Diagnostic accuracy study of routine echocardiography for bicuspid aortic valve: a retrospective study and meta-analysis
Introduction
Bicuspid aortic valve (BAV) disease is the disease where the aortic valve has only two cusps instead of three tricuspid aortic valve (TAV). With a prevalence of 0.5% to 1.39% in the general population BAV is the most frequent congenital heart disease (1). There is a considerable anatomical variance of BAV, and several systems exist to classify this variance (1-4). The morbidity of BAV is high already at younger adulthood, and 25% of affected persons experience in the course of their life severe aortic valve dysfunction, ascending aortic aneurysm, cardiac death, hospital admission for heart failure, and aortic dissection or rupture (5). Therefore, it is important to diagnose BAV before severe complications develop. Guidelines recommend transthoracic echocardiography (TTE) as the standard procedure to diagnose BAV (6). Indeed, since the initial report of in vivo diagnosis of BAV by TTE in 1974 studies continued to report high diagnostic accuracy for BAV (7). However, these studies were performed under ideal conditions, with expert investigators having a special interest in BAV, with usage of high-end TTE equipment, and with exclusion of patients having suboptimal imaging conditions.
We performed the current study in the setting of echocardiography prior to elective surgical replacement of the aortic valve. We aimed at assessing the diagnostic accuracy of routine TTE for BAV. First, we assessed diagnostic accuracy for BAV according to (I) original reports upon preoperative routine echocardiography. Then we assessed diagnostic accuracy for BAV according to (II) expert re-evaluation of all available original TTE recordings. Finally, we performed a meta-analysis of previously published studies to assess accuracy of TTE for diagnosing BAV according to PRISMA recommendations (8). All diagnostic results were validated against the standard reference of intraoperative inspection.
Methods
Patients
We analysed all consecutive individuals with BAV who underwent aortic valve replacement at our tertiary care centre between March 2009 and March 2014. We excluded one individual with intraoperative confirmation of a unicuspid aortic valve, and seven individuals with aortic valves that exhibited two cusps but three sinuses with three interleaflet triangles indicating an acquired rather than congenital bicuspid structure of the aortic valve. We did not identify other congenital aortic valve malformations such as a quadricuspid aortic valve (9-11). Therefore, a total of 108 individuals with BAV confirmed during surgical inspection qualified for inclusion in this study. We established a diagnostic control group by matching each individual with intraoperative diagnosis of BAV with one individual with intraoperative diagnosis of TAV. We identified these control patients by matching according to age at operation, and date of operation and institution where TTE had been performed. Therefore our final study group comprised a total of 216 individuals including 132 men and 84 women at a mean age of 62±14 years (range, 19–82 years). All patients underwent TTE and uncomplicated standard aortic valve replacement surgery. Given the retrospective, observational study design and anonymous data analysis, the Hamburg review board waived the requirement of individual patient consent.
Two diagnostic groups
We assessed TTE in two patient groups. First, we assessed the diagnostic results in the (I) group of all 216 individuals, where we assessed the diagnosis of BAV according to the original diagnoses as documented by the primary investigators during original TTE examination. Primary investigators comprised board certified cardiologists from private practices or from referring hospitals in 42 patients or interns under the supervision of expert investigators in our hospital in 174 patients.
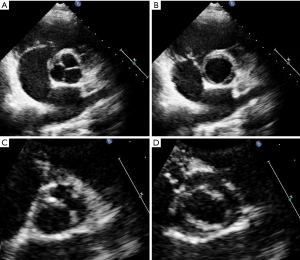
Second, we performed an (II) expert re-evaluation of all 158 available original TTE recordings. For this re-evaluation we applied the diagnostic criteria of Nistri et al. for BAV, where we separated BAV from TAV depending on visualization of two versus three aortic valve cusps in systole and diastole in the short-axis view (Figure 1) (12). Two expert-readers with ≥10 years of echocardiographic experience jointly re-evaluated TTE recordings in all individuals with diagnosis of BAV or TAV on a consensus basis. Both examiners were aware that they took part in a study on the accuracy of TTE for BAV. However, they were blinded to intraoperative findings and results from other imaging procedures.
Diagnostic criteria
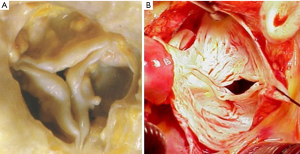
We considered findings on TTE as true positive when BAV was diagnosed on TTE and confirmed at surgery, as false negative when TAV was found on TTE but BAV was identified at surgery, as true negative when TAV was suggested by TTE and excluded at surgery, and as false positive when BAV was diagnosed by TTE but TAV was identified at surgery (Figure 2). We considered intraoperative diagnosis by the attending surgeon as standard reference for BAV and TAV, because this diagnosis is superior even to aortic tissue examination by a pathologist (13). The classification of BAV according to Schaefer. was type 1 with fused right and left coronary cusp, type 2 with fused right and non-coronary cusps, and type 3 with fused non-coronary and left coronary cusps as documented by the attending surgeon (3). All TTE examinations were performed within 30 days prior to aortic valve replacement using standard commercially available systems with current M-mode and 2-dimensional scanning technology.
Clinical variables
Preoperative variables from patients’ charts included age, sex, body height, body weight, and body surface area according to Du Bois (14). We documented whether TTE was performed at our institution or at referring institutions. We assessed indications for valve surgery according to surgical records as isolated or predominant aortic valve stenosis, isolated or predominant aortic valve regurgitation or mixed aortic valve disease when stenosis and regurgitation contributed equally to valve dysfunction. We noted aortic aneurysm with diameters ≥4.0 cm at the level of the aortic sinuses or the ascending aorta as documented on preoperative TTE or tomographic imaging, severe calcification of the aortic valve with extensive thickening and calcification of all aortic cusps with grade 4 calcification according to Rosenhek et al. on TTE (15), and with grade 5 calcification according to Yousry et al. on surgical inspection (16).
Systematic review and meta-analysis
We performed a systematic review of the literature to assess published frequencies of true positive, false negative, true positive and true negative diagnoses of BAV as target condition and TAV as control condition on standard two-dimensional echocardiography as index test with intraoperative diagnosis or exclusion of BAV on surgical inspection as reference test. We considered studies published in English, French, or German language with inclusion of individuals of all ages with confirmation of BAV or TAV at surgery of the aortic valve. We used the following key words to search MEDLINE: BAV, echocardiography, diagnosis, sensitivity, specificity, accuracy, and we screened the literature cited in all articles that we retrieved from this search. In this way we identified a total of 68 studies. Of these, we excluded 39 studies, because they did not provide information on sensitivity and specificity, 10 studies, because they did not use intraoperative inspection as reference test, 7 studies because they did not present diagnostic data adequately, and 3 studies because the articles uses another language than English, French or German. We provide the details of these studies in a flow sheet according to PRISMA recommendations (Supplementary, Figure S1) (8). Finally, we identified a total of nine studies for inclusion in our systematic review. Two investigators assessed STARD quality scores (17) of all nine studies independently from each other. They jointly discussed and reconciled discrepant scorings (Table 1).
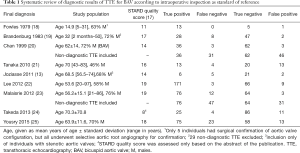
Full table
Statistical methods
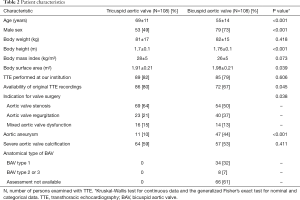
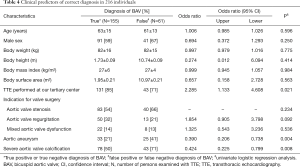
Unless otherwise specified, we expressed quantitative data as means ± standard deviation (SD) and qualitative data as numbers (percentage). For comparison of baseline characteristics we employed the Kruskal-Wallis test for continuous data and the generalized Fisher’s exact test for nominal and categorical data (Table 2). We derived sensitivity, specificity, accuracy, and positive and negative likelihood ratios of TTE from the number of true positive, false negative, true negative and false negative diagnostic results using standard formulas (26), where we calculated “exact” Clopper-Pearson confidence intervals (CI) (Table 3) (27). We used logistic regression to identify clinical variables that related to a correct diagnosis of BAV or TAV (Table 4). We considered P values <0.05 as significant, and we included in our multivariate model all variables with significant P value on univariate testing (Table 4). For meta-analysis we derived sensitivity, specificity, accuracy, and positive and negative likelihood ratios of TTE and their respective 95% CIs from the number of true positive, false negative, true negative and false negative diagnostic results with the same methods as described above (Table 1). We used forest plots to display the results of meta-analysis where we computed 95% CIs, weight of studies and pooled sensitivity and specificity. We used IBM-SPSS software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA: IBM Corp) for all statistical tests, with the exception of meta-analysis including Forest plots, where we used Stata, version 14 (StataCorp, College Station, Texas, USA).
Full table

Full table
Full table
Results
Patients
This study included 108 adults with BAV and 108 adults with TAV. The indication for aortic valve replacement surgery was isolated or predominant stenosis in 123 (56.9%), isolated or predominant regurgitation in 63 (29.2%), and mixed stenosis and regurgitation in 30 individuals (13.9%). At surgery, BAVs exhibited type 1 morphology in 32%, type 2 or type 3 in 7%. Individuals with BAV were younger, they were predominantly male, and they had larger body surface area as compared to their tricuspid counterparts. Moreover, proximal aortic aneurysm was more common in the BAV group (Table 2).
Diagnostic accuracy
In the (I) group of 216 individuals with diagnoses based on original records of primary investigators, the sensitivity was 46.3%, the specificity was 97.2%, the accuracy was 71.8%, the positive likelihood ratio was 16.667, and the negative likelihood ratio was 0.552. In the (II) group of 158 individuals with availability of original TTE recordings, expert re-evaluation yielded a sensitivity of 59.7%, a specificity of 93%, an accuracy of 77.8%, a positive likelihood ratio of 8.560, and a negative likelihood ratio of 0.433. The sensitivity was higher on (II) re-evaluation than on (II) primary documentation (P<0.001), but the specificity was similar in both groups (P=0.07, Table 3).
Predictors of incorrect diagnosis
Univariate analysis did not identify an impact of age, sex, body weight, body height, body mass index, body surface area, or the type of indication for aortic valve surgery on the accuracy of routine TTE for BAV. However, multivariate analysis identified as independent predictors of incorrect diagnosis of BAV a TTE performed at a non-tertiary care center [odds ratio (OR) 2.587, 95% CI, 1.228–5.453; P=0.012], presence of aortic aneurysm (OR 0.305, 95% CI, 0.153–0.607; P=0.001) and presence of severe aortic valve calcification (OR 0.363, 95% CI, 0.185–0.711; P=0.003; Table 4).
Systematic review and meta-analysis
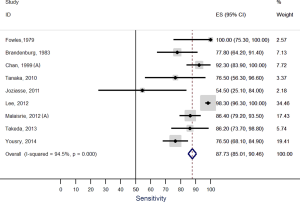
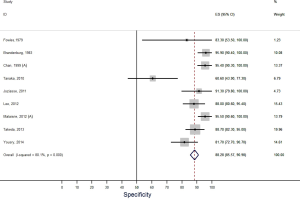
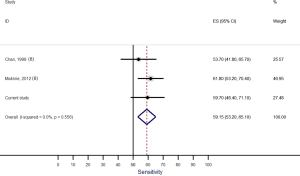
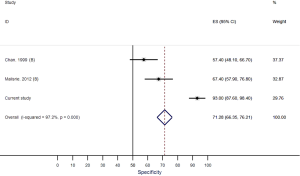
Our systematic review identified nine studies that qualified for inclusion in the meta-analysis (Table 1). The meta-analysis of these nine studies identified a pooled sensitivity of 87.7% (95% CI, 85.0–90.5%; Figure 3) and a pooled specificity of 88.3% (95% CI, 85.6–90.9%; Figure 4). Of these nine studies two additionally presented diagnostic accuracy of TTE with inclusion of non-diagnostic TTE images (Table 1). We considered the results from these two studies in a separate meta-analysis to assess the diagnostic accuracy of TTE including non-diagnostic TTE results. In the analysis of these two studies we included our own results with expert re-evaluation of TTE recordings. The pooled sensitivity of these three studies for BAV was 59.2% (95% CI, 53.2–65.1%; Figure 5) and the pooled specificity was 71.3% (95% CI, 66.4–76.2%; Figure 6).
Discussion
Main results
The study showed that the primary investigators’ sensitivity of routine echocardiography for BAV was only 46.3%. The sensitivity was significantly higher with expert re-evaluation of original TTE recordings, but with 59.7% it still remained suboptimal. The probability of an inaccurate diagnosis of BAV increased with TTE performed at a non-tertiary care center, with presence of an aortic aneurysm and with presence of severe aortic valve calcification. In contrast to these findings the meta-analysis of previously published studies demonstrated a high pooled sensitivity of 87.7%. However, a sub-analysis of published studies with inclusion of suboptimal diagnostic results yielded a pooled sensitivity of only 59.2%.
Low sensitivity of routine echocardiography
Classical studies of the diagnostic accuracy of TTE suggested a high sensitivity of up to 100% for BAV (18,22). However, these studies were performed at tertiary care centers, with careful selection of patients according to age, adequate image quality, absence of previous surgery or intervention, and with expert readers who jointly evaluated images for presence of BAV (18-22). Conversely, we aimed at assessing the diagnostic accuracy of primary investigators during routine echocardiography. Our results reflect the diagnostic performance in a wide spectrum of referring institutions, with investigators at different levels of experience and expertise, and with a varying awareness of BAV as a relevant underlying pathology of aortic valve dysfunction. Moreover, routine conditions in process-optimized clinical settings today include time constraints that discourage detailed investigations and profound discussions of diagnostic findings (28,29). Therefore, the low sensitivity of only 46.3% was not surprising, but rather underpinned that TTE is an investigator-dependent diagnostic tool.
The current study showed that the sensitivity increased significantly when experts re-evaluated the original TTE recordings. Classical studies support the importance of being an expert to exploit the diagnostic potential of TTE (30). In addition, recent studies documented the role of training and experience to increase cognitive knowledge and psychomotor skills for echocardiography (31,32). However, with 59.7% the sensitivity of expert re-evaluation still remained below optimal study results (18,22). The most likely explanation for these suboptimal results of our expert re-evaluation was the suboptimal interpretability of images, where multiple factors are known to contribute. On the one hand, unpreventable patient-related factors are known to play an important role. It is clear that patients with obesity, chest wall deformities, narrow intercostal spaces and pulmonary emphysema yield suboptimal imaging quality. On the other hand, suboptimal imaging quality may have resulted from preventable investigator-related factors. Such factors include sub-optimal positioning of the patient, sub-optimal angulation of the ultrasound probe, incomplete assessment of all aortic valve cusp structures, and incomplete use of all available views, imaging modalities and software options. The important role of experience to assess interpretable images was documented recently for focused cardiac ultrasound (32).
High specificity of routine echocardiography
In contrast to the relatively low sensitivity to diagnose BAV, routine TTE yielded a relatively high specificity of 97.2% for excluding BAV. This high specificity was even higher than the pooled specificity of 88.3% in our meta-analysis. We suggest that the low rate of false positive diagnoses of BAV in our routine TTE examinations resulted at least in part from investigators who may not have actively considered BAV when describing “TAV”. Indeed, in the present study the specificity dropped to 93% with expert re-evaluation. The experts yielded a higher number of false positive findings most likely because of their deliberate search for BAV.
Predictors of inaccurate diagnosis
In this study we aimed at identifying factors that predicted an inaccurate diagnosis of BAV on routine evaluation. We did not identify an impact of age, sex, body weight, body height, body mass index, and body surface area on diagnostic accuracy. Conversely, TTE was less accurate when performed at non-tertiary care centers. This finding may suggest an impact of specialization on echocardiographic results. Moreover, when an ascending aortic aneurysm was present the accuracy for BAV was lower. This is surprising, since aortopathy is a well-known marker of BAV (1,2,33). Strictly speaking, the association seems to reflect some type of diagnostic error (34). Such error may be based in a lack of medical knowledge as unawareness of aortopathy as hallmark of BAV. Alternatively, the error may result from cognitive bias such as anchoring. The investigator has an initial impression, such as: “This routine patient simply has a severe aortic stenosis”. Anchoring happens, when the investigator disqualifies subsequent information as corroboration of his initial impression, for example he may think: “There is an aneurysm. This is a post-stenotic dilatation that corroborates my initial diagnosis of severe aortic stenosis” (29).
The diagnosis of BAV was less accurate with severe aortic valve calcification. The negative relationship of aortic valve calcification and echocardiographic detection of BAV has been documented previously (25). The most likely reason is that calcified masses can overly and erode the aortic valve anatomy and thereby conceal the underlying cusp structure. Finally, Brandenburg et al. pointed out in their classical study of TTE for BAV that in diastole a raphe in BAV may appear like a commissure of TAV (19). Therefore, BAV without a raphe may be easier to diagnose than BAV with a raphe (4). Unfortunately, most of our intraoperative descriptions of the aortic valve did not explicitly exclude presence of a raphe. Similarly, the studies included in our meta-analysis did not investigate the impact of a raphe on the diagnostic accuracy of BAV.
Meta-analysis
The meta-analysis of previously published studies demonstrated a pooled sensitivity of 87.7% for BAV which was significantly higher than in the current study. These series utilized an “idealized” design to assess the best possible diagnostic results rather than that they aimed at assessing the diagnostic results under routine conditions. However, we performed a sub-analysis of published studies that included results from echocardiographic examinations with limited diagnostic quality. This sub-analysis yielded a pooled sensitivity of only 59.2%. This sensitivity was almost identical to the sensitivity that we obtained from re-evaluation of routine TTE recordings. Therefore, this meta-analysis supports the view that TTE yields almost ideal diagnostic accuracy when ideal investigators examine ideal patients. However, the meta-analysis also provides evidence that TTE yields suboptimal diagnostic accuracy when non-expert investigators examine patients who exhibit suboptimal conditions for transthoracic examination.
Study limits
Our study aimed at assessing the diagnostic accuracy of TTE for diagnosing BAV in a routine clinical setting. For matching individuals with BAV, we were unable to identify individuals with TAV who were as young as those with BAV. Despite some age differences between the BAV and the TAV group logistic regression analysis excluded an impact of age on the accuracy of TTE. Moreover, we focused on a surgical patient cohort, and by doing so, we selected only patients with a severe aortic valve dysfunction. By this design, we also excluded inoperable individuals with severe obesity, marked lung diseases or major chest wall deformities and therefore diagnostic accuracy may have been biased towards individuals with fair transthoracic imaging conditions. These limitations also applied to the studies which we included in our meta-analysis. All studies for meta-analysis including our own study relied on surgical inspection as reference standard to distinguish BAV from TAV. However, even though surgical inspection is widely accepted as standard of reference, some studies suggest that diagnoses can differ between surgeon and pathologist, where the quality of both diagnoses may depend on the awareness and experience of individual investigators (13,35).
Conclusions
The current study shows that TTE yields almost ideal diagnostic accuracy when ideal investigators examine ideal patients in ideal settings. However, the study also shows that TTE yields suboptimal diagnostic accuracy under routine conditions. Echocardiography in non-tertiary care settings, concomitant aortic aneurysm, and presence of severe aortic valve calcification predict an inaccurate diagnosis of BAV.
For the optimal exploit of the diagnostic potential of TTE for BAV a broader approach to improve the setting of routine evaluation appears necessary. The discourse of diagnostic accuracy in clinical routine settings provides an innovative opportunity to connect the scientific discourse of diagnostic accuracy studies (17) with the scientific discourse of reducing diagnostic errors (29,34,36). Such connection of discourses may open alleys to develop improved concepts for optimal use of imaging modalities in routine settings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Given the retrospective, observational study design and anonymous data analysis, the Hamburg review board waived the requirement of individual patient consent and ethical approval.
References
- von Kodolitsch Y, Kaemmerer H. Bicuspid aortic valve. In: Niwa K, Kaemmerer H. editors. Aortopathy. Tokyo: Springer Japan, 2017:229-56.
- Girdauskas E, Borger MA. Bicuspid aortic valve and associated aortopathy: an update. Semin Thorac Cardiovasc Surg 2013;25:310-6. [Crossref] [PubMed]
- Schaefer BM, Lewin MB, Stout KK, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008;94:1634-8. [Crossref] [PubMed]
- Kang JW, Song HG, Yang DH, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging 2013;6:150-61. [Crossref] [PubMed]
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317-25. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-132. [Crossref] [PubMed]
- Nanda NC, Gramiak R, Manning J, et al. Echocardiographic recognition of the congenital bicuspid aortic valve. Circulation 1974;49:870-5. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Mookadam F, Thota VR, Lopez AM, et al. Unicuspid aortic valve in children: a systematic review spanning four decades. J Heart Valve Dis 2010;19:678-83. [PubMed]
- Khan SK, Tamin SS, Araoz PA. Quadricuspid aortic valve by cardiac magnetic resonance imaging: a case report and review of the literature. J Comput Assist Tomogr 2011;35:637-41. [Crossref] [PubMed]
- Angelini A, Ho SY, Anderson RH, et al. The morphology of the normal aortic valve as compared with the aortic valve having two leaflets. J Thorac Cardiovasc Surg 1989;98:362-7. [PubMed]
- Nistri S, Basso C, Marzari C, et al. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol 2005;96:718-21. [Crossref] [PubMed]
- Joziasse IC, Vink A, Cramer MJ, et al. Bicuspid stenotic aortic valves: clinical characteristics and morphological assessment using MRI and echocardiography. Neth Heart J 2011;19:119-25. [Crossref] [PubMed]
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303-11; discussion 12-3. [PubMed]
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7. [Crossref] [PubMed]
- Yousry M, Rickenlund A, Petrini J, et al. Real-time imaging required for optimal echocardiographic assessment of aortic valve calcification. Clin Physiol Funct Imaging 2012;32:470-5. [Crossref] [PubMed]
- Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med 2003;138:40-4. [Crossref] [PubMed]
- Fowles RE, Martin RP, Abrams JM, et al. Two-dimensional echocardiographic features of bicuspid aortic valve. Chest 1979;75:434-40. [Crossref] [PubMed]
- Brandenburg RO Jr, Tajik AJ, Edwards WD, et al. Accuracy of 2-dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: echocardiographic-anatomic correlation in 115 patients. Am J Cardiol 1983;51:1469-73. [Crossref] [PubMed]
- Chan KL, Stinson WA, Veinot JP. Reliability of transthoracic echocardiography in the assessment of aortic valve morphology: pathological correlation in 178 patients. Can J Cardiol 1999;15:48-52. [PubMed]
- Tanaka R, Yoshioka K, Niinuma H, et al. Diagnostic value of cardiac CT in the evaluation of bicuspid aortic stenosis: comparison with echocardiography and operative findings. AJR Am J Roentgenol 2010;195:895-9. [Crossref] [PubMed]
- Lee SC, Ko SM, Song MG, et al. Morphological assessment of the aortic valve using coronary computed tomography angiography, cardiovascular magnetic resonance, and transthoracic echocardiography: comparison with intraoperative findings. Int J Cardiovasc Imaging 2012;28 Suppl 1:33-44. [PubMed]
- Malaisrie SC, Carr J, Mikati I, et al. Cardiac magnetic resonance imaging is more diagnostic than 2-dimensional echocardiography in determining the presence of bicuspid aortic valve. J Thorac Cardiovasc Surg 2012;144:370-6. [Crossref] [PubMed]
- Takeda H, Muro T, Saito T, et al. Diagnostic accuracy of transthoracic and transesophageal echocardiography for the diagnosis of bicuspid aortic valve: comparison with operative findings. Osaka City Med J 2013;59:69-78. [PubMed]
- Yousry M, Rickenlund A, Petrini J, et al. Aortic valve type and calcification as assessed by transthoracic and transoesophageal echocardiography. Clin Physiol Funct Imaging 2015;35:306-13. [Crossref] [PubMed]
- Jaeschke R, Guyatt G, Lijmer J. Diagnostic tests. In: Guyatt G, Rennie D. editors. Users’ guides to the medical literature. Chicago: AMA Press, 2002.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404-13. [Crossref]
- von Kodolitsch Y, Bernhardt AM, Kölbel T, et al. Maximizing therapeutic success: The key concepts of individualized medical strategy (IMS). Cogent Medicine 2015;2:1109742. [Crossref]
- Lee CS, Nagy PG, Weaver SJ, et al. Cognitive and system factors contributing to diagnostic errors in radiology. AJR Am J Roentgenol 2013;201:611-7. [Crossref] [PubMed]
- Picano E, Lattanzi F, Orlandini A, et al. Stress echocardiography and the human factor: the importance of being expert. J Am Coll Cardiol 1991;17:666-9. [Crossref] [PubMed]
- Montealegre-Gallegos M, Mahmood F, Kim H, et al. Imaging skills for transthoracic echocardiography in cardiology fellows: The value of motion metrics. Ann Card Anaesth 2016;19:245-50. [Crossref] [PubMed]
- Bobbia X, Pradeilles C, Claret PG, et al. Does physician experience influence the interpretability of focused echocardiography images performed by a pocket device? Scand J Trauma Resusc Emerg Med 2015;23:52. [Crossref] [PubMed]
- Girdauskas E, Rouman M, Disha K, et al. Morphologic and Functional Markers of Aortopathy in Patients With Bicuspid Aortic Valve Insufficiency Versus Stenosis. Ann Thorac Surg 2017;103:49-57. [Crossref] [PubMed]
- Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what's the goal? Acad Med 2002;77:981-92. [Crossref] [PubMed]
- Roberts WC, Vowels TJ, Ko JM. Comparison of interpretations of valve structure between cardiac surgeon and cardiac pathologist among adults having isolated aortic valve replacement for aortic valve stenosis (+/- aortic regurgitation). Am J Cardiol 2009;103:1139-45. [Crossref] [PubMed]
- Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med 2003;78:775-80. [Crossref] [PubMed]
- Osler W. The biscuspid condition of the aortic valves. Trans Assoc Am Physicians 1886;2:185-92.
- Radford DJ, Bloom KR, Izukawa T, et al. Echocardiographic assessment of bicuspid aortic valves. Angiographic and pathological correlates. Circulation 1976;53:80-5. [Crossref] [PubMed]
- Leech G, Mills P, Leatham A. The diagnosis of a non-stenotic bicuspid aortic valve. Br Heart J 1978;40:941-50. [Crossref] [PubMed]
- Folger GM Jr, Alam M, Stein PD. M-mode echocardiographic failure to consistently identify the equally bicuspid aortic valve: correlation with orifice-view aortography. Angiology 1982;33:111-8. [Crossref] [PubMed]
- Zema MJ, Caccavano M. Two dimensional echocardiographic assessment of aortic valve morphology: feasibility of bicuspid valve detection. Prospective study of 100 adult patients. Br Heart J 1982;48:428-33. [Crossref] [PubMed]
- Pachulski RT, Chan KL. Progression of aortic valve dysfunction in 51 adult patients with congenital bicuspid aortic valve: assessment and follow up by Doppler echocardiography. Br Heart J 1993;69:237-40. [Crossref] [PubMed]
- Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol 1997;30:1809-12. [Crossref] [PubMed]
- Lazar JM, Smith RH, Scott WC. Intraoperative evaluation of a bicuspid aortic valve. Journal of Cardiothoracic and Vascular Anesthesia 1997;11:253-5. [Crossref] [PubMed]
- Epperlein S, Mohr-Kahaly S, Erbel R, et al. Aorta and aortic valve morphologies predisposing to aortic dissection. An in vivo assessment with transoesophageal echocardiography. Eur Heart J 1994;15:1520-7. [Crossref] [PubMed]
- Nistri S, Sorbo MD, Marin M, et al. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 1999;82:19-22. [Crossref] [PubMed]
- Steinberger J, Moller JH, Berry JM, et al. Echocardiographic diagnosis of heart disease in apparently healthy adolescents. Pediatrics 2000;105:815-8. [Crossref] [PubMed]
- Dod HS, Nanda NC, Agrawal GG, et al. Three-dimensional transesophageal echocardiographic assessment of aortic valve pathology. Am J Geriatr Cardiol 2003;12:209-13. [Crossref] [PubMed]
- Novaro GM, Mishra M, Griffin BP. Incidence and echocardiographic features of congenital unicuspid aortic valve in an adult population. J Heart Valve Dis 2003;12:674-8. [PubMed]
- Basso C, Boschello M, Perrone C, et al. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol 2004;93:661-3. [Crossref] [PubMed]
- Gurvitz M, Chang RK, Drant S, et al. Frequency of aortic root dilation in children with a bicuspid aortic valve. Am J Cardiol 2004;94:1337-40. [Crossref] [PubMed]
- Ostberg JE, Brookes JA, McCarthy C, et al. A comparison of echocardiography and magnetic resonance imaging in cardiovascular screening of adults with Turner syndrome. J Clin Endocrinol Metab 2004;89:5966-71. [Crossref] [PubMed]
- Niinuma H, Yoshioka K, Ogino Y, et al. Three-dimensional demonstration of bicuspid aortic valve by 16-row multidetector computed tomography: comparison with transesophageal echocardiography. Eur J Cardiothorac Surg 2005;27:346. [Crossref] [PubMed]
- Tirrito SJ, Kerut EK. How not to miss a bicuspid aortic valve in the echocardiography laboratory. Echocardiography 2005;22:53-5. [Crossref] [PubMed]
- Movahed MR, Hepner AD, Ahmadi-Kashani M. Echocardiographic prevalence of bicuspid aortic valve in the population. Heart Lung Circ 2006;15:297-9. [Crossref] [PubMed]
- Ahmed S, Honos GN, Walling AD, et al. Clinical outcome and echocardiographic predictors of aortic valve replacement in patients with bicuspid aortic valve. J Am Soc Echocardiogr 2007;20:998-1003. [Crossref] [PubMed]
- Debl K, Djavidani B, Buchner S, et al. Unicuspid aortic valve disease: a magnetic resonance imaging study. Rofo 2008;180:983-7. [Crossref] [PubMed]
- Singh P, Dutta R, Nanda NC. Live/real time three-dimensional transthoracic echocardiographic assessment of bicuspid aortic valve morphology. Echocardiography 2009;26:478-80. [Crossref] [PubMed]
- Stefani L, De Luca A, Maffulli N, et al. Speckle tracking for left ventricle performance in young athletes with bicuspid aortic valve and mild aortic regurgitation. Eur J Echocardiogr 2009;10:527-31. [Crossref] [PubMed]
- Albano AJ, Mitchell E, Pape LA. Standardizing the method of measuring by echocardiogram the diameter of the ascending aorta in patients with a bicuspid aortic valve. Am J Cardiol 2010;105:1000-4. [Crossref] [PubMed]
- Piccoli G, Slavich G, Gianfagna P, et al. Cardiovascular flashlight. Cleft bicuspid aortic valve: the Achilles' heel of echocardiography? Eur Heart J 2010;31:2140. [Crossref] [PubMed]
- Hope MD, Hope TA, Crook SE, et al. 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc Imaging 2011;4:781-7. [Crossref] [PubMed]
- Sadron Blaye-Felice MA, Seguela PE, Arnaudis B, et al. Usefulness of three-dimensional transthoracic echocardiography for the classification of congenital bicuspid aortic valve in children. Eur Heart J Cardiovasc Imaging 2012;13:1047-52. [Crossref] [PubMed]
- Cognet T, Seguela PE, Thomson E, et al. Assessment of valvular surfaces in children with a congenital bicuspid aortic valve: preliminary three-dimensional echocardiographic study. Arch Cardiovasc Dis 2013;106:295-302. [Crossref] [PubMed]
- Koh TW. Diagnosis of bicuspid aortic valve: role of three-dimensional transesophageal echocardiography and multiplane review analysis. Echocardiography 2013;30:360-3. [Crossref] [PubMed]
- Lee M, Sung J, Cho SJ, et al. Aortic dilatation and calcification in asymptomatic patients with bicuspid aortic valve: analysis in a Korean health screening population. Int J Cardiovasc Imaging 2013;29:553-60. [Crossref] [PubMed]
- Panayotova R, Macnab A, Waterworth PD. A pilot project of familial screening in patients with bicuspid aortic valve disease. J Heart Valve Dis 2013;22:150-5. [PubMed]
- Torres FS, Windram JD, Bradley TJ, et al. Impact of asymmetry on measurements of the aortic root using cardiovascular magnetic resonance imaging in patients with a bicuspid aortic valve. Int J Cardiovasc Imaging 2013;29:1769-77. [Crossref] [PubMed]
- Kemaloglu Oz T, Karadeniz FO, Gundlapalli H, et al. Coexisting bicuspid aortic and pulmonary valves with normally related great vessels diagnosed by live/real time three-dimensional transesophageal echocardiography. Echocardiography 2014;31:218-21. [Crossref] [PubMed]
- Michelena HI, Prakash SK, Della Corte A, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014;129:2691-704. [Crossref] [PubMed]
- Naseem T, Song M, Ianchulev S, et al. The echocardiographic evaluation of a bicuspid aortic valve: the effect of jet eccentricity and left ventricular outflow tract geometry on the effective orifice area. J Cardiothorac Vasc Anesth 2014;28:423-7. [Crossref] [PubMed]
- Chamberland CR, Sugeng L, Abraham S, et al. Three-Dimensional Evaluation of Aortic Valve Annular Shape in Children With Bicuspid Aortic Valves and/or Aortic Coarctation Compared With Controls. Am J Cardiol 2015. [Crossref] [PubMed]
- Anabwani GM, Bonhoeffer P. Prevalence of heart disease in school children in rural Kenya using colour-flow echocardiography. East Afr Med J 1996;73:215-7. [PubMed]
- Padial LR, Oliver A, Sagie A, et al. Two-dimensional echocardiographic assessment of the progression of aortic root size in 127 patients with chronic aortic regurgitation: role of the supraaortic ridge and relation to the progression of the lesion. Am Heart J 1997;134:814-21. [Crossref] [PubMed]
- Alegret JM, Duran I, Palazon O, et al. Prevalence of and predictors of bicuspid aortic valves in patients with dilated aortic roots. Am J Cardiol 2003;91:619-22. [Crossref] [PubMed]
- Alegret JM, Palazon O, Duran I, et al. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005;21:213-7. [Crossref] [PubMed]
- Tutar E, Ekici F, Atalay S, et al. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005;150:513-5. [Crossref] [PubMed]
- Alkadhi H, Leschka S, Trindade PT, et al. Cardiac CT for the differentiation of bicuspid and tricuspid aortic valves: comparison with echocardiography and surgery. AJR Am J Roentgenol 2010;195:900-8. [Crossref] [PubMed]
- Galanti G, Stefani L, Toncelli L, et al. Effects of sports activity in athletes with bicuspid aortic valve and mild aortic regurgitation. Br J Sports Med 2010;44:275-9. [Crossref] [PubMed]
- Tsai SF, Trivedi M, Daniels CJ. Comparing Imaging Modalities for Screening Aortic Complications in Patients with Bicuspid Aortic Valve. Congenital Heart Disease 2012;7:372-7. [Crossref] [PubMed]
- van der Linde D, Rossi A, Yap SC, et al. Ascending Aortic Diameters in Congenital Aortic Stenosis: Cardiac Magnetic Resonance versus Transthoracic Echocardiography. Echocardiography 2013;30:497-504. [Crossref] [PubMed]
- Murphy DJ, McEvoy SH, Iyengar S, et al. Bicuspid aortic valves: diagnostic accuracy of standard axial 64-slice chest CT compared to aortic valve image plane ECG-gated cardiac CT. Eur J Radiol 2014;83:1396-401. [Crossref] [PubMed]
- Espinola-Zavaleta N, Muñoz-Castellanos L, Attié F, et al. Anatomic three-dimensional echocardiographic correlation of bicuspid aortic valve. Journal of the American Society of Echocardiography 2003;16:46-53. [Crossref] [PubMed]
- Makkar A, Siddiqui TS, Stoddard MF, et al. Impact of Valvular Calcification on the Diagnostic Accuracy of Transesophageal Echocardiography for the Detection of Congenital Aortic Valve Malformation. Echocardiography 2007;24:745-9. [Crossref] [PubMed]
- Chu JW, Picard MH, Agnihotri AK, et al. Diagnosis of congenital unicuspid aortic valve in adult population: the value and limitation of transesophageal echocardiography. Echocardiography 2010;27:1107-12. [Crossref] [PubMed]
- Zegdi R, Ciobotaru V, Huerre C, et al. Detecting aortic valve bicuspidy in patients with severe aortic valve stenosis: high diagnostic accuracy of colour Doppler transoesophageal echocardiography. Interactive CardioVascular and Thoracic Surgery 2013;16:16-20. [Crossref] [PubMed]
- Dall'Aglio V, Nicolosi GL, Burelli C, et al. Value and limitations of the echocardiographic definition of the bicuspid aortic valve. G Ital Cardiol 1986;16:155-61. [PubMed]
- Veyrat C, el Yafi W, Gourtchiglouian C, et al. Characteristics of jets in adult bicuspid aortic valve by color Doppler imaging. Arch Mal Coeur Vaiss 1991;84:1803-8. [PubMed]
- Sawada H, Shibata Y, Shionoya M, et al. Echocardiographic assessment of aortic regurgitation and aortic root dilatation in bicuspid aortic valve. J Cardiol 1992;22:495-501. [PubMed]
- Somelli A, Carrino G, De Majo AM. A bicuspid aortic valve. Usefulness of echocardiography in its identification. Minerva Cardioangiol 1988;36:59-66. [PubMed]
- Joo I, Park EA, Kim KH, et al. MDCT differentiation between bicuspid and tricuspid aortic valves in patients with aortic valvular disease: correlation with surgical findings. Int J Cardiovasc Imaging 2012;28:171-82. [Crossref] [PubMed]
- Espinal M, Fuisz AR, Nanda NC, et al. Sensitivity and specificity of transesophageal echocardiography for determination of aortic valve morphology. American Heart Journal 2000;139:1071-6. [Crossref] [PubMed]
- Brandenburg RO Jr, Tajik AJ, Edwards WD, et al. Accuracy of 2-dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: echocardiographic-anatomic correlation in 115 patients. Am J Cardiol 1983;51:1469-73. [Crossref] [PubMed]