Bioengineered in vitro models of thrombosis: methods and techniques
Introduction
Thrombosis is a prevailing vascular disorder that may readily occur in both arteries and veins (1-4). Thrombosis is often the result of vascular injuries, featured by obstruction, to different degrees, of vascular lumens by coagulated blood and thus disturbance/cessation of blood flows at the site surrounding the thrombus (5,6). In healthy vessels, the endothelium functions to prevent coagulation, through secretion of anti-thrombotic agents such as nitric oxide and expression of binding sites for similar molecules such as antithrombin (5,7,8). Therefore, injuries of the endothelium usually lead to the activation of primary homeostasis (platelet binding and aggregation) and the coagulation cascade (fibrin formation and crosslinking) (Figure 1A) (5,7,8,10). While acute thrombosis may be resolved via administration of thrombolytic agents such as tissue plasminogen activator (tPA) that degrade fibrin (11-14), this is not always effective. The invasion and propagation of fibroblasts and smooth muscle cells from the site of endothelial injury into the mass of thrombus usually result in its fibrosis and matrix organization, turning the thrombus to a permanent clot that cannot be resolved (Figure 1B) (15-20). Subsequently, the vessel wall stiffens and blood pressure builds up (21,22), further developing post-thrombotic syndrome, clinically manifesting as symptoms including edema, pain, and ulceration, among others (15,18).
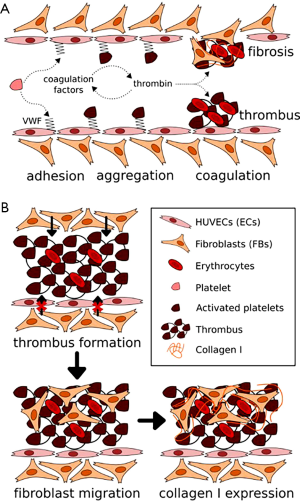
While animals have been historically used as models to study thrombosis (23-25), these in vivo models typically differ from the human system, leading to biased understanding and inaccurate predictions of treatment effects in humans. In addition, imaging and characterizing thrombosis in animals are cumbersome, and they cannot achieve high throughput that is needed in cases such as screening of therapeutics. Here, we discuss some recent advances in methods and techniques used for generating in vitro biomimetic models of human thrombosis, termed thrombosis-on-a-chip systems, which to a large extent recapitulate the important pathophysiology of their in vivo counterparts in terms of vascular structures, extracellular matrix properties, and cellular composition. These miniaturized, usually transparent devices also allow for convenient analyses at much increased throughputs than animals. We finally conclude with future perspectives. It should be noted that, only hydrogel-based thrombosis-on-a-chip models will be illustrated in the current Review, as they typically present better biomimetic features than those fabricated with silicone elastomers and plastics.
Thrombosis-on-a-chip models generated using soft lithography
Soft lithography is a long-established technique for patterning a variety of (bio)materials at microscales (26). It is generally conducted through a replica molding process, i.e., a master mold with a desired pattern is first fabricated, on top of which the secondary material is cast and crosslinked; this secondary material is then detached from the master mold to achieve the replication of the pattern in the reverse mode. As such, the interconnected microfluidic channels inside a bulk material can be readily produced to mimic the perfusable vascular structure (27,28). Soft lithography is easy to operate, capable of high-resolution patterning, and highly reproducible, but is sometimes limited by the reliance on the need for the master molds with pre-designed primary patterns.
In a prominent example, Zheng and colleagues utilized a vascular network generated with soft lithography to study angiogenesis and thrombosis (29). In their procedure, a polydimethylsiloxane (PDMS) stamp containing a surface pattern of interconnected ridges was cast with a bath of collagen solution; upon gelation, the PDMS stamp was removed to expose the patterned grooves at the bottom of the collagen hydrogel, followed by attachment to another layer of flat collagen at the bottom to form a closed vascular structure within the collagen matrix (Figure 2A). Subsequently, endothelial cells were seeded into this hollow vascular pattern, leading to the formation of a monolayer of endothelium on the interior surface of the microchannels (Figure 2A,B).
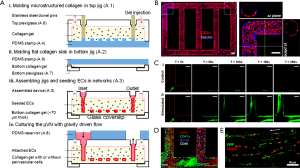
With this perfusable vascular device, the authors were able to further study the interactions of whole blood and the endothelium. As expected, under normal conditions, the vast majority of blood cells flowed past the endothelial surfaces without noticeable adherence, and only a small amount of platelets were observed to roll along the endothelium during the blood flow (Figure 2C). On the contrary, when the vascular chip was primed with phorbol-12-myristate-13-acetate (PMA), a known secretagogue for von Willebrand factor (vWF), the platelets started to significantly aggregate and adhere to the endothelial surface (Figure 2C). After 1 h of perfusion, leukocytes were also shown to attach to the lumen walls with signs of migration through the barrier into the surrounding collagen hydrogel (Figure 2D). Mechanistic investigation revealed the secretion of vWF by the endothelial cells on surfaces, which served to bound to platelets in the blood (Figure 2E), indicating that the engineered vascular model was biologically functional and responsive to externally applied signaling molecules, capable of inducing thrombosis formation.
Thrombosis-on-a-chip model generated with bioprinting
As aforementioned, while soft lithography is convenient, it has limited flexibility in fabrication of vascular patterns due to the requirement of master molds. To this end, three-dimensional (3D) bioprinting has recently emerged as a versatile technology to fabricate volumetric tissue constructs possessing complex architectures, including those that are vascularized (30-36).
Among the various bioprinting systems such as inkjet bioprinter, microextrusion bioprinter, laser-assisted bioprinter, and stereolithography (32-34), sacrificial bioprinting strategies based on extrusion have been most widely used for generation of vascularized tissue constructs at relatively high resolutions. In a typical procedure, a microfibrous pattern is first deposited in an arbitrary shape, and cast by the hydrogel matrix that is subsequently crosslinked; the initially bioprinted microfibrous network is selectively removed from the hydrogel block to induce the formation of an interconnected microvascular network that resembles the blood vessels. The use of a bioprinting system allows significantly improved flexibility of such a method in comparison with soft lithography, as the deposition of the sacrificial template can be automated simply by altering the digital input patterns for the bioprinters. A variety of sacrificial biomaterials have been developed to enable extrusion bioprinting of vascularized constructs, ranging from carbohydrate lattices that can be removed by dissolution by perfusing medium (37), mechanical extraction of stiffened agarose microfibers (38-40), as well as thermoresponsive materials that liquefy upon temperature change, such as Pluronic F127 that transforms from the hydrogel state at room temperature to a liquid at <4 °C (41,42), or gelatin that gels at room temperature or lower but becomes a liquid 37 °C (43). Similar to soft lithography, these sacrificially bioprinted microchannels could also be functionalized with a layer of endothelial cells to introduce biological functionality.
Using a modified sacrificial bioprinting strategy based on Pluronic, we and co-workers recently reported the fabrication of a thrombosis-on-a-chip model (9). A Pluronic template, containing the vascular pattern and the outside frame, was first bioprinted and then dried in air overnight; this bioprinted structure was placed on a substrate, filled with a gelatin methacryloyl (GelMA) hydrogel pre-polymer, and photocrosslinked; eventually, the entire construct was placed in a cold buffer bath to rehydrate the Pluronic and to dissolve it out (Figure 3A,B). The bioprinting of not only the vascular template but also the surrounding frame followed by desiccation served as the container to hold the hydrogel block in place, eliminating the need for additional molds. Both straight and branching microchannels could be generated using this sacrificial bioprinting method, which could be further endothelialized (Figure 3C). The obtained microchannels resembling the blood vessels were filled with human whole blood induced to clot, leading to formation of a biomimetic thrombosis model with aggregated blood cells (Figure 3D). By comparing with a human clot, the bioengineered thrombosis-on-a-chip model exhibited strong similarity when an endothelial barrier was present (Figure 3E). It was further demonstrated that, the acute clots in these bioprinted thrombosis models could be dissolved away by perfusing with tPA, while fibroblasts embedded within the surrounding GelMA hydrogel matrix were able to migrate into the thrombus in the absence of an intact endothelium, depositing collagens that resulted in the aging of the clot on the chip.
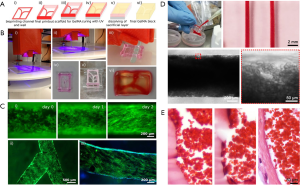
Conclusions
We have discussed recent advances in methods and techniques, primarily based on soft lithography and 3D bioprinting, for engineering biomimetic in vitro models of thrombosis. These models feature a hydrogel matrix mimicking the extracellular matrix of the biological tissue, endothelialized microchannels resembling the blood vessels, and the ability to flow human whole blood through microchannels stimulated with specific agents to induce platelet aggregation in situ or to directly infuse blood induced to coagulate within the microchannels. These thrombosis-on-a-chip models could faithfully reproduce this important vascular disorder in vitro, enabling accurate investigations into their biology and treatment. Although still preliminary, we foresee that further development of these models will eventually allow for personalized screening of intravascular interventions for treatment of thrombosis in a patient-specific manner using cells and imaging data derived from individual patients.
Acknowledgements
Funding: The authors acknowledge funding from the National Institutes of Health (K99CA201603, R21EB021148, R01HL137193, and R01EB024403). YS Zhang further acknowledges support from the Lush Prize and the Science and Technology Commission of Shanghai Municipality (STCSM) 17JC 1400200.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. New Engl J Med 1984;310:1137-40. [Crossref] [PubMed]
- Falk E, Fernández-Ortiz A. Role of thrombosis in atherosclerosis and its complications. Am J Cardiol 1995;75:3B-11B. [Crossref] [PubMed]
- Mai C, Hunt D. Upper-extremity deep venous thrombosis: a review. Am J Med 2011;124:402-7. [Crossref] [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost 2001;86:452-63. [PubMed]
- Owens AP 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost 2010;104:432-9. [Crossref] [PubMed]
- Furie B, Furie BC. Mechanisms of thrombus formation. New Engl J Med 2008;359:938-49. [Crossref] [PubMed]
- Breitenstein A, Tanner FC, Luscher TF. Tissue Factor and Cardiovascular Disease: Quo Vadis? Circ J 2010;74:3-12. [Crossref] [PubMed]
- Versteeg HH, Heemskerk JW, Levi M, et al. New fundamentals in hemostasis. Physiol Rev 2013;93:327-58. [Crossref] [PubMed]
- Zhang YS, Davoudi F, Walch P, et al. Bioprinted Thrombosis-on-a-Chip. Lab Chip 2016;16:4097-105. [Crossref] [PubMed]
- Oklu R, Albadawi H, Watkins MT, et al. Detection of extracellular genomic DNA scaffold in human thrombus: implications for the use of deoxyribonuclease enzymes in thrombolysis. J Vasc Interv Radiol 2012;23:712-8. [Crossref] [PubMed]
- Jaff MR, McMurtry MS, Archer SL, et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension. Circulation 2011;123:1788-830. [Crossref] [PubMed]
- Kunadian V, Gibson CM. Thrombolytics and Myocardial Infarction. Cardiovasc Ther 2012;30:e81-8. [Crossref] [PubMed]
- American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, O'Gara PT, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. [Crossref] [PubMed]
- Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012;379:2364-72. [Crossref] [PubMed]
- Deatrick KB, Eliason JL, Lynch EM, et al. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg 2005;42:140-8. [Crossref] [PubMed]
- Fineschi V, Turillazzi E, Neri M, et al. Histological age determination of venous thrombosis: A neglected forensic task in fatal pulmonary thrombo-embolism. Forensic Sci Int 2009;186:22-8. [Crossref] [PubMed]
- Hara T, Truelove J, Tawakol A, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography enables the detection of recurrent same-site deep vein thrombosis by illuminating recently formed, neutrophil-rich thrombus. Circulation 2014;130:1044-52. [Crossref] [PubMed]
- Nosaka M, Ishida Y, Kimura A, et al. Time-dependent appearance of intrathrombus neutrophils and macrophages in a stasis-induced deep vein thrombosis model and its application to thrombus age determination. Int J Legal Med 2009;123:235-40. [Crossref] [PubMed]
- Saha P, Humphries J, Modarai B, et al. Leukocytes and the Natural History of Deep Vein Thrombosis Current Concepts and Future Directions. Arterioscler Thromb Vasc Biol 2011;31:506-12. [Crossref] [PubMed]
- Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008;28:387-91. [Crossref] [PubMed]
- Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg 2011;53:500-9. [Crossref] [PubMed]
- Kahn SR, Comerota AJ, Cushman M, et al. The Postthrombotic Syndrome: Evidence-Based Prevention, Diagnosis, and Treatment Strategies A Scientific Statement From the American Heart Association. Circulation 2014;130:1636-61. [Crossref] [PubMed]
- Fahed R, Raymond J, Ducroux C, et al. Testing flow diversion in animal models: a systematic review. Neuroradiology 2016;58:375-82. [Crossref] [PubMed]
- Raj JA, Stoodley M. Experimental Animal Models of Arteriovenous Malformation: A Review. Vet Sci 2015;2:97-110. [Crossref] [PubMed]
- Lysgaard Poulsen J, Stubbe J, Lindholt JS. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur J Vasc Endovasc Surg 2016;52:487-99. [Crossref] [PubMed]
- Qin D, Xia Y, Whitesides GM. Soft lithography for micro-and nanoscale patterning. Nat Protoc 2010;5:491. [Crossref] [PubMed]
- Khademhosseini A, Suh KY, Jon S, et al. A soft lithographic approach to fabricate patterned microfluidic channels. Anal Chem 2004;76:3675-81. [Crossref] [PubMed]
- Ling Y, Rubin J, Deng Y, et al. A cell-laden microfluidic hydrogel. Lab Chip 2007;7:756-62. [Crossref] [PubMed]
- Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 2012;109:9342-7. [Crossref] [PubMed]
- Sheth R, Balesh ER, Zhang YS, et al. Three-Dimensional Printing: An Enabling Technology for IR. J Vasc Interv Radiol 2016;27:859-65. [Crossref] [PubMed]
- Zhang YS, Duchamp M, Oklu R, et al. Bioprinting the Cancer Microenvironment. ACS Biomater Sci Eng 2016;2:1710-21. [Crossref] [PubMed]
- Zhang YS, Yue K, Aleman J, et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng 2017;45:148-63. [Crossref] [PubMed]
- Malda J, Visser J, Melchels FP, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 2013;25:5011-28. [Crossref] [PubMed]
- Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773-85. [Crossref] [PubMed]
- Li YC, Zhang YS, Akpek A, et al. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016;9:012001. [Crossref] [PubMed]
- Zhang YS, Pi Q, van Genderen AM. Microfluidic Bioprinting for Engineering Vascularized Tissues and Organoids. J Vis Exp 2017;(126).
- Miller JS, Stevens KR, Yang MT, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 2012;11:768-74. [Crossref] [PubMed]
- Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014;14:2202-11. [Crossref] [PubMed]
- Massa S, Sakr MA, Seo J, et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017;11:044109. [Crossref] [PubMed]
- Zhang YS, Duchamp M, Ellisen LW, et al. Recapitulating Mammary Ductal Carcinoma Microenvironment in vitro Using Sacrificial Bioprinting. Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1-5; Washington DC, USA. Philadelphia: AACR; Cancer Res 2017;77:Abstract nr 4828.
- Kolesky DB, Truby RL, Gladman AS, et al. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv Mater 2014;26:3124-30. [Crossref] [PubMed]
- Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 2016;113:3179-84. [Crossref] [PubMed]
- Lee VK, Kim DY, Ngo H, et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014;35:8092-102. [Crossref] [PubMed]


