Procedural challenge of coronary catheterization for ST-segment elevation myocardial infarction in patient who underwent transcatheter aortic valve replacement using the CoreValveTM
Introduction
Aortic valve stenosis is an atherosclerotic disease-causing heart failure despite medical therapies. It is frequently accompanied by atherosclerotic coronary artery disease which affects clinical outcomes in patients with aortic valve stenosis (1,2). Surgical aortic valve replacement has been considered as the standard therapeutic option in patients with severe aortic valve stenosis. Recently, transcatheter aortic valve replacement (TAVR) is becoming a feasible and effective therapeutic option for extremely high-risk or “inoperable” patients and is an alternative therapy for many high-risk but “operable” patients. This is supported by recent randomized clinical trials which have demonstrated the clinical efficacy of TAVR in patients with symptomatic severe aortic stenosis who are inoperable or at high risk as well as at intermediate risk for surgical aortic valve replacement (3-6). Currently, different types of trans-catheter valves including balloon-expandable and self-expandable ones are available and their efficacies have been reported in the aforementioned clinical trials (3-6). The self-expanding Medtronic CoreValveTM device (Medtronic Inc., Minneapolis, MN, USA) is characterized as a long funnel-shaped nitinol stent frame which completely covers the aortic root (7). Given that the atherosclerotic disease substrate of aortic valve stenosis likely exhibits the formulation and progression of coronary atherosclerosis, this CoreValveTM-related feature emerges the concern that the implanted valve may interfere the access to the coronary ostium when a patient receiving TAVR requires coronary catheterization to treat coronary artery disease. This case report describes the procedural challenge of coronary angiography in a patient who had ST-segment elevation myocardial infarction 4 years after TAVR using CoreValveTM.
Case presentation
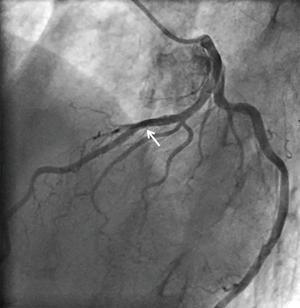
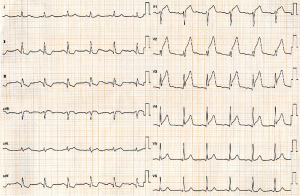
A 73-year-old man was hospitalized due to congestive heart failure. Echocardiography demonstrated severe aortic valve stenosis (aortic valve area =0.55 cm2, peak and mean pressure gradient =91 and 58 mmHg, respectively) with preserved ejection fraction (left ventricular ejection fraction =51%). Due to a concomitant severe chronic obstructive pulmonary disease (The Society of Thoracic Surgeon score =5.8%), our heart team decided to conduct TAVR. Coronary angiography prior TAVR showed the presence of mild stenosis in the middle segment of the left anterior descending artery (Figure 1). Twenty-nine-mm CoreValveTM was successfully implanted without any major complication (Figure S1). Four years later after the procedure, he visited our emergency department due to severe prolonged chest pain. Electrocardiogram showed a marked ST-segment elevation at precordial leads (Figure 2). Emergent coronary catheterization was conducted for prompt revascularization of infarct-related artery. However, the implanted CoreValveTM profoundly interfered manipulation of catheters and guidewires. In particular, a RadifocusTM guidewire (Terumo, Tokyo, Japan) did not went through the CoreValveTM and migrated into the space between the CoreValveTM and the aorta wall (Figure S2). Following substantial attempts, a RadifocusTM guidewire was successfully advanced into the left coronary cusp by positioning a 5-French diagnostic catheter just above the CoreValveTM (Figure S3). We delivered a diagnostic catheter along the RadifocusTM guidewire. However, Judkins left (JL) 4, Amplatz-left 1 and multipurpose catheters were unfeasible for selective intubation of the stent mesh which was closest to the left coronary ostium. The left coronary artery was barely engaged by a JL3.5 (Figure S4). Coronary angiography identified a severe stenosis in the middle segment of the left anterior descending artery (Figure 3). A JL3.5 guide-catheter (6-French, BritetipTM, Cordis, Milpitas, CA, USA) was engaged into the left coronary artery. This culprit lesion was successfully treated by an implantation of cobalt-chromium everolimus-eluting stent without any complication (Figure 3). Door-to-balloon time was 105 minutes.




Discussion
In our case, despite the need for prompt restoration of coronary flow in the setting of ST-segment elevation myocardial infarction, the implanted CoreValveTM considerably made it difficult to control catheters, resulting in a long door-to-balloon time over 90 minutes. This case raises a potential concern that a supracoronary implanted valve would become a major obstacle in conducting coronary angiography in patients who previously received TAVR.
CoreValveTM is a self-expanding frame which extends superiorly to anchor in the supracoronary aorta (7). This feature of CoreValveTM substantially interferes guidewire manipulation and catheter engagement as shown in our case. Similar observation has been reported by several case reports (8-10), which illustrates the challenges to perform coronary angiography and percutaneous coronary intervention (PCI) after the implantation of transcatheter valve. Given that even other self-expandable and balloon-expandable transcatheter valves which are placed in a fully or partially supracoronary position could also have risks to impede coronary catheterization procedures, this prosthesis-related feature should be recognized when coronary catheterization is needed.
The most challenging procedure to perform coronary catheterization is to achieve the selective intubation of diagnostic and PCI catheter into the left or right coronary artery. Since the CoreValveTM completely covers the ascending aorta, this modified the geometry of the entire aortic root which results in more difficult situation to manipulate catheters. Normally, the Judkins-left catheter is advanced over the J-wire to the aortic root. After the guidewire is removed, a subtle advancement or retraction of the catheter with counter clockwise rotation enables to engage the catheter into the coronary ostia. In our case, however, a subtle control of catheter itself without the J-wire was not possible in the modified aortic root due to the implanted valve. After we left the J-wire inside the catheter, its precise manipulation was possible and successfully engaged into the left coronary artery. Our case underscores careful planning to conduct coronary catheterization under the presence of the implanted transcatheter valve.
Based on our experiences in this case, the following tips and procedures seem to be important to achieve successful PCI in patients who previously received TAVR (Table 1). Since the space within aorta is limited due to the implanted valve, JL and JR are more appropriate rather than Amplatz type and back up catheters. Minor modification of their catheter’s tip is possible and effective to properly engage into coronary artery. Multipurpose type may be also applicable because it’s almost straight-shape catheter is easy to manipulate despite the presence of the prosthetic valve. Moreover, by attaching this catheter to the skirt of the implanted valve, catheter shape is modified and then potentially intubated into coronary ostia. In some cases, even if the aforementioned catheter selected, its intubation into coronary artery may be impossible. In this situation, after placing tip of guiding catheter close to the coronary ostia as much as possible, crossing guidewire under the use of microcatheter is required. Another possible way is to cross the guidewire through the cannulated diagnostic catheter and then exchange to 5 or 6 French size guiding catheter over the extension wire (Figure 4). With regard to the size of guiding catheter, 5 or 6 French size is better than larger ones. This is because bulky catheter is very hard to subtle control in this situation. Smaller size of catheters is more controllable to adapt its shape to the coronary ostia. If stronger buck-up is needed to deliver stent, mother-and-child catheter extension technique or deep engage of guiding catheter with balloon anchoring technique is possible options during PCI (11,12). It is also important to select stent with superior deliverability such as Resolute OnyxTM drug-eluting stent.

Full table
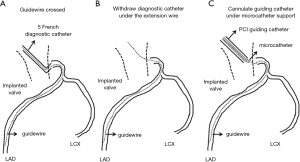
Selecting proper access site for cardiac catheterization procedure is another important part. Since very tortuous iliac and femoral arteries are not uncommon in patients with severe aortic valve stenosis, radial or brachial artery use should be always considered. In our case, left radial artery was used due to severely calcified and tortuous femoral arteries. Considering that smaller size of catheters is more suitable to cannulate into coronary artery, trans-radial PCI is applicable option in patients who previously treated with TAVR.
As shown in our case, the need for emergent coronary angiography and PCI makes the procedure tougher in patients treated with TAVR. This is because this urgent situation does not provide enough time to plan strategies prior to the actual procedure. In our case, the door-to-balloon time was over 90 minutes because this was our first case to conduct coronary angiography and PCI in the setting of acute coronary syndrome in a patient with the implanted transcatheter aortic valve. This indicates that interventionalists need to fully understand the three-dimensional geometry of the prosthetic valve and its relation to the coronary ostia, and they should have the ability to quickly strategize the procedure of coronary catheterization under the presence of the implanted transcatheter aortic valve.
Aortic valve stenosis has been considered as atherosclerotic disease caused by lipoprotein deposition, chronic inflammation and active leaflet calcification. Given that these atherogenic stimuli also contributes to the formation and progression of coronary atherosclerosis, the risk for future coronary events always exists in patients with aortic valve stenosis. In our case, mild stenotic lesion in the left descending artery substantially progressed for 4 years, leading to the occurrence of acute coronary syndrome. Due to the progressive nature of coronary atherosclerosis in patients with aortic valve stenosis (13), further widespread adoption of TAVR using different types of valves predicts that a substantial number of cases will need diagnostic and interventional procedures after TAVR. This underscores the urgent needs to establish better procedural strategies for coronary catheterization and to develop new transcatheter valve with larger stent meshes which would not disturb coronary ostia. The concomitant use of anti-atherosclerotic medications including high-intensity statin and proprotein convertase subtilisin/kexin type 9 inhibitor after TAVR would be another important additional therapies to slow plaque progression, thereby potentially avoid future coronary catheterization.
In conclusion, the implanted CoreValveTM substantially interfered the manipulation of RadifocusTM guidewire and catheters in patients who previously received TAVR. Considering the progressive nature of coronary atherosclerosis in patients with aortic valve stenosis, our challenging situation is not uncommon and the interventionalist should establish the applicable procedures which enable for prompt selective intubations of catheters and conducting PCI in patients with aortic valve stenosis who previously treated with TAVR using CoreValveTM.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Vandeplas A, Willems JL, Piessens J, et al. Frequency of angina pectoris and coronary artery disease in severe isolated valvular aortic stenosis. Am J Cardiol 1988;62:117-20. [Crossref] [PubMed]
- Rapp AH, Hillis LD, Lange RA, et al. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am J Cardiol 2001;87:1216-7; A7.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321-31. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Ielasi A, Latib A, Tespili M. Current and new-generation transcatheter aortic valve devices: an update on emerging technologies. Expert Rev Cardiovasc Ther 2013;11:1393-405. [Crossref] [PubMed]
- Geist V, Sherif MA, Khattab AA. Successful percutaneous coronary intervention after implantation of a CoreValve percutaneous aortic valve. Catheter Cardiovasc Interv 2009;73:61-7. [Crossref] [PubMed]
- Michiels V, Vrints C, Bosmans J. Percutaneous coronary intervention after transcatheter aortic valve implantation. Heart 2011;97:1458. [Crossref] [PubMed]
- Greenberg G, Kornowski R. Coronary angioplasty after self-expandable transcatheter aortic valve implantation. J Invasive Cardiol 2013;25:361-3. [PubMed]
- Di Mario C, Ramasami N. Techniques to enhance guide catheter support. Catheter Cardiovasc Interv 2008;72:505-12. [Crossref] [PubMed]
- Takahashi S, Saito S, Tanaka S, et al. New method to increase a backup support of a 6 French guiding coronary catheter. Catheter Cardiovasc Interv 2004;63:452-6. [Crossref] [PubMed]
- Milin AC, Vorobiof G, Aksoy O, et al. Insights into aortic sclerosis and its relationship with coronary artery disease. J Am Heart Assoc 2014;3:e001111. [Crossref] [PubMed]
- Aikawa Y, Kataoka Y, Kanaya T, et al. Successful implantation of CoreValve. Asvide 2018;5:390. Available online: http://asvidett.amegroups.com/article/view/24185
- Aikawa Y, Kataoka Y, Kanaya T, et al. Manipulation of Radifocus guidewire through CoreValve. Asvide 2018;5:391. Available online: http://asvidett.amegroups.com/article/view/24187
- Aikawa Y, Kataoka Y, Kanaya T, et al. Delivery of diagnostic catheters along Radifocus guidewire for selective intubation of the stent mesh. Asvide 2018;5:392. Available online: http://asvidett.amegroups.com/article/view/24188
- Aikawa Y, Kataoka Y, Kanaya T, et al. Left coronary angiography. Asvide 2018;5:393. Available online: http://asvidett.amegroups.com/article/view/24189


