Coronary CT angiography—future directions
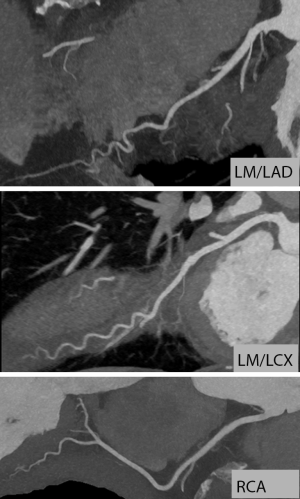
With the exception of some early attempts using Electron beam tomography in the 1990s (1-3), coronary CT angiography (CTA) has not become possible until recently. Around the year 2000, multi-detector CT systems were introduced (4) and provided mechanical CT with gantry rotation times below one second. The combination with specific ECG-gated algorithms for partial-scan image reconstruction provided the prerequisite for cardiac imaging with high temporal resolution and, as a consequence, coronary visualization relatively free of motion artefact. Technology has since then evolved to an astonishing extent. Substantially faster gantry rotation times (now below 300 ms), detector arrays comprising between 62 and 320 detector rows, dual source CT and substantial = tube technology improvements combined with advanced image reconstruction algorithms have led to significant improvements in image quality. These are mainly due to increased resolution (spatial and temporal) and image noise. Furthermore, wider detectors reduce artefacts that are caused by “stitching” of data sets from multiple cardiac cycles. Using a combination of these techniques and algorithms and after intravenous injection of iodinated contrast agent, current CT systems permit to robustly image the coronary arteries (Figure 1). With carefully selected image acquisition protocols, the contemporary estimated effective dose of coronary CTA will typically range between approximately 1.5 and 5.0 mSv (5). However, patient characteristics (e.g., body weight) and the type of CT system used will have relevant influence on dose. In selected cases, tailored protocols may provide estimated effective radiation doses of well below 1.0 mSv (Figure 2) (6-8).
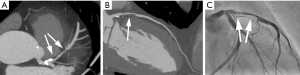
It should of course be mentioned that—even with current technology—coronary CTA does not perform equally well in all patients. If patients cannot hold their breath even for a short period of time, if patients are severely overweight, and in patients with arrhythmias or whose heart rate cannot be slowed below a threshold of 60–65 beats/min, the likelihood to obtain poor quality image data sets is high and the diagnostic use of coronary CTA should be carefully weighed against available alternative diagnostic methods such as stress myocardial perfusion imaging.
What is the current situation?
Coronary CTA, in general, has high accuracy for detecting coronary artery stenoses. It is relevant to note that specificity is typically lower than sensitivity—stenoses are over—rather than underestimated. Published research trials and their meta-analyses consistently report sensitivity to be above 90% and specificity to be in the lower 80% range. A recent meta-analysis even indicated a sensitivity of 95.6% to identify patients with coronary artery stenoses (9). Obviously, these performances will influence the way coronary CTA is used clinically. With its high sensitivity, resulting in a high negative predictive value (NPV), coronary CTA is particularly well suited to reliably rule out coronary stenoses. However, in patients with unfavorable conditions for high image quality, the accuracy of coronary CTA is reduced and this, in particular, concerns specificity. As outlined above, this may typically be the case in patients with irregular or with high heart rates, in patients who have pronounced coronary calcification and especially in patients with a combination of both. Also, studies demonstrated that the accuracy of coronary CTA is reduced in patients who have a high pre-test likelihood of CAD as compared to those with lower pre-test likelihood (10).
Hence, now and in the future, clinical applications of coronary CTA will typically be based on the method´s high sensitivity in the detection of coronary stenoses, under the prerequisite that image quality is good and that the patient’s pre-test likelihood is in the lower range. The known high sensitivity results in a very high negative predictive value, meaning that CT is able to extremely reliably rule out stenoses in patients with symptoms that suggest the possibility of coronary artery disease. This will most likely remain the most prominent application of cardiac CT well into the future and it is well supported by data. Large-scale registries and prospective trials have consistently shown that prognosis regarding survival and cardiovascular events is excellent and that there is no need for further testing if there is no coronary artery lesion in CT—both in patients with acute and stable chest pain. Such data has been derived, for example, from the CONFIRM registry which included >25,000 patients (11,12) and from the large, prospective randomized PROMISE trial which included 10,000 patients (13). In PROMISE CT angiography performed just as good as stress testing when used as the initial test for patients in whom coronary disease was suspected. Event rates during follow-up were not different between the stress-testing and the CT group at 2 years (3.3% vs. 3.0%). The utilization rate of invasive coronary angiography was higher in patients who underwent CT as the initial test (12.2% vs. 8.3%) but the rate of coronary angiograms that were performed but did not show any relevant coronary stenoses was significantly lower (28% vs. 53%, P=0.02).
Importantly, initial data show that in the future, the use of coronary CTA may not only be able to replace other forms of diagnostic testing, but, in fact, may improve patient outcome. Such evidence was found in the SCOT-HEART trial. Within the trial, 4,146 patients with suspected CAD were randomized to receive either a standard workup (typically, stress testing) or to receive the same standard workup plus an additional coronary CTA investigation (14). After a mean follow-up period of 1.7 years, the rate of myocardial infarction (both fatal and non-fatal) was reduced by 38% in patients assigned to additionally receiving contrast-enhanced coronary CTA (which, however, just failed to achieve significance at P=0.0527). Speculating about the future of coronary CT angiography, this trial may indeed be a particularly strong piece of evidence. It indicates that patient management may actually benefit from adding CT, making a more prominent role of coronary CTA in the future rather likely.
Guidelines
Future, widespread clinical application of coronary CTA will require the incorporation of cardiac CT into the respective management guidelines by large professional societies, since most clinical care algorithms and diagnostic decisions are based on this guidance. In fact, a systematic review of professional guidelines revealed that CT imaging is rather widely incorporated at present time (15). In particular, coronary CTA increasingly penetrates guidelines on national and international levels regarding the diagnostic workup of patients with stable and acute chest pain.
For example, in their guidelines for stable coronary artery disease published in the year 2012, the American Heart Association/American College of Cardiology assign a “Class IIa” recommendation (meaning: “should be considered”) to the use of coronary CTA if patients with low-to-intermediate pre-rest likelihood of disease are unable to exercise. A “Class IIb” recommendation (“may be considered”) is given to coronary CTA in patients who can exercise and who have what is estimated to be an intermediate pre-test likelihood of disease. Coronary CTA is also endorsed with a “Class IIa” recommendation if patients who present with an intermediate pre-test likelihood of disease have a non-conclusive exercise test, have ongoing symptoms in spite of a normal exercise test, or if they are unable to undergo stress testing by stress echocardiography or nuclear medicine myocardial perfusion imaging (16). The European Society of Cardiology (ESC) assigns a “Class IIa” recommendation to coronary CTA in patients who present with acute chest pain but without ECG changes or elevated enzymes (17). The ESC also assigns a “Class IIa” recommendation for coronary CTA as a first-line test in patients with suspected stable CAD and a low-to-intermediate pre-test likelihood of CAD (18,19). As a very reasonable disclaimer, the guidelines add that the respective centre must HAVE adequate equipment as well as experience in coronary CTA and the patient characteristics should be well suited to expect good image quality for coronary CTA. This includes the absence of extensive coronary arterial calcification (18).
Coronary CTA and ischemia
Coronary CTA is an anatomic test, which does not permit to evaluate the hemodynamic significance of coronary artery stenoses. This is a potential downside that CT angiography shares with the invasive coronary angiogram. In fact, the development of coronary CTA during the past 15 years has coincided with a period in which the ischemic extent, rather than the degree of stenosis, was increasingly recognized as the parameter that predicts a benefit of revascularization (for example, the FAME trials) (20-25). As a consequence, that time period saw purely anatomic testing falling somewhat into disgrace. To some extent, future applications of coronary CTA will hinge on this relationship between anatomic disease on one hand and functional consequences (ischemia) on the other. Interestingly, for a large part of the “candidate cohort” for coronary CTA examinations, this is not relevant. These are the patients who have a low pre-test likelihood of CAD and if coronary CTA performed to exclude coronary stenosis is “negative”; no further testing is necessary. However, if stenoses is present, ischemia testing will be necessary in most cases to decide about optimal further management (with the rare exception of cases were anatomy alone implies a benefit of revascularization, such as left main coronary artery stenosis or triple vessel disease with inclusion of the proximal left anterior descending coronary artery). Referral to another test for assessment of ischemia is a possibility (either a non-invasive test or an invasive angiography with FFR), but CT itself also offers opportunities that may play an increasingly important role in the future. CT myocardial perfusion imaging is one option (26). In the literature, numerous evaluations of such an approach can be found. For example, in the Core 320 trial, coronary CTA plus CT myocardial perfusion showed a high accuracy to predict hemodynamically relevant stenosis when tested against a gold standard of combined SPECT imaging plus invasive coronary angiography (27). All the same, there is no relevant clinical use of CT myocardial perfusion at present and its future utilization will depend on the development of robust, low-dose image acquisition protocols and reliable methods for evaluation. If these problems are solved, more widespread application can be expected.
As an alternative to myocardial perfusion imaging, computational fluid dynamic modeling can be used to simulate the effect of coronary artery stenoses on downstream myocardial perfusion under stress from anatomical coronary CTA dataset obtained under resting conditions (“FFR-CT”, see Figure 3). According to published data, simulated FFR-CT results correlate quite closely with invasively measured FFR values (28). In the largest such study, the “NXT Trial”, the sensitivity of FFR-CT to identify coronary lesions with significant stenosis (i.e., invasive FFR ≤0.80) was 86%, specificity was 79% and overall accuracy was 81% (29).
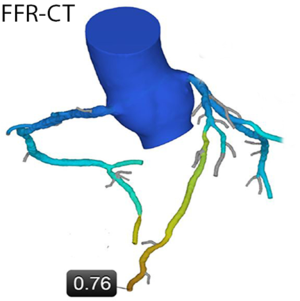
The extent to which coronary CTA can be combined with ischemia imaging and the logistics around it will be a major determinant of the use of coronary CTA in the future. The better CT is suited to identify ischemia in addition to anatomic stenosis, and the more robust the results of techniques such as FFR-CT or CT myocardial perfusion imaging, the more likely it will be that clinical applications of CT imaging in the context of CAD will expand into the spectrum of patients with a higher pre-test likelihood of stenoses, or to patients with previously known CAD.
Plaque
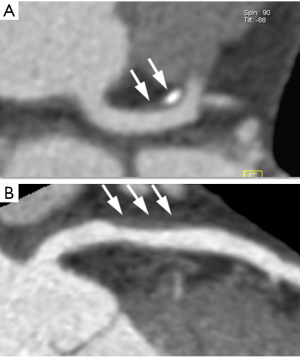
Coronary CTA allows to visualize not only stenosis, but also the coronary atherosclerotic plaque, even without stenosis (Figure 4). The ability to visualize plaque is strongly dependent on image quality. Given that the vast majority of cardiac events are caused by rupture of the atherosclerotic plaque, the detection and characterization of plaque components has for many years been regarded as a superbly interesting approach to improved, individualized risk stratification beyond traditional risk factors. Plaque characterization is possible to some degree with CT since some of these CT parameters indicate the “vulnerability” of coronary atherosclerotic lesions. These parameters include a “spotty” pattern of calcification, low CT attenuation (<30 HU) and large degree of positive remodelling (30). In several trials, the overall plaque extent in CT on one hand and above-named features on the other hand were both associated with future coronary atherosclerotic events (31,32). However, all these trials were characterized by low event rates and even in the presence of plaque with “advanced” feature, the overall prognosis is still rather good, which precludes the use of coronary CTA as a screening method in unselected populations. A future extension of CT into unselected “screening” to identify more patients who are candidates for risk-modifying therapy, e.g., with statins is therefore unlikely. The opposite, however, may be the case: if CT is normal, prognosis is good and even in patients with a somewhat elevated risk profile, statin therapy may not be required. This harbors the chance of a tremendous clinical role for coronary CTA. In an evaluation of the CONFIRM registry that included 10,418 symptomatic individuals without previously known coronary artery disease (a typical “primary prevention” population), Chow et al. were able to show that as far as future cardiovascular events are concerned, only individuals with detectable plaque in coronary CTA benefitted from statin therapy, while in the cohort without any detectable plaque, statin therapy was not beneficial (33). This may offer an opportunity to withhold expensive, long-term therapy in a substantial amount of patients, with corresponding economic implications for the individual and the healthcare system.
Challenges
The major challenge of coronary CTA is the need for high image quality to allow accurate and clinically reliable diagnosis. This requires careful image acquisition which must include adequate preparation of the patient and, if necessary, the use of beta blockers to lower heart rate. If image quality is impaired, an increased rate of false-positive results is usually the consequence. Severe calcification (higher in older patients and those with a high pre-test likelihood) and stents are further conditions which make coronary CTA unreliable.
Summary of future perspectives
An increasing number of clinical trials will become available evaluating the use of coronary CTA in several clinical contexts, particularly for the diagnosis of CAD. In addition, it can be expected that entirely new areas will be explored of application for coronary CTA. For example, there is continuously expanding interest to use coronary CTA in the context of percutaneous coronary intervention (PCI) (34). This includes the use of coronary CTA in the context of interventional revascularization for chronic total coronary occlusions (35-37), which has been shown to provide valuable information not readily obtainable from invasive coronary angiography. With ever more complex coronary interventions performed percutaneously, and an increasing diversity of devices available for such treatment, a growing future role of cardiac CT in the context of coronary intervention is predictable.
CT technology will have further developments, but these will not be as groundbreaking or fast as the rapid progress of the past 10–15 years. All the same, continuous, progress can be expected. Recent evidence, for example, is the widespread introduction of iterative reconstruction algorithms that increase the image quality, allow reduced radiation exposure, or achieve a combination of both. More importantly, potentially the availability of high-end CT throughout healthcare systems will increase. Importantly, there is a requirement to provide adequate training and quality assurance in a cardiac CT. This is one of the major issues that need to be urgently addressed. In addition, the ability to perform high-quality coronary CTA will be available at an increasing amount of institutions across the globe. Uniform approaches for interpretation (38) and reporting systems may be useful to support imaging quality and quality control (39).
At the moment, it is uncertain the extent to which CT-FFR and CT perfusion imaging will become clinically robust diagnostic tools endorsed by official guidelines. As mentioned above, these two techniques are likely not relevant for the vast majority of patients at the lower end of the spectrum of pre-test probability of CAD, in which coronary CTA is an extremely useful test if adequately performed, and in whom a “negative” coronary CTA permits to forego any further testing. Major future developments will therefore probably be the wider dissemination and availability of CTA, and use of coronary CTA as a diagnostic test to rule out coronary disease even in patients with higher pre-test likelihood or those with known disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Moshage WE, Achenbach S, Seese B, et al. Coronary artery stenoses: three-dimensional imaging with electrocardiographically triggered, contrast agent-enhanced, electron-beam CT. Radiology 1995;196:707-14. [Crossref] [PubMed]
- Schmermund A, Rensing BJ, Sheedy PF, et al. Intravenous electron-beam computed tomographic coronary angiography for segmental analysis of coronary artery stenoses. J Am Coll Cardiol 1998;31:1547-54. [Crossref] [PubMed]
- Achenbach S, Moshage W, Ropers D, et al. Value of electron-beam computed tomography for the noninvasive detection of high-grade coronary-artery stenoses and occlusions. N Engl J Med 1998;339:1964-71. [Crossref] [PubMed]
- Achenbach S, Ulzheimer S, Baum U, et al. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation 2000;102:2823-8. [Crossref] [PubMed]
- Chinnaiyan KM, Boura JA, DePetris A, et al. Advanced Cardiovascular Imaging Consortium Coinvestigators. Progressive radiation dose reduction from coronary computed tomography angiography in a statewide collaborative quality improvement program: results from the Advanced Cardiovascular Imaging Consortium. Circ Cardiovasc Imaging 2013;6:646-54. [Crossref] [PubMed]
- Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 2010;31:340-6. [Crossref] [PubMed]
- Schuhbaeck A, Achenbach S, Layritz C, et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur Radiol 2013;23:597-606. [Crossref] [PubMed]
- Hell MM, Bittner D, Schuhbaeck A, et al. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: Feasibility, image quality, radiation dose, and effect of iterative reconstruction. J Cardiovasc Comput Tomogr 2014;8:418-25. [Crossref] [PubMed]
- Menke J, Kowalski J. Diagnostic accuracy and utility of coronary CT angiography with consideration of unevaluable results: A systematic review and multivariate Bayesian random-effects meta-analysis with intention to diagnose. Eur Radiol 2016;26:451-8. [Crossref] [PubMed]
- Meijboom WB, van Mieghem CA, Mollet NR, et al. 64-Slice Computed Tomography Coronary Angiography in Patients With High, Intermediate, or Low Pretest Probability of Significant Coronary Artery Disease. J Am Coll Cardiol 2007;50:1469-75. [Crossref] [PubMed]
- Otaki Y, Arsanjani R, Gransar H, et al. What have we learned from CONFIRM? Prognostic implications from a prospective multicenter international observational cohort study of consecutive patients undergoing coronary computed tomographic angiography. J Nucl Cardiol 2012;19:787-95. [Crossref] [PubMed]
- Min JK, Dunning A, Lin FY, et al. CONFIRM Investigators. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849-60. [Crossref] [PubMed]
- Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291-300. [Crossref] [PubMed]
- SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383-91. [Crossref] [PubMed]
- Al-Mallah MH, Aljizeeri A, Villines TC, et al. Cardiac computed tomography in current cardiology guidelines. J Cardiovasc Comput Tomogr 2015;9:514-23. [Crossref] [PubMed]
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354-471. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003. [Crossref] [PubMed]
- Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900-7. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. [Crossref] [PubMed]
- Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis: 5-Year Follow-Up of the DEFER Study. J Am Coll Cardiol 2007;49:2105-11. [Crossref] [PubMed]
- Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;36:3182-8. [Crossref] [PubMed]
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N Engl J Med 2009;360:213-24. [Crossref] [PubMed]
- De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208-17. [Crossref] [PubMed]
- Pelgrim GJ, Dorrius M, Xie X, et al. The dream of a one-stop-shop: Meta-analysis on myocardial perfusion CT. Eur J Radiol 2015;84:2411-20. [Crossref] [PubMed]
- Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 2014;35:1120-30. [Crossref] [PubMed]
- Min JK, Taylor CA, Achenbach S, et al. Noninvasive Fractional Flow Reserve Derived From Coronary CT Angiography: Clinical Data and Scientific Principles. JACC Cardiovasc Imaging 2015;8:1209-22. [Crossref] [PubMed]
- Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145-55. [Crossref] [PubMed]
- Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. [Crossref] [PubMed]
- Min JK, Shaw LJ, Devereux RB, Okin PM, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161-70. [Crossref] [PubMed]
- Hadamitzky M, Täubert S, Deseive S, et al. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J 2013;34:3277-85. [Crossref] [PubMed]
- Chow BJ, Small G, Yam Y, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter registry) registry. Arterioscler Thromb Vasc Biol 2015;35:981-9. [Crossref] [PubMed]
- Achenbach S. Coronary CTA and percutaneous coronary intervention – a symbiosis waiting to happen. J Cardiovasc Comput Tomogr 2016;10:384-85. [Crossref] [PubMed]
- Opolski MP, Achenbach S. CT Angiography for Revascularization of CTO: Crossing the Borders of Diagnosis and Treatment. JACC Cardiovasc Imaging 2015;8:846-58. [Crossref] [PubMed]
- Li Y, Xu N, Zhang J, et al. Procedural success of CTO recanalization: Comparison of the J-CTO score determined by coronary CT angiography to invasive angiography. J Cardiovasc Comput Tomogr 2015;9:578-84. [Crossref] [PubMed]
- Sugaya T, Oyama-Manabe N, Yamaguchi T, et al. Visualization of collateral channels with coronary computed tomography angiography for the retrograde approach in percutaneous coronary intervention for chronic total occlusion. J Cardiovasc Comput Tomogr 2016;10:128-34. [Crossref] [PubMed]
- Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342-58. [Crossref] [PubMed]
- Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10:269-81. [Crossref] [PubMed]


