Pulmonary hypertension: diagnosis, imaging techniques, and novel therapies
Introduction
Pulmonary hypertension (PH) encompasses a heterogeneous group of clinical disorders characterized by increased pulmonary artery pressure. By definition, PH exists in patients with a mean pulmonary arterial pressure (mPAP) greater than or equal to 25 mmHg as assessed by right heart catheterization (RHC) (1). While the exact prevalence of PH is unknown, registry data offers important mortality and hospitalization data for clinicians. In the United States of America between 2001 and 2010, the death rate for PH, based on the International Classification of Disease, 10th Revision codes for mortality from primary and secondary PH, was estimated at 6.5 deaths per 100,000 people and with 131 hospitalizations per 100,000 discharges (2).
Classification
To improve management of a pathophysiologically diverse group of related diseases, a common classification for PH was created in 1998 (3). The groupings separate patients by mechanism of disease, which ultimately help guide management. These guidelines were updated in 2013 to reflect improved understanding of PH (Figure 1). The 2013 World Health Organization (WHO) classification divides PH into the following categories: group 1, pulmonary arterial hypertension (PAH); group 2, PH due to left heart disease; group 3, PH due to lung diseases and/or hypoxia; group 4, chronic thromboembolic PH (CTEPH) and other pulmonary artery obstructions; and group 5, PH with unclear and/or multifactorial mechanisms (4).

Group 1 PH, also known as PAH, includes idiopathic; heritable; drug/toxin induced PAH; and PAH associated with connective tissue disease, human immunodeficiency virus, portal hypertension, congenital heart disease, or schistosomiasis. PAH is rare, with an observed prevalence of 15 cases per million adults according to French registry data (5). Within the PAH population, 50 percent belong to the first three sub-types. RHC demonstrates a pulmonary artery wedge pressure (PAWP) less than or equal to 15 mmHg, thus excluding left heart failure as the cause of elevated mPAP. In addition, pulmonary vascular resistance (PVR) is greater than 3 Woods units (6). PAH is precapillary PH because the level of obstruction to flow is at the precapillary level (as opposed to group 2 where it is at the postcapillary level).
Group 2 PH can be attributed to left-sided heart disease, including heart failure with reduced ejection fraction (HFrEF), heart failure with preserved ejection fraction (HFpEF), mitral or aortic valve disease, or congenital/acquired left inflow or outflow tract obstruction and congenital cardiomyopathies. Since left-sided heart disease is a relatively common condition, especially in the United States, group 2 PH is by far the most common category. Approximately 60% of patient with severe HFrEF and 70% of patient with HFpEF, present with PH symptoms. Furthermore, PH is found in nearly 100% of patients with symptomatic mitral valve disease and up to 65% of patients with severe symptomatic aortic stenosis (7). Nearly half of patients with heart failure have HFpEF (8). In a study of 244 patients with HFpEF, followed with annual transthoracic echocardiograms (TTE) over a three year period, PH was present in 83% of patients. This cohort had pulmonary artery systolic pressures (PASPs) which were higher than those with hypertension without diastolic dysfunction, suggesting a component of PAH in addition to pulmonary venous hypertension (9).
The WHO classification scheme characterizes PH secondary to lung disorders and chronic hypoxia as group 3 hypertension. Chronic obstructive lung disease, interstitial lung disease, mixed restrictive/obstructive lung disease, sleep-disordered breathing, alveolar hypoventilation disorders, chronic high altitude exposure, and developmental lung diseases all belong to group 3 PH. Severe PH is relatively uncommon in this sub-type, except in the setting of coexistent emphysema and fibrosis (10).
CTEPH and pulmonary artery obstructions, such as those caused by angiosarcoma, other intravascular tumors, arteritis, congenital pulmonary artery stenosis, and hydatidosis comprise group 4 PH. The Spanish PH registry found a prevalence of CTEPH of 3.2 cases per million and incidence of 0.9 cases per million per year (4,11).
Group 5 encompasses all the remaining causes of PH. This group includes various disease processes and includes hematological disorders such as the hemolytic anemias, systemic disorders, metabolic disorders, and others.
Since the classification schema was formalized, the inclusion criteria for each group have been subject to change, based on improved understanding of the disease. The 2013 WHO classification (12) and the 2015 European Society of Cardiology and the European Respiratory PH guidelines highlight these alterations in detail—clinically significant changes include newly identified gene mutations in group 1 PAH, reclassification of several pediatric pulmonary conditions, and the inclusion of pulmonary artery obstructions in group 4 PH and the hemolytic anemias including sickle cell anemia in group 5 (7).
Diagnosis
Typical symptoms of PH include dyspnea, fatigue, angina, or syncope. The presence of symptoms is related mainly to progressive right ventricular (RV) failure (7). However, the presenting symptom may mirror the underlying pathophysiology. PAH (group 1 PH) patients often complain of progressively worsening dyspnea on exertion. One should look for a collagen vascular disorder, HIV, exposure to anorexigen drugs or illicit drugs (amphetamines, cocaine), dasatinib, and for a family history (10). Group 2 patients can suffer from typical heart failure symptoms, including dyspnea on exertion, chest pain, orthopnea, paroxysmal nocturnal dyspnea, and lower extremity edema. In group 3 PH, patients may have a known lung disorder or risk factors, such as smoking, which contribute to shortness of breath. History of a hypercoagulable state, splenectomy, pacemaker leads, family or personal history of frequent miscarriages, or recurrent deep vein thrombosis may provide clues to a diagnosis of CTEPH (group 4 PH).
Physical exam findings in PH correlate to the degree of right heart failure (10). Blood pressure is often low to low-normal owing to RV pump failure. There can be compensatory tachycardia. Oxygen saturation may be low to normal. Jugular venous pulsation is often elevated with an elevated ‘a’ wave, corresponding to decreased RV compliance. Auscultation may reveal a loud P2, corresponding to elevated PA systolic pressures. Right-sided valvular murmurs, such as tricuspid regurgitation may reflect a dilated right heart; while left-sided murmurs such as aortic stenosis or mitral stenosis may point to an underlying cause. The peripheral exam may reveal hepatomegaly, ascites, and lower extremity edema.
Numerous laboratory parameters have been analyzed as potential biomarkers for the diagnosis and monitoring of PH. Brain natriuretic peptide (BNP) and pro-BNP have been used for years as surrogates for mortality risk (13). Uric acid levels in the blood have found to be inversely proportional to PVR, but the clinical implication of this association is unknown (14). In a recent meta-analysis, Foris et al. examined markers related to heart failure, inflammation, hemostasis, remodeling, and endothelial cell-smooth muscle cell interaction. Unfortunately, there is no clear clinically established role for these tests currently (15). Finally, high sensitivity troponin (hsTn), a marker for myocyte necrosis, is detectable in 95% of patient with PH. The degree of hsTn elevation is positively correlated with an increase in mortality, and therefore may have prognostic value (16).
Other tests commonly done are the 6-minute walk test to assess functional performance, pulmonary function testing and arterial blood gases. Depending on the clinical situation, serologies for autoimmune diseases and genetic testing may be considered.
Imaging
Electrocardiogram (ECG)
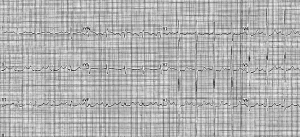
The initial work-up of suspected PH should include a screening 12-lead ECG (Figure 2). ECG tracings may show left-sided heart disease, such as left ventricular hypertrophy or left atrial enlargement, which helps point to an underlying cause. In more advanced disease, signs of right heart disease evolve. Rightward axis deviation often accompanies RV hypertrophy. Similarly, higher R waves in V1, V2, I, II, III, and aVF, and deeper S waves in I, aVL, and V3–V6 reflect a larger RV mass. An increased S wave in lead I with an associated Q wave and T wave inversion in lead III (S1Q3T3 pattern), may be suggestive of RV strain such as seen in thromboembolic disorders (17). Pulmonary disease or left heart disease may be accompanied by frequent atrial premature contractions and multifocal atrial tachycardia or atrial fibrillation.
TTE
In patients with suspected PH based on clinical history, physical exam findings, and with or without ECG evidence of right heart failure, TTE is the preferred, non-invasive screening test (18). TTE provides a multitude of reliable parameters to assess pulmonary hemodynamics (Figure 3). The calculation of systolic PAP (PASP) and mPAP relies on three easily obtained values, tricuspid regurgitation peak velocity (TRV), peak pulmonary regurgitation velocity (PRV), and right atrial pressure (RAP). TRV is positively correlated with PASP and mPAP values found on invasive RHC. PASP can be calculated with the equation 4*TRV2+RAP, where RAP is estimated using the absolute inferior vena cava (IVC) size and the amount of collapsibility during respiration in non-intubated individuals. mPAP is estimated using the formula 4*PRV2+RAP (19). Measurement of PVR relies on the recognition that PVR is directly related to changes in pressure, but inversely related to pulmonary flow, as estimated by the time velocity integral of the RVOT (TVIRVOT). Thus, TRV/TVIRVOT*10+0.16 can predict PVR (20).
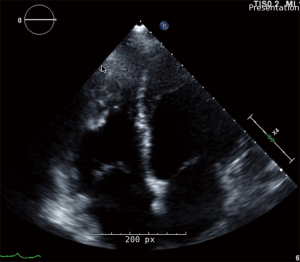
The Tei myocardial performance index (MPI) is a dimensionless index which measures the efficiency of the RV during systole. Tei MPI is the ratio between the sum of the isovolumic contraction time and the isovolumic relaxation time over the ejection time. More time spent ejecting blood from the RV means better function of the chamber; thus the lower the index, the more efficient the RV. In patients with idiopathic PAH (iPAH), the Tei index has been shown to be significantly higher than in healthy controls (0.83 vs. 0.28). Those patients with a Tei MPI greater than 0.83 had a five-year event-free survival rate less than 10%, compared to greater than 70% if Tei MPI less than 0.83 (21).
In addition to pulmonary hemodynamics, TTE evaluation of the right ventricle provides important indirect evidence of PH. The right ventricle can be difficult to study precisely with TTE owing to its irregular shape and non-uniform contraction pattern (13). Tricuspid annular plane systolic excursion (TAPSE) is a surrogate marker for RV function using M-mode on TTE. As the RV dilates and function declines, the tricuspid annulus has less movement per cardiac cycle. Other markers of right heart failure include increased RV wall thickness, RV dilation, RA dilation, and RV outflow tract dilation. Increased pressure on the right side of the heart can cause the intraventricular septum to flatten and giving rise to a “D-shaped” appearance of the left ventricle (LV) (22). The majority of patients with PAH have echocardiographic evidence of chronic RV overload at time of diagnosis. This is manifest as RV hypertrophy, intraventricular septum abnormalities, and decreased RV systolic function. Visually, the presence of a large pericardial effusion, large right atrium, large right ventricle, and an inter-atrial septum bowing from right to left suggests the diagnosis of PH (23). A mid-systolic notch pattern is particularly noteworthy, and more likely to be seen in patients with PAH and CTEPH (24). The presence of RV dysfunction, diminished LV cavity size, and RV dysfunction are also markers of a poorer overall prognosis (25).
Two-dimensional strain (TDS) analysis uses the concept of speckle-tracking to assess RV function. TDS is an attractive adjunct to traditional TTE given its easy attainability, cost-effectiveness, and angle independence. However, as with any novel technology, its adoption suffers from a lack of robust outcomes data and the question of reproducibility (18). Several smaller studies have analyzed TDS in the PH population. Declining peak RV longitudinal strain parameters were correlated with increased mortality (26) and worsening New York Heart Association (NYHA) functional class (FC) (27). While initial studies of TDS have been promising, further research is required before full-scale implementation in PH.
Postcapillary (elevated PCWP >15 mmHg) PH is a defining feature of group 2 PH (28). The current classification system divides WHO group 2 PH into the subcategories of systolic heart failure, diastolic heart failure, and valvular heart disease. A recent study showed that in patients with HFrEF, the probability of PH was correlated with abnormal diastolic parameters, but not systolic ejection fraction (29). Thus, a potentially more useful classification might include passive, reactive and mixed PH, which takes into account the presence or absence of pulmonary vascular disease. This classification relies on the transpulmonary pressure gradient (TPG) and the PVR.
TPG is defined as mPAP minus PCWP and the PVR is calculated as TPG divided by the cardiac output (30). Passive PH refers to elevated mPAP as a result of downstream pressure exerted on the pulmonary arteries. By definition, TPG and PVR are normal (6±2 mmHg and 0.9±0.4 Wood units) (31) because the abnormality does not include the pulmonary arteries. These patients are best treated with standard heart failure treatment in an effort to decrease PCWP. When TPG and PVR are elevated in group 2 PH, patients necessarily have an elevated mPAP as a consequence of elevated PCWP in addition to structural abnormalities of the pulmonary vasculature (7). However, the TPG can be unreliable as it is sensitive to changes in cardiac output, distension of the pulmonary vessels, and the pulsatility of pulmonary blood flow. By measuring the pressure gradient between the PA in diastole and the PCWP, known as the diastolic pressure gradient (DPG), many of these confounding parameters are eliminated. Thus, DPG may in fact be superior to TPG in patients with mixed PH (32). Contrary to the current guidelines, if group 2 patients have an element of pulmonary arterial disease in combination with elevated PCWP, pulmonary arterial specific agents may have utility (33).
Cardiac computed tomography (CT)
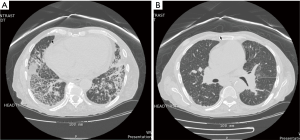
Both high-resolution computed tomography (HRCT) and computed tomography angiography (CTA) have a role in the diagnosis and management of PH. Specifically, PH may be manifested as main pulmonary artery dilatation greater than or equal to 29 mm, a pulmonary artery:ascending aorta ratio greater than or equal to 1.0, and/or a segmental artery:bronchus ratio greater than 1.0 in three or four lung lobes on CT (33). A case series of nearly 300 patients examined the statistical performance of CT-derived mean pulmonary artery diameter (mPAD) in the diagnosis of PH. The authors concluded that an mPAD greater than 29.5 mm had a sensitivity of 70.8% and a specificity of 79.4% for PH. When the cutoff is changed to an mPAD greater than 31.5 mm, the sensitivity decreases to 52.0%, but the specificity increases to 90.2% (34). Given the relatively poor sensitivity performance at both mPAD cutoffs, the test is likely not useful for screening patients with presumed PH. HRCT is especially useful for characterization of changes to lung parenchyma seen in WHO group 3 diseases such as interstitial lung disease or COPD (Figure 4) (35). In patients with CTEPH, CTA often shows central ground-glass opacifications, thickened lung septa, and pleural effusions. Changes in the pulmonary arteries include webbing, blind pouches, synechiae, abrupt termination and organized fibrotic thrombus. While CTA information may prove useful clinically in WHO group 4, a ventilation-perfusion nuclear medicine scan is the preferred screening modality due to its improved sensitivity (36). In fact, up to 25% of cases of CTEPH may be missed if the workup includes only a CTA without a VQ scan (1,37-39). CTA also adds utility in determining the surgical candidacy of CTEPH patients (40). Changes limited to the distal 1/3rd of the pulmonary arterial tree makes a surgical approach (thromboendarterectomy) to the treatment of CTEPH difficult.
Magnetic resonance imagining (MRI)
In the past decade, cardiac magnetic resonance imaging (CMR) has become the “gold-standard” in the assessment of cardiac structure and function (41). Compared to standard 2-dimensional TTE, CMR has superior reproducibility regarding ventricular mass and function analysis. Since CMR acquires 3-dimensional images, it does not rely on geometrical assumptions like TTE (42). For example, compared to the TAPSE method in TTE, MRI relies on geometric shortening in longitudinal and transverse planes, which more accurately estimates RV ejection fraction (43).
In addition to the standard RV assessment, CMR has several techniques unique to the modality. Late gadolinium enhancement (LGE) is the delayed uptake of MRI contrast which can detect fibrosis/scarring. LGE at the RV insertion points on the interventricular septum is commonly seen in advanced PH. A higher degree of enhancement correlates with worse RV performance parameters (44). While the degree of LGE is useful in prognosis in advanced disease, an earlier marker of “at risk” myocardium could decrease morbidity and mortality—in this regard, myocardial T1 being a measurement of its longitudinal relaxation properties is prolonged at an earlier stage in most forms of cardiac pathology (45). Mapping myocardial T1 values before and after gadolinium administration allows for extracellular volume (ECV) assessment. ECV quantifies the expansion of the extracellular matrix as a result of diffuse reactive fibrosis. In an animal model of PH, native T1 and Eq-ECV values at the RV insertion points were high in the PH arm, before the onset of overt RV failure. Thus T1 mapping may allow for early detection of PH (Figure 5) (46).
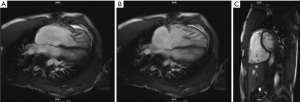
MRI can also provide information on abnormalities in pulmonary artery (PA) flow profiles that occur at an early stage in PH, as well as arterial compliance, pulsatility and distensibility which are reduced and have prognostic significance. 4D flow MRI can show abnormal flow vortices in the PA and provide measures of wall shear-stress. Aside from RV indices such as volumes and EF, MRI can assess abnormalities in the septal curvature and time to peak ejection and assess RV perfusion and strain. In CTEPH, it provides adjunctive imaging of the pulmonary vasculature, useful in determining operability.
The current PH guidelines suggest CMR can be useful as an adjunct to TTE and RHC. Baseline and follow-up CMR has important prognostic value in PAH (47). CMR will likely see and increasing role in the diagnosis and management of PH in the near future (48).
RHC
RHC is required to diagnose PAH and CTEPH, but also may be useful in assessing the severity of PH, and in determining reversibility according to the most recent PAH guidelines. Despite the invasive nature of RHC the rate of complication is low; morbidity is estimated at 1.1% and mortality at 0.055% (49). A complete study should include pressure measurements in the RV, RA, PA, and PA wedge positions; blood oximetry measurements from the IVC, SVC, RA, RV, PA, and systemic artery; and cardiac output measured via thermodilution or Fick. The diagnosis of PAH is supported by a PCWP less than or equal to 15 mmHg and PVR greater than 3 Wood units, along with an mPAP of >25 mmHg. RHC is recommended in patients with congenital cardiac shunts to help guide treatment. Finally, patient with group 2 and 3 PH should have RHC if heart and/or lung transplantation is being considered.
Vasoreactivity testing (VT) is a useful adjunct to RHC in certain patient populations. VT is recommended in patients with suspected group 1 PAH (50). Vasoreactivity is defined as a reduction of mPAP of greater than or equal to 10 mmHg to reach an absolute value less than or equal to 40 mmHg, without a reduction in CO (23). In patients with a positive VT, the administration of calcium channel blockers (CCBs) provides a survival benefit (51).
Treatment
Medical therapy
Treatment of PAH follows a step-wise approach. It is prudent to consider referring patients to an expert center well versed in the diagnosis, follow up and treatment of patients with PAH. As mentioned above, CCBs are effective in patients with positive VT. Most studies have focused on nifedipine, diltiazem, and amlodipine (52). Guidelines recommend that vasoreactive patients should receive aggressively titrated CCB therapy, with assessment of response following 3–4 months of treatment.
PAH patients with inadequate response to CCB or with negative VT qualify for advanced PH therapies, including endothelin receptor antagonists (ERAs), phosphodiesterase 5 inhibitors (PDE-5i) and prostacyclin analogues (PAs). The current advanced PH treatments work by decreasing PVR, lowering PAP, and relieving symptoms, but have no curative intent. Low to intermediate risk patients are typically started on oral monotherapy or oral combination therapy. No drug class has been shown to be superior for initial monotherapy. However, initial combination therapy with ambrisentan with tadalafil has been shown to be superior to either medication alone, thus it has received a higher grade of recommendation (53).
ERAs include ambrisentan, bosentan, and macitentan. As a class, ERAs work by binding to endothelin receptor A and/or receptor B, which inhibits the vasoconstriction caused by endothelin-1 (13). ERAs have the added advantage of oral administration and are recommended for NYHA class II and III (7). PDE-5i such as tadalafil, sildenafil, and vardenafil were initially marketed for the treatment of erectile dysfunction. The mechanism of action is the inhibition of the enzyme responsible for the degradation of cyclic guanosine monophosphate (cGMP). The resultant increase in cGMP leads to pulmonary and systemic vasodilation via the effect of nitric oxide (NO) on arteries. Compared to the ERAs, sildenafil has a similar effect on exercise duration, hemodynamics, and symptoms (54). PDE-5is are approved in NYHA class II and III. Finally, PAs replace the natural prostaglandin that is reduced in PH patients. Epoprostenol and treprostinil are approved for NYHA class II and IV, largely owing to their intravenous administration and potency (55). Iloprost is an inhaled PA that is administered 6–9 times a day. Selexipag is an oral selective prostacyclin IP receptor agonist that functions similarly to endogenous prostacyclin. Use of selexipag alone or as part of combination therapy lead to a 40% reduction in a combined endpoint of all-cause death, hospitalization, need for surgical intervention or initiation of IV therapy (31). As PAH progresses, the majority of patients will receive combination therapy, escalating to parenteral therapy.
Effective treatment for HFpEF remains difficult, especially when PH coexists. The RELAX trial was the first to study the effect of PDE-5 inhibitors on the clinical status of patients with HFpEF. In a subgroup analysis of those patients with concomitant PH, defined as PASP greater than or equal to 40 mmHg, there was no difference in the change in peak VO2 at 24 weeks in the sildenafil arm compared to placebo (56). Long-acting nitrates have also been examined as an initial treatment in the HFpEF population. Specifically, in patients with NYHA class II–III HF, the use of long-acting nitrates were shown in 110 patients to reduce, not improve, daily total activity levels (57). Treatment of HFpEF with PH remains particularly challenging for clinicians.
The treatment of inoperable CTEPH has been revolutionized by the discovery of soluble guanylate cyclase (sGC) stimulators like riociguat. Riociguat was approved by the Federal Drug Administration for the treatment of PAH and CTEPH in October 2013 (58). Normally, NO binds to sGC, which stimulates the production of cGMP. As described above, increased cGMP leads to pulmonary arterial vasodilation via vascular smooth muscle cell activation. This class of compounds directly stimulates sGC in the absence of NO, producing anti-aggregatory, anti-proliferative, and vasodilatory effects. The CHEST-1 phase 3 clinical trial for Riociguat, found that in 261 patients with inoperable CTEPH, exercise capacity and PVR were significantly improved at 16 weeks of therapy (59).
Current PH treatment guidelines advocate for the routine administration of long-term anticoagulation (LTAC) in patients with iPAH (60). This recommendation is based on observational studies suggesting a pro-thrombotic state in this patient population. Using data from the REVEAL registry, the effect of LTAC was assessed in patients with iPAH and in those with PAH related to systemic sclerosis (SSc-PAH). Survival analysis in 187 participants suggested no advantage for LTAC in iPAH and a survival disadvantage for LTAC in SSc-PAH (61). Thus, the recommendation for LTAC in iPAH remains somewhat controversial. LTAC plays no role in the management of other types of PH, except for group 4. In patients with CTEPH, lifelong anticoagulation is recommended.
Novel agents for the treatment of PAH are under investigation. Imatinib, a monoclonal antibody used in the treatment of chronic myeloid leukemia, has also been shown to have inhibitory effect on platelet derived growth factor and c-KIT signaling, which have been implicated in the development of PH. Used as add-on therapy, Imatinib significantly improved exercise tolerance and hemodynamics in advanced PAH. Despite these benefits, imatinib had no effect on mortality, FC, or time to clinical worsening (39). Likewise, inhaled vasoactive intestinal peptide and serotonin antagonists have also been explored with unsatisfactory results. Further research is being directed at rho kinase inhibitors, vascular endothelial growth factor receptor inhibitors, angiopoietin-1 inhibitors, and elastase inhibitors. Gene and stem cell therapy also shows promise (7).
Invasive/surgical interventions
Patients who fail combination medical therapy may be candidates for invasive and or surgical interventions for treatment of severe PH. Interventional techniques are generally regarded as palliative in nature or serving as a bridge therapy to eventual lung transplantation (62).
Balloon atrial septostomy (BAS) is the only approved interventional treatment in PH, though experimental modalities are being developed (63). The concept of BAS is the creation of a communication between the right and left atria, similar to an atrial septal defect. The resultant right-to-left shunt functionally decompresses the right heart chambers, increases left ventricular preload, and ultimately increases cardiac output (64). BAS involves an atrial septal puncture, insertion of a standard valvuloplasty balloon, and progressive dilation with larger balloons (65). Newer techniques involve the deployment of stents or modified septal occluder devices to prevent AS closure. BAS has been shown to improve cardiac index, decrease RAP, and improve six-minute walk test duration. However, the effect of BAS on long-term survival has not been subject to a randomized controlled trial (66).
Research into potential percutaneous therapies for PH is ongoing. The creation of a direct connection between the left pulmonary artery, and the descending aorta, known as a Pott’s shunt, has been achieved in animal models and a small number of humans (67). Theoretically, this method of decompression has benefits over BAS because deoxygenated blood bypasses the coronary arteries and brain, but remains in the experimental phase (68).
The final option for patients who fail aggressive medical and interventional therapies is definitive heart-lung transplantation and bilateral lung transplantation. These therapies are considered in patients with NYHA class III–IV FC despite combination therapy (69). In patients with iPAH and severe secondary PH, bilateral transplant is preferred over single lung transplant due to decreased risk of allograft edema (70). Post-transplant survival can be stratified based on pre-operative 6-minute walk test distance and peak myocardial oxygen consumption, cardiac index <2.0 L/min/m2 and RAP >15 mmHg (44). In patients who survive the first year post-transplantation, mortality is approximately fifty percent at 9 years (71). Despite the relatively favorable results from transplantation, only approximately thirty percent of patients with PAH are ultimately referred (72).
Pulmonary endarterectomy (PEA) is the treatment of choice for group 4 PH patients with WHO FC II–IV with surgically accessible thrombi in the proximal pulmonary arteries. PEA requires thoracic surgeons to cut through the medial layer of the bilateral pulmonary arteries with patients under deep hypothermia and circulatory arrest (73). Post-operatively, patients are typically maintained on extracorporeal membrane oxygenation (74). PEA is tremendously efficacious with significant symptomatic relief and restoration of near normal hemodynamics post-operatively (75). In high volume centers, in-hospital mortality for PEA is less than 4.7% (76). PEA is a highly invasive procedure and can only be performed for lesions located proximally or in lobar branches (77). As such, there is significant interest in developing a minimally invasive technique to treat CTEPH, especially when distal disease is present. Percutaneous transluminal pulmonary angioplasty (PTPA) may fulfill this need. In a study of 29 patients undergoing PTPA, patients had statistically significant improved in NYHA-FC, plasma BNP levels, and pulmonary hemodynamics at 6 months (78). Further study and clinical experience is necessary before PTPA becomes a mainstream treatment of CTEPH.
Predicting survival
While PAH is universally fatal, the course of disease in patients with group 1 PAH is variable; therefore, identifying predictors of survival is clinically important. The National Institutes of Health (NIH) derived a prognostic equation based on patients enrolled in the Registry of Primary Pulmonary Hypertension from 1983–1987 to aid clinicians in risk-stratifying PAH patients (79). However, the usefulness of this equation lessened as new methods of diagnosis and therapeutics were discovered. Using the modern PAH collected in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL registry), researchers aimed to identify factors that determine survival in PAH. The REVEAL database is a multi-center, prospective, observational registry that enrolled approximately 3,000 consecutively diagnosed patients with WHO group 1 PH (80). The 1-year survival was 91.0%. Nearly 40 characteristics were evaluated for their prognostic values. The variables most strongly associated with increased hazard ratio (>2-fold increase) included: portal hypertension, family history of PAH, men >60 years old, NYHA FC IV, and PVR >32 wood units. Other variables with a significant associated increased hazard ratio included: underlying connective tissue disease, renal insufficiency, NYHA FC III, resting SBP <110 mmHg, resting HR >92 BPM, 6MWD <165 m, BNP >180 pg/mL, presence of pericardial effusion, percent predicted DLCO <32% and mean RAP >20 mmHg. Only four variables were associated with increased 1-year survival: NYHA FC I, 6MWD >440 m, BNP <50 pg/mL, and percent predicted DLCO >80% (81). Using a Cox proportional-hazard multivariable analysis, the investigators developed a prognostic equation for patients with PAH which discriminated between high and low risk patients considerably better than the previous NIH model (0.772 to 0.588). In addition to clinical judgment, this tool aims to help risk stratify patient at time of diagnosis and throughout a patient’s course. More investigation is needed to further explore its utility.
Conclusions
PH comprises a diverse group of diseases which lead to elevation in pulmonary pressures. The WHO classification systems helps to categorize PH based on the underlying pathophysiology. Clinicians must have a high clinical suspicion for PH based on history, physical exam, EKG, and laboratory findings. TTE is the preferred screening test for patients with suspected PH. CT, MRI, and RHC can provide useful adjunctive data for the diagnosis and management of PH. Medical therapy is first line for PH and depends on the underlying mechanism of disease. Research into novel therapeutic agents is robust. In patients who fail combination drug therapy, BAS serves as potential bridge to transplant or as palliative maneuver. Ultimately, bilateral lung transplant or lung heart transplant can allow a significant improvement in mortality. In select CTEPH patients, PEA improves symptoms and hemodynamics. Given the significant morbidity and mortality associated with PH, more research is necessary to diagnose PH earlier in its course, to discover novel therapeutic agents with the potential to reverse the underlying pathology, and to refine interventional and surgical procedures to reduce complications and improve outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62:D42-50. [Crossref] [PubMed]
- George MG, Schlieb LJ, Ayala C, et al. Pulmonary Hypertension Surveillance. Chest 2014;146:476-95. [Crossref] [PubMed]
- Primary pulmonary hypertension executive summary from the world symposium. 1998.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated Clinical Classification of Pulmonary Hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypetension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023-30. [Crossref] [PubMed]
- Rubenfire M. Guidelines for diagnosis and treatment of pulmonary hypertension. J Am Coll Cardiol 2015.
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 2016;69:177. [Crossref] [PubMed]
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9. [Crossref] [PubMed]
- Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community based study. J Am Coll Cardiol 2009;53:1119-26. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219-63. [Crossref] [PubMed]
- Escribano-Subias P, Blanco I, Lopes-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 2012;40:596-603. [Crossref] [PubMed]
- Rich S. Pulmonary Hypertension. In: Lilly LS, Braunwald E. editors. Braunwald's heart disease: a textbook of cardiovascular medicine. Elsevier Health Sciences, 2012:1696-718.
- Voelkel MA, Wynne KM, Badesch DB, et al. Hyperuricemia in pulmonary hypertension. Chest 2000;117:19-24. [Crossref] [PubMed]
- Foris V, Kovacs G, Tscherner M, et al. Biomarkers in pulmonary hypertension; What do we know? Chest 2013;144:274-83. [Crossref] [PubMed]
- Vélez-Martínez M, Ayers C, Mishkin JD, et al. Association of Cardiac Troponin I With Disease Severity and Outcomes in Patients With Pulmonary Hypertension. Am J Cardiol 2013;111:1812-7. [Crossref] [PubMed]
- Haeck ML, Vliegen HW. Diagnosis and treatment of pulmonary hypertension. Heart 2015;101:311-9. [Crossref] [PubMed]
- Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: an essential tool. Chest 2007;131:339-41. [Crossref] [PubMed]
- Milan A, Magnino C, Veglio F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr 2010;23:225-39. [Crossref] [PubMed]
- Abbas AE, Fortuin FD, Schiller NB, et al. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 2003;41:1021-7. [Crossref] [PubMed]
- Otto CM. editor. The practice of clinical echocardiography. Elsevier Health Sciences 2012:634.
- Armstrong WF, Ryan T. editors. Figenbaum’s Echocardiography. 7th ed. Philadelphia, OA: Lippincott Williams and Wilkins, 2009.
- Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in Pulmonary arterial hypertension: from diagnosis to Prognosis. J Am Soc Echocardiogr 2013;26:1-14. [Crossref] [PubMed]
- Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart functionin pulmonary hypertension. Am J Respir Crit Care Med 2011;183:268-76. [Crossref] [PubMed]
- Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002;39:1214-9. [Crossref] [PubMed]
- Sachdev A, Villarraga HR, Frantz RP, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011;139:1299-309. [Crossref] [PubMed]
- Haeck ML, Scherptong RW, Marsan NA, et al. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging 2012;5:628-36. [Crossref] [PubMed]
- Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med 2007;28:233-41. x. [Crossref] [PubMed]
- Miller WL, Mahoney DW, Michelena HI, et al. Contribution of ventricular diastolic dysfunction to pulmonary hypertension complicating chronic systolic heart failure. JACC Cardiovasc Imaging 2011;4:946-54. [Crossref] [PubMed]
- Hoeper MM. Definition, classification, and epidemiology of pulmonary arterial hypertension. Semin Respir Crit Care Med 2009;30:369-75. [Crossref] [PubMed]
- Kovacs G, Olschewski A, Berghold A, et al. Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J 2012;39:319-28. [Crossref] [PubMed]
- Naeije R, Vachiaery JL, Yerly P, et al. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013;41:217-23. [Crossref] [PubMed]
- Devaraj A, Wells AU, Meister MG, et al. Detection of Pulmonary Hypertension with Multidetector CT and Echocardiography Alone and in Combination. Radiology 2010;254:609-16. [Crossref] [PubMed]
- Mahammedi A, Oshmyansky A, Hassoun PM, et al. Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. J Thorac Imaging 2013;28:96-103. [Crossref] [PubMed]
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Sociaty, Inc.;and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-619. [Crossref] [PubMed]
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013;62:D92-9. [Crossref] [PubMed]
- Bresser P, Pepke-Zaba J, Jais X, et al. Medical therapies for chronic thromboembolic pulmonary hypertension: an evolving treatment paradigm. Proc Am Thorac Soc 2006;3:594-600. [Crossref] [PubMed]
- Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Circulation 2011;124:1973-81. [Crossref] [PubMed]
- Galiè N, Kim NH. Pulmonary microvascular disease in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc 2006;3:571-6. [Crossref] [PubMed]
- Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the IMPRES study. Circulation 2013;127:1128-38. [Crossref] [PubMed]
- Benza R, Biederman R, Murali S, et al. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol 2008;52:1683-92. [Crossref] [PubMed]
- Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29-34. [Crossref] [PubMed]
- Kind T, Mauritz G, Marcus JT, et al. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson 2010;12:35. [Crossref] [PubMed]
- Peacock AJ, Noordegraf AV. Cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. Eur Respir Rev 2013;22:526-34. [Crossref] [PubMed]
- Taylor AJ, Salerno M, Dharmakuma R, et al. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc Imaging 2016;9:67-81. [Crossref] [PubMed]
- García-Álvarez A, García-Lunar I, Pereda D, et al. Association of Myocardial T1-Mapping CMR With Hemodynamics and RV Performance in Pulmonary Hypertension. JACC Cardiovasc Imaging 2015;8:76-82. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156-63. [Crossref] [PubMed]
- Peacock AJ, Crawley S, McClure L, et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging 2014;7:107-14. [Crossref] [PubMed]
- Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006;44:1502-10. [Crossref] [PubMed]
- Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 2011;365:44-53. [Crossref] [PubMed]
- Tonelli AR, Alnuaimat H, Mubarak K. Pulmonary vasodilator testing and use of calcium channel blockers in pulmonary arterial hypertension. Respir Med 2010;104:481-96. [Crossref] [PubMed]
- Fonseca GH, Souza R, Salemi VM, et al. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J 2012;39:112-8. [Crossref] [PubMed]
- Galiè N, Barbera JA, Frost AE, et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med 2015;373:834-44. [Crossref] [PubMed]
- Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148-57. [Crossref] [PubMed]
- Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996;334:296-301. [Crossref] [PubMed]
- Galiè N, Channick R, Chin K, et al. Effect of selexipag on morbidity/mortality in pulmonary arterial hypertension: results of the GRIPHON Study. J Am Coll Cardiol 2015;65:A380.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure With Preserved Ejection Fraction. A Randomized Clinical Trial. JAMA 2013;309:1268-77. [Crossref] [PubMed]
- Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med 2015;373:2314-24. [Crossref] [PubMed]
- FDA approves Adempas to treat pulmonary hypertension. FDA New Release. Oct. 8, 2013.
- Ghofrani HA, D’Amiri AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369:319-29. [Crossref] [PubMed]
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62:D60-72. [Crossref] [PubMed]
- Preston IR, Roberts KE, Miller DP, et al. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the registry to evaluate early and long-term PAH disease management (REVEAL). Circulation 2015;132:2403-11. [Crossref] [PubMed]
- Keogh AM, Mayer E, Benza RL, et al. Interventional and surgical modalities for treatment in pulmonary hypertension. J Am Coll Cardiol 2009;54:S67-77. [Crossref] [PubMed]
- Althoff TF, Knebel F, Panda A, et al. Long-term follow-up of a fenestrated Amplatzer atrial septal occluderin pulmonary arterial hypertension. Chest 2008;133:283-5. [Crossref] [PubMed]
- Sandoval J, Gaspar J, Pulido T, et al. Graded balloon dilation atrial septostomy in severe primarypulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol 1998;32:297-304. [Crossref] [PubMed]
- Rich S, Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol 1983;51:1560-1. [Crossref] [PubMed]
- Kurzyna M, Dabrowski M, Bielecki D, et al. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest 2007;131:977-83. [Crossref] [PubMed]
- Esch JJ, Shah PB, Cockrill BA, et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant 2013;32:381-7. [Crossref] [PubMed]
- Baruteau AE, Serraf A, Levy M, et al. Potts shunt in children with idiopathic pulmonary arterial hypertension: long-term results. Ann Thorac Surg 2012;94:817-24. [Crossref] [PubMed]
- Bhamra-Ariza P, Keogh AM, Muller DW. Percutaneous interventional therapies for the treatment of patient with severe pulmonary hypertension. J Am Coll Cardiol 2014;63:611-8. [Crossref] [PubMed]
- Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med 2011;184:159-71. [Crossref] [PubMed]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073-86. [Crossref] [PubMed]
- de Perrot M, Granton JT, McRae K, et al. Outcome of patients with pulmonary arterial hypertension referred for lung transplantation: a 14-year single-center experience. J Thorac Cardiovasc Surg 2012;143:910-8. [Crossref] [PubMed]
- Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg 2012;94:97-103. [Crossref] [PubMed]
- Berman M, Tsui S, Vuylsteke A, et al. Successful extracorporeal membrane oxygenation support after pulmonary thromboendarterectomy. Ann Thorac Surg 2008;86:1261-7. [Crossref] [PubMed]
- Jenkins D, Mayer E, Screaton N, et al. State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev 2012;21:32-9. [Crossref] [PubMed]
- Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141:702-10. [Crossref] [PubMed]
- Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2004;23:637-48. [Crossref] [PubMed]
- Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:756-62. [Crossref] [PubMed]
- D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115:343-9. [Crossref] [PubMed]
- McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc 2008;83:923-31. [Crossref] [PubMed]
- Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting Survival in Pulmonary Arterial Hypertension: Insights From the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164-72. [Crossref] [PubMed]


