it highlights a potential role that imaging studies might play in drug development, via their ability to optimize the design of the next step. Accordingly, the impact of CER-001 3 mg/kg will be directly compared with placebo in ACS patients with baseline PAV ≥30% in the CER-001 Atherosclerosis Regression ACS Trial (CARAT: NCT02484378).
Multivariate analysis identified that change in glycated hemoglobin was associated with plaque progression in our study subjects. While our previous IVUS studies showed diabetes mellitus as an independent contributor to plaque progression, there was no significant relationship of glycated hemoglobin with change in PAV in diabetic patients with CAD (16,26). Whether infusion of CER-001 modulates the association between glycemic control and progression of coronary atherosclerosis will require further investigation.
Limitations
A number of caveats should be noted. Our findings are a post hoc analysis of films from a clinical trial that failed to meet its primary efficacy end point of elucidating differences in coronary atheroma regression between placebo and CER-001 at three different doses (10). Therefore, current results should be considered as hypothesis generating. Given the post hoc nature of our analysis, there were some differences in body mass index, risk factors and medication use between patients with different degrees of plaque burden. However, the differences in propensity to regression with CER-001 persisted after controlling for these differences. Additional residual confounding may have influenced the degree of atheroma regression, biasing our results. We could not evaluate the association of atheroma regression with CER-001 and clinical outcomes due to relatively small study population, this will ultimately require a large clinical outcomes trial. It should also be noted that gray scale IVUS does not have enough capability to visualize plaque composition.
Conclusions
In summary, the presence of more extensive baseline coronary atheroma burden was associated with its regression under the infusion of CER-001 in ACS subjects. Administration of 3 mg/kg CER-001 induced the greatest atheroma regression in ACS subjects with baseline PAV ≥30%. Our findings indicate ACS patients with more extensive atherosclerotic disease most likely to benefit from HDL mimetic therapy at this dose. Whether this agent ultimately comes to clinical practice will require further investigation in large clinical trials.
Acknowledgements
The CHI-SQUARE study and this independent and blinded analysis were funded by Cerenis Therapeutics.
Footnote
Conflicts of Interest: SJ Nicholls is a Principal Research Fellow of the National Health and Medical Research Council (NHMRC) of Australia and has received speaking honoraria from AstraZeneca, Amgen, Pfizer, Eli Lilly, Merck and Takeda, consulting fees from Amgen, AstraZeneca, Eli Lilly, Pfizer, Merck, Takeda, Roche, NovoNordisk, LipoScience and Anthera and research support from AstraZeneca, Cerenis, Amgen, Eli Lilly, Anthera, Sanofi-Regeneron, Novartis, Resverlogix and Lipid Sciences. R Puri is supported by the Neil Hamilton Fairley NHMRC Overseas Early Career Research Fellowship and has received consulting fees from Sanofi-Regeneron and Cerenis. Y Kataoka has received honoraria from Takeda, Kowa and Amgen Astellas BioPharma, and research support from Cerenis. Constance Keyserling, JF Paolini and JL Dasseux are employed by Cerenis Therapeutics. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by each participating center’s institutional review board (Sponsor Protocol No.: CER-001-CLIN-002; WIRB Protocol No.: 20110078; WIRB Study No.: 1122902) and all patients provided written, informed consent prior to entering the study.
References
- Mineo C, Shaul PW. Novel biological functions of high-density lipoprotein cholesterol. Circ Res 2012;111:1079-90. [Crossref] [PubMed]
- Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res 2004;95:764-72. [Crossref] [PubMed]
- Fisher EA, Feig JE, Hewing B, et al. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol 2012;32:2813-20. [Crossref] [PubMed]
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012;125:1905-19. [Crossref] [PubMed]
- Lüscher TF, Landmesser U, von Eckardstein A, et al. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res 2014;114:171-82. [Crossref] [PubMed]
- Mineo C, Deguchi H, Griffin JH, et al. Endothelial and antithrombotic actions of HDL. Circ Res 2006;98:1352-64. [Crossref] [PubMed]
- Kingwell BA, Chapman MJ. Future of high-density lipoprotein infusion therapies: potential for clinical management of vascular disease. Circulation 2013;128:1112-21. [Crossref] [PubMed]
- Tardy C, Goffinet M, Boubekeur N, et al. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis 2014;232:110-8. [Crossref] [PubMed]
- Kootte RS, Smits LP, van der Valk FM, et al. Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J Lipid Res 2015;56:703-12. [Crossref] [PubMed]
- Tardif JC, Ballantyne CM, Barter P, et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 2014;35:3277-86. [Crossref] [PubMed]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292-300. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol 2006;47:992-7. [Crossref] [PubMed]
- Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001;37:1478-92. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071-80. [Crossref] [PubMed]
- Puri R, Nissen SE, Ballantyne CM, et al. Factors underlying regression of coronary atheroma with potent statin therapy. Eur Heart J 2013;34:1818-25. [Crossref] [PubMed]
- Bayturan O, Kapadia S, Nicholls SJ, et al. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol 2010;55:2736-42. [Crossref] [PubMed]
- Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;368:2004-13. [Crossref] [PubMed]
- Puri R, Nissen SE, Shao M, et al. Antiatherosclerotic effects of long-term maximally intensive statin therapy after acute coronary syndrome: insights from Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin. Arterioscler Thromb Vasc Biol 2014;34:2465-72. [Crossref] [PubMed]
- Kataoka Y, Wolski K, Balog C, et al. Progression of coronary atherosclerosis in stable patients with ultrasonic features of high-risk plaques. Eur Heart J Cardiovasc Imaging 2014;15:1035-41. [Crossref] [PubMed]
- Puri R, Madder RD, Madden SP, et al. Near-Infrared Spectroscopy Enhances Intravascular Ultrasound Assessment of Vulnerable Coronary Plaque: A Combined Pathological and In Vivo Study. Arterioscler Thromb Vasc Biol 2015;35:2423-31. [Crossref] [PubMed]
- Tian J, Dauerman H, Toma C, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol 2014;64:672-80. [Crossref] [PubMed]
- Kataoka Y, Hammadah M, Puri R, et al. Plaque microstructures in patients with coronary artery disease who achieved very low low-density lipoprotein cholesterol levels. Atherosclerosis 2015;242:490-5. [Crossref] [PubMed]
- Tardy C, Goffinet M, Boubekeur N, et al. HDL and CER-001 Inverse-Dose Dependent Inhibition of Atherosclerotic Plaque Formation in apoE-/- Mice: Evidence of ABCA1 Down-Regulation. PLoS One 2015;10:e0137584. [Crossref] [PubMed]
- Puri R, Nissen SE, Shao M, et al. Coronary atheroma volume and cardiovascular events during maximally intensive statin therapy. Eur Heart J 2013;34:3182-90. [Crossref] [PubMed]
- Barter PJ, Rye KA. Targeting High-density Lipoproteins to Reduce Cardiovascular Risk: What Is the Evidence? Clin Ther 2015;37:2716-31. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008;52:255-62. [Crossref] [PubMed]


