Not quite an acute ST segment elevation myocardial infarction
Introduction
Acute ST-Segment Elevation Myocardial Infarction (MI) has long been known as one of the most serious emergencies cardiologists face on a daily basis. Typically, patient’s present with crushing chest pain and shortness of breath compounded with classic ECG changes and positive TROPONIN level. Definitive management in such patients involves emergent cardiac catheterization with the intent to diagnose and treat any form of coronary obstruction. However, there are times when despite these findings there is an alternative etiology which may not be cardiac after all. One such etiology includes cardiac lymphoma where involvement of the heart muscle can result in ECG changes mimicking underlying myocardial ischemia or infarction. This review and case report provides insight into this rare but documented cardiac condition.
Case report
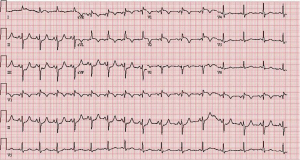
A 58 year-old African American male with a past medical history significant for a questionable previous antero-septal myocardial infarction in 1992, emphysema, Hepatitis C, and non-Hodgkin Lymphoma (diffuse large B cell lymphoma) treated with 6 cycles of R-CHOP with successful remission presented to the ER with complaints of worsening chest pain for over 1 month. Physical Exam revealed a patient in moderate distress with vital signs demonstrating slightly elevated blood pressure and heart rate. Further exam revealed a right testicular swelling, right lower extremity weakness but no reproducible chest wall tenderness. Routine lab work revealed a normal CBC and BMP, but elevated troponin I (1.3) and elevated D-dimer. Subsequent ECGs demonstrated 1-2 mm concave ST elevation predominantly over the anterolateral leads (Figure 1). Given his acute presentation of chest pain and ST-T wave changes suggestive of an ACUTE MI in addition to his previous CAD history and risk factors the patient underwent an urgent left heart catheterization to evaluate his coronary anatomy.
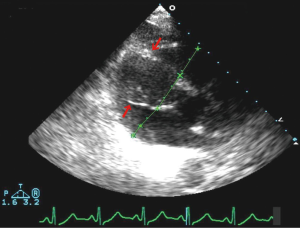
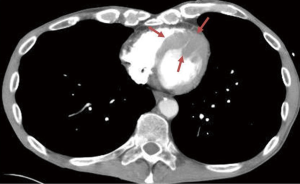
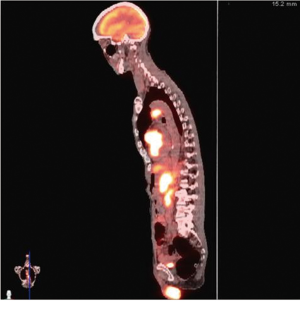
Urgent left heart catheterization revealed mild non-obstructive coronary artery disease with no obvious culprit lesion. Given these results, Anterior ST elevation MI was ruled out and further investigation was carried out to determine the etiology of his chest pain and elevated troponin. Transthoracic Echocardiography demonstrated a unique finding of profound antero-septal wall thickening resulting in near obliteration of the left ventricular cavity with marked reduction in left ventricular function (Figure 2). Further CHEST CT revealed multiple enlarged mediastinal lymph nodes suggestive of recurrent non-Hodgkins lymphoma (Figure 3). With the above results, it appeared the patient likely had a spread of his lymphoma into his myocardium. Further investigative tests including CT Head and Abdomen and Whole body PET scan revealed multiple organ involvement including liver, testicular, adrenal and massive leptomeningeal spread (Figure 4). With his clinical picture and radiological findings the likely etiology of his chest pain, elevated troponin and evolving ECG changes were attributed to secondary cardiac lymphoma – a rare manifestation. The patient was initiated on aggressive chemotherapy which was modified in view of the extensive spread of his condition.
Given this unusual clinical scenario it was hard to find an ideal regimen to treat him; repeating R-CHOP was not a feasible option considering that his disease was much more aggressive and there was no evidence in literature on using R-CHOP in cases of relapse. The decision was made to treat him with RICE regimen (rituxan, ifosfamide, carboplatin and etopside), intrathecal methotrexate and testicular XRT after chemotherapy was completed. However, due to the side effects and overall toxicity of these agents, the patient refused further therapy and died a few months later.
Discussion
Lymphoma, a hematological cancer, affects thousands of people yearly and can be predominantly divided into two major groups—Hodgkins and Non-Hodgkins Lymphoma with the latter being far more common. Both forms of lymphoma can be staged using the Ann Arbor method where Stage I-II encompasses predominantly lymph node involvement and III-IV involves extra-lymphatic organs or sites. Overall this particular malignancy is characterized by nonspecific symptoms such as fever, chills, weight loss, poor appetite, lymphadenopathy, night sweats etc. Non-Hodgkins lymphoma consists of 16 different conditions that have little in common with each other (1). This group of lymphomas can vary in their severity ranging from indolent to very aggressive with diffuse large B-cell lymphoma being a more aggressive form and attributing to 30-40% of all adult non-Hodgkins Lymphoma (2), as seen in our case.
Cardiac malignancies can be further divided into primary or secondary tumors with metastatic involvement often being the culprit in the latter group. Primary cardiac neoplasms are exceedingly rare (0.0001-0.5% incidence in autopsy series) making diagnosis and treatment challenging (3). In contrast, secondary cardiac tumors often characterized by metastatic involvement of the heart are far more common. Malignant melanoma, leukemia, lymphoma, lung, soft tissue sarcomas, renal, esophageal, hepatocellular, thyroid and breast cancers are commonly associated with cardiac involvement (4). Therefore, cardiac or pericardial metastasis should be considered whenever a patient with a known malignancy develops cardiovascular symptoms.
Most cases of cardiac lymphoma demonstrate either one or more chamber involvement with the right heart being the most common site (5-7). Hypertrophy or thickening of the ventricular septum without subsequent cardiomyopathy can result in characteristic hemodynamic disturbances (8). Spread to the myocardium has been hypothesized to be secondary to either hematogenous spread through coronary arteries, epicardial and pericardial lymphatics or through vena caval spread to the right-sided heart chambers. Contiguous spread can also occur from directly adjacent tissues including the lung, esophagus, lymph nodes etc(9).
Clinical symptoms of cardiac lymphoma are nonspecific ranging from being silent (as seen in B cell lymphomas) to producing chest pain, worsening shortness of breath, palpitations etc(10,11). Physical signs include an array of findings based on the structural involvement and subsequent hemodynamic disturbances. Examples include a pericardial friction rub; appearance of distended neck veins or even signs of florid heart failure. Diagnostic evaluation should mainly include echocardiography, MRI and CT. Echocardiography, often the first line imaging modality can reveal a mass like appearance or even infiltration of the pericardium, epicardium, myocardium or endocardium. One such study demonstrated trans-esophageal echocardiography as a modality to obtain tissue diagnosis via endomyocardial biopsy (12). However, despite its ease of use echocardiography also has its limitations for diagnosis secondary to operator dependence and restricted field of views. To combat these limitations other imaging modalities such as CT and MRI have been utilized with MRI providing a more detailed diagnosis. Features such as variable signal intensity and delayed contrast enhancement help to identify areas of myocardial necrosis and fibrosis often seen in cardiac malignancies whether primary or secondary (13). In most cases, however, conclusive diagnosis via tissue sampling is usually obtained on post-mortem examination (14).
This case shows that lymphomatous involvement of the heart can mimic an acute ST segment elevation MI, yet is extremely rare and therefore the clinical presentation, results of diagnostic studies and outcome following therapeutic interventions are largely unknown. Early detection and treatment of Cardiac Lymphoma in cases of recurrent Non-Hodgkin Lymphoma is imperative to prevent serious complications such as outflow obstruction and to develop therapeutic regimens in the future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Harris NL, Jaffe ES, Diebold J, et al. Lymphoma classification--from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol 2000;11:3-10. [PubMed]
- Lee PW, Woo KS, Chow LT, et al. Images in cardiovascular medicine. Diffuse infiltration of lymphoma of the myocardium mimicking clinical hypertrophic cardiomyopathy. Circulation 2006;113:e662-4. [PubMed]
- Butany J, Nair V, Naseemuddin A, et al. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219-28. [PubMed]
- Batchelor WB, Butany J, Liu P, et al. Cardiac metastasis from primary cervical squamous cell carcinoma: three case reports and a review of the literature. Can J Cardiol 1997;13:767-70. [PubMed]
- Rolla G, Calligaris-Cappio F, Burke AP, et al. Cardiac lymphoma. Travis WD, Brambilla E, Muller-Hermelink HK, et al. eds. Pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon, France, 2004:282-3.
- Chim CS, Chan AC, Kwong YL, et al. Primary cardiac lymphoma. Am J Hematol 1997;54:79-83. [PubMed]
- Ikeda H, Nakamura S, Nishimaki H, et al. Primary lymphoma of the heart: case report and literature review. Pathol Int 2004;54:187-95. [PubMed]
- Wynne J, Kasper DL, Braunwald E, et al. Cardiomyopathy and myocarditis. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill Co, 2004.
- Jeudy J, Kirsch J, Tavora F, et al. From the radiologic pathology archives: cardiac lymphoma: radiologic-pathologic correlation. Radiographics 2012;32:1369-80. [PubMed]
- Chinen K, Izumo T. Cardiac involvement by malignant lymphoma: a clinicopathologic study of 25 autopsy cases based on the WHO classification. Ann Hematol 2005;84:498-505. [PubMed]
- Saotome M, Yoshitomi Y, Kojima S, et al. Primary cardiac lymphoma--a case report. Angiology 2002;53:239-41. [PubMed]
- Burling F, Devlin G, Heald S. Primary cardiac lymphoma diagnosed with transesophageal echocardiography-guided endomyocardial biopsy. Circulation 2000;101:E179-81. [PubMed]
- O'Donnell DH, Abbara S, Chaithiraphan V, et al. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol 2009;193:377-87. [PubMed]
- Ban-Hoefen M, Zeglin MA, Bisognano JD. Diffuse large B cell lymphoma presenting as a cardiac mass and odynophagia. Cardiol J 2008;15:471-4. [PubMed]


