Value of neutrophil to lymphocyte ratio as a predictor of mortality in patients undergoing aortic valve replacement
Introduction
Calcific aortic valve stenosis is the most common valvular heart disease in the western world (1). Surgical aortic valve replacement (AVR) represents the gold standard of treatment, when indicated, with around 67,500 procedures done annually in the United States (2).
Calcific aortic valve disease is an indolent disease with a spectrum that ranges from mild valve thickening, aortic sclerosis, to severe calcification and impaired leaflet motion, aortic stenosis. This process was thought to be degenerative and a consequence of time-dependent leaflet damage and calcium deposition (3). Clinical aortic stenosis doesn’t uniformly affect the elderly, with an estimated prevalence of only 3% to 5% in patients aged 75 or more (4,5). This suggests that other factors, besides age-related degeneration, play a major role in the disease pathogenesis. Evidence of chronic inflammation has been found, by immunohistochemical studies, in aortic valve leaflets; with the presence of macrophages and T lymphocytes, which release inflammatory cytokines. These inflammatory cells and cytokines help stimulate fibrotic and calcific processes, which in turn increase valve stiffness (3). Presence of chronic inflammation is also supported by studies demonstrating increased systemic C-reactive protein in patients with aortic stenosis (6), increased temperature in stenotic aortic valve cusps (7), and increased 18F-sodium fluoride (18F-NaF) uptake with the use of positron emission tomography, with progressive rise in activity with increasing valve severity (8).
Several studies showed that elevated neutrophil to lymphocyte ratio (NLR) is a significant predictor of adverse outcomes for patients with cardiovascular disease including stable coronary artery disease, acute coronary syndrome, heart failure and aortic stenosis. Additionally, a relationship between NLR and the severity of calcific aortic stenosis has been found (9-13).
Our objective in this study is to examine the utility of NLR as a predictor of short- and long-term mortality in patients with calcific aortic stenosis undergoing surgical AVR.
Methods
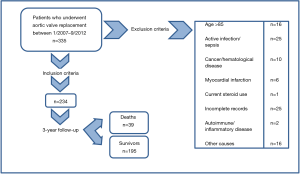
This longitudinal retrospective observational study examined the usefulness of NLR value as a predictor of survival in 335 patients who underwent AVR between January 2007 and September 2011. Patients undergoing non-emergent, AVR, secondary to aortic valve stenosis, were evaluated for study inclusion. Study exclusion criteria included age >85 years, clinical evidence of infective endocarditis or any other active infection, cancer, hematopoietic disease, autoimmune or inflammatory diseases, current steroid or chemotherapy use, emergent valve replacement, acute coronary syndrome on admission, and incomplete medical records. Two hundred and thirty-four patients out of the 335 were eligible for the study inclusion (Figure 1). All-cause short-term (30 days), 6-month, and 3-year mortality were obtained from electronic medical records and Social Security Death Index. This study was approved by the institutional review board of Saint Joseph Regional Medical Center.
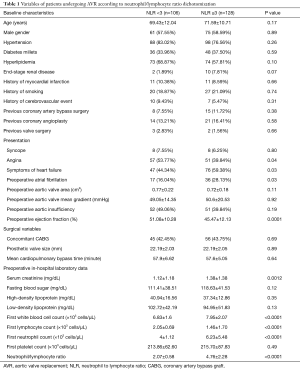
White blood cell (WBC) counts and differentials were obtained from the first, pre-operative blood tests on index admissions. Differential leukocyte counts were obtained using automated blood cell counter. Electronic medical records were reviewed, independently, by two physicians for baseline characteristics, presenting symptoms, preoperative, in-hospital laboratory values, preoperative echocardiographic findings, and AVR surgical reports (Table 1). Aortic valve area, mean aortic flow gradient, presence of aortic insufficiency and other valvular pathologies were obtained from preoperative transthoracic and/or transesophageal echocardiograms. Smoking history included any current or previous smoking.
Full table
The patients were dichotomized based on their NLR, NLR ≥3 and <3. The cutoff value for NLR was calculated by testing all possible cutoffs that would discriminate between mortality by Cox proportional analyses. The cutoff value was then rounded to a clinically convenient value. Variables were assessed for the normality of distribution using D’Agostino-Pearson test. Continuous variables were presented as means ± SDs and categorical variables were presented as frequencies and percentages. Group comparisons used chi-square analysis for categorical variables. Continuous variables were compared using analysis of variance or Mann-Whitney U-test, depending on the probability distribution of the variable. All probabilities were 2-sided and P values <0.05 were considered statistically significant.
Univariate Cox proportional hazard models were used to examine the association of NLR and all other variables with long-term mortality. Then, a multivariate Cox proportional hazard model, adjusted for all predictors of long-term mortality of the univariate analysis and significant baseline characteristics was done. We confirmed that the proportionality of hazards assumption was met. Kaplan-Meier curves were used to illustrate the difference in 3-year survival function between the two groups.
Leukocytes and platelets parameters were investigated for superiority of mortality prediction in two ways. First, each leukocyte parameter was compared between survivor and mortality groups using Kruskal-Wallis test or analysis of variance, depending on normality of data distribution. Second, multivariate Cox proportional hazard model using leukocyte and platelets parameters was done.
All data were analyzed using MedCalc version 15.2.2 (MedCalc Software bvba, Belgium) and SPSS 22 (IBM Corp., USA).
Results
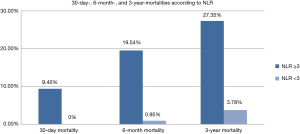
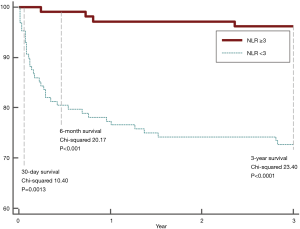
The primary outcome was all-cause 3-year mortality. Thirty-nine deaths occurred in the 234 patients (16.7%). Figure 2 illustrates significant higher short-term (9.40% vs. 0, P=0.0006), 6-month (19.54% vs. 0.95%, P<0.0001), and 3-year mortality (27.35% vs. 3.78%, P<0.0001) in patients with NLR ≥3, when compared to those with NLR <3. Three-year Kaplan-Meier curves shows that patients with NLR ≥3 had significantly worse short, 6-month, and 3-year mortalities than patients with NLR <3 (Mantel-Cox Chi-square 10.4, 20.17, 23.40 respectively, P<0.001; Figure 3).
In the high NLR group, there was a significantly higher prevalence of symptoms of heart failure (44.34% vs. 59.38%, P=0.03), lower ejection fraction (51.08±10.28 vs. 45.47±12.13, P=0.0001), preoperative presence of atrial fibrillation (16.04% vs. 28.13%, P=0.03), and higher serum creatinine levels (1.12±1.18 vs. 1.38±1.38, P=0.0012) when compared to those with lower NLR. However, angina was significantly higher in the lower NLR group (53.77% vs. 39.84%, P=0.04).
Leukocyte parameters, including neutrophils and lymphocytes, total WBC, and NLR significantly differed by patients’ vital status (P=0.02452, P=0.00015, P=0.03460, P<0.0001, respectively; Table 2). Of all, NLR was the best predictor of mortality (Kruskal-Wallis Chi-square 22.03, P<0.0001). Leukocyte parameters, WBC, and NLR adjusted mortality hazards also indicated that NLR significantly predict long term mortality [hazard ratio (HR) =1.27, 95% CI, 1.17–1.39], whereas the rest failed to demonstrate a significant mortality hazard.

Full table
In the univariate Cox regression analysis, patients with an NLR ≥3 had an 8.39 fold increase risk of 3-year mortality (HR =8.39; 95% CI, 3.00–23.48, P=0.0001). Univariate analysis also identified end-stage renal disease, hypertension, history of cerebrovascular accident, preoperative ejection fraction, history of bypass surgery, and serum creatinine as prognosticators of mortality (Table 3). The rest of variables failed to show any statistical significance.
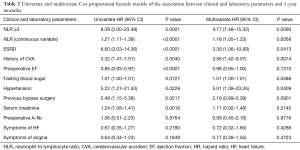
Full table
In a multivariate Cox regression analysis NLR was independently associated with a higher 3-year mortality with a HR increase by a factor of 1.3 (HR =1.3, 95% CI, 1.18–1.44; P<0.0001) and a NLR ≥3 was associated with 4.77-fold increase in 3-year mortality. The multivariate analysis was adjusted for the independent predictors of outcome: history of end stage renal disease, cerebrovascular event, hypertension, and bypass surgery; symptoms of heart failure, angina and A-fib; and ejection fraction, fasting blood sugar, and serum creatinine as continuous variables. Additionally, previous cerebrovascular accident and end-stage renal disease were also found to be independent prognosticators of 3-year mortality (Table 3).
Discussion
The pathophysiology of calcific aortic valve disease is characterized by three primary processes: lipid accumulation, inflammation, and calcification (14). This disease process shares similar risk factors with atherosclerosis such as smoking, advanced age, male gender, metabolic syndrome, elevated lipoprotein (a) level, increased serum creatinine level, hypertension, diabetes mellitus, high low density lipoprotein-cholesterol and low level of high density lipoprotein-cholesterol (4,15-20). The calcification of the aortic valve leaflets in AS are usually located on the aortic side of the leaflets, at the cusps coaptation lines and at the cusps attachment sites to the aortic wall and extend to the cusps. This pattern suggests that calcifications develop at sites of maximal mechanical stress. This was postulated to be the initiating factor in the disease process, in a similar manner to that of atherosclerosis, by causing endothelial injury (3). Histological studies have shown similarities between AS valvular lesions and atherosclerosis lesions. Inflammation manifests with T lymphocytes and macrophages which infiltrate the endothelium and release cytokines. These cytokines promote cellular proliferation and extracellular matrix remodeling by acting on valvular fibroblasts (3,21). Despite that, prospective randomized trials have failed to show a reduction in aortic valve calcific stenosis progression with anti-inflammatory, statin therapy (22,23). This might be a result of late initiation of statin therapy in the disease process, after reaching irreversible stages.
NLR was studied as a prognostic marker in atherosclerotic heart disease and cancer. Many authors reported that higher NLR values were associated with worse outcomes in patients with acute coronary syndrome, stable coronary artery disease, ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction and in patients undergoing percutaneous coronary interventions (12,24,25). Calcific aortic valve stenosis is a progressive disease, where the underlying pathophysiology of inflammation parallels that of atherosclerosis. Our study is the first study, to our knowledge, to examine the use of NLR value as a risk predictor for short and long-term mortality in patients diagnosed with severe AS undergoing AVR surgery.
NLR is a nonspecific marker of systemic inflammation. It represents a ratio between two different subtypes of leukocytes; neutrophils, which are part of the non-specific, innate immune system, and lymphocytes, which are part of the specific, regulatory pathway of the immune system. One major difficulty in using NLR value as a prognosticator in disease processes is determining an appropriate cut-off value around which disease outcomes differ. The appropriate NLR cut-off value is likely to change with the stage of illness, laboratory techniques employed, concomitant infection and patient demographic characteristics (26). In our study, a statistically significant difference in all-cause mortality was found between patients using the cutoff value of 3.
Despite the normal range of the index white blood cell counts in both of our NLR groups, the high NLR group had statistically significant higher normal white blood cell count values. In addition, the high NLR group had lower lymphocyte counts, as well as higher neutrophil counts, leading to the statistically significant higher NLR values. It is clear that NLR carries more information than its constituent elements especially when the absolute counts of these elements are in the normal range.
In our study, there was a higher prevalence of certain co-morbid conditions in the high NLR group (NLR ≥3). Those co-morbid conditions are symptomatic heart failure, lower ejection fraction (EF), preoperative atrial fibrillation, and elevated serum creatinine level. This is consistent with prior studies that showed an elevated NLR in these conditions (27-30). There was a higher prevalence of end-stage renal disease (ESRD) in the high NLR group; however, this was not statistically significant in our sample. The prevalence of angina was higher in the low NLR group; this is consistent with the fact that angina is associated with the longest median survival (approximately 5 years) in patients with severe AS when compared to heart failure and syncope (31).
Prior studies have demonstrated that risk factors such as tobacco use, diabetes mellitus, dyslipidemia, hypertension and coronary artery disease, have significant correlations (positive or negative) with NLR (32-35), however, both NLR groups in our study did not statistically differ in the prevalence of these conditions.
In our study, pre-operative NLR was demonstrated to predict short- and long-term mortality after AVR surgery in patients diagnosed with severe AS. It is not clear whether high NLR is related to the pathogenic process of AS or it is a component or marker of one or many unrelated deleterious systemic pathologic conditions that continue unabated after AVR increasing mortality rates in the high NLR group. It is also unclear whether high NLR has an etiological role in the increased mortality rates or it is just a marker of the etiological condition(s) of increased mortality. Assuming that high NLR is related to the pathogenesis of AS, the fact that higher mortality rates continue to occur after having a new prosthetic aortic valve in place, in the high NLR group, suggests that the disease process of AS is systemic and not confined to the aortic valve. Another possibility is that high NLR is a component or marker of a systemic abnormality that has many manifestations, with AS being one manifestation, but other manifestations continue to evolve over time after AVR leading to increased mortality.
The main limitation of our study is the non-randomized, retrospective, single-center, observational design. Our study included a small number of patients which may have led to selection bias. A large multicenter prospective study that uses a clinically relevant NLR cut-off value is needed to establish NLR as a true prognostic factor in patients diagnosed with severe AS undergoing AVR surgery.
Conclusions
NLR is an independent predictor of short- and long-term mortality in patients with aortic stenosis undergoing AVR surgery. Patients with a NLR ≥3 had a statically significant mortality. We strongly suggest the use of NLR as a tool to risk stratify patients with aortic stenosis undergoing AVR surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Saint Joseph Regional Medical Center.
References
- Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 2011;8:162-72. [Crossref] [PubMed]
- Clark MA, Duhay FG, Thompson AK, et al. Clinical and economic outcomes after surgical aortic valve replacement in Medicare patients. Risk Manag Healthc Policy 2012;5:117-26. [Crossref] [PubMed]
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005;111:3316-26. [Crossref] [PubMed]
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease: Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630-4. [Crossref] [PubMed]
- Lindroos M, Kupari M, Heikkilä J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993;21:1220-5. [Crossref] [PubMed]
- Galante A, Pietroiusti A, Vellini M, et al. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol 2001;38:1078-82. [Crossref] [PubMed]
- Toutouzas K, Drakopoulou M, Synetos A. In vivo aortic valve thermal heterogeneity in patients with nonrheumatic aortic valve stenosis the: first in vivo experience in humans. J Am Coll Cardiol 2008;52:758-63. [Crossref] [PubMed]
- Dweck MR, Jones C, Joshi NV. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76-86. [Crossref] [PubMed]
- Wang X, Zhang G, Jiang X, et al. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis 2014;234:206-13. [Crossref] [PubMed]
- Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther 2013;11:55-9. [Crossref] [PubMed]
- Uthamalingam S, Patvardhan EA, Subramanian S, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol 2011;107:433-8. [Crossref] [PubMed]
- Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 2010;106:470-6. [Crossref] [PubMed]
- Avci A, Elnur A, Göksel A, et al. The relationship between neutrophil/lymphocyteratio and calcific aortic stenosis. Echocardiography 2014;31:1031-5. [Crossref] [PubMed]
- Otto CM, Kuusisto J, Reichenbach DD, et al. Characterization of the early lesion in “degenerative” valvular aortic stenosis: histological and immunohistochemical studies. Circulation 1994;90:844-53. [Crossref] [PubMed]
- Baumgartner H. Aortic stenosis: medical and surgical management. Heart 2005;91:1483-8. [Crossref] [PubMed]
- Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol 1987;59:998-9. [Crossref] [PubMed]
- Mohler ER, Sheridan MJ, Nichols R, et al. Development and progression of aortic valve stenosis: atherosclerosis risk factors—a causal relationship? A clinical morphologic study. Clin Cardiol 1991;14:995-9. [Crossref] [PubMed]
- Lindroos M, Kupari M, Valvanne J, et al. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J 1994;15:865-70. [Crossref] [PubMed]
- Boon A, Cheriex E, Lodder J, et al. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart 1997;78:472. [Crossref] [PubMed]
- Peltier M, Trojette F, Sarano ME, et al. Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with a three-cuspid aortic valve. Am J Cardiol 2003;91:97-9. [Crossref] [PubMed]
- Wallby L, Janerot-Sjöberg B, Steffensen T, et al. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart 2002;88:348-51. [Crossref] [PubMed]
- Rossebø AB, Pedersen TR, Boman K, et al. SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343-56. [Crossref] [PubMed]
- Cowell SJ, Newby DE, Prescott RJ, et al. Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352:2389-97. [Crossref] [PubMed]
- Núñez J, Nunez E, Bodi V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol 2008;101:747-52. [Crossref] [PubMed]
- He Jingyu, Li Jing, Wang Yunfei, et al. Neutrophil-to-lymphocyte ratio (NLR) predicts mortality and adverse-outcomes after ST-segment elevation myocardial infarction in Chinese people. Int J Clin Exp Pathol 2014;7:4045-56. [PubMed]
- Lee GK, Lee LC, Chong E, et al. The long-term predictive value of the neutrophil-to-lymphocyte ratio in Type 2 diabetic patients presenting with acute myocardial infarction. QJM 2012;105:1075-82. [Crossref] [PubMed]
- Cooper HA, Exner DV, Waclawiw MA, et al. White blood cell count and mortality in patients with ischemic and nonischemic left ventricularsystolic dysfunction (an analysis of the Studies Of Left Ventricular Dysfunction (SOLVD)). Am J Cardiol 1999;84:252-7. [Crossref] [PubMed]
- Engström G, Melander O, Hedblad B. Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail 2009;2:217-22. [Crossref] [PubMed]
- Acet H, Ertaş F, Akıl MA, et al. New inflammatory predictors for non-valvular atrial fibrillation: echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio. Int J Cardiovasc Imaging 2014;30:81-9. [Crossref] [PubMed]
- Solak Y, Yilmaz MI, Sonmez A, et al. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronickidney disease. Clin Exp Nephrol 2013;17:532-40. [Crossref] [PubMed]
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation 1968;38:61-7. [Crossref] [PubMed]
- MacNee W, Wiggs B, Belzberg AS, et al. The effect of cigarette smoking on neutrophil kinetics in human lungs. N Engl J Med 1989;321:924-8. [Crossref] [PubMed]
- Sefil F, Ulutas KT, Dokuyucu R, et al. of neutrophil lymphocyte ratio and blood glucose regulation in patients with type 2 diabetes mellitus. J Int Med Res 2014;42:581-8. [Crossref] [PubMed]
- Prajapati JH, Sahoo S, Nikam T, et al. Association of high density lipoprotein with platelet to lymphocyte and neutrophil to lymphocyte ratios in coronary artery disease patients. J Lipids 2014;2014:686791. [Crossref] [PubMed]
- Gillum RF, Mussolino ME. White blood cell count and hypertension incidence: The NHANES I Epidemiologic Follow-up Study. J Clin Epidemiol 1994;47:911-9. [Crossref] [PubMed]