Pulmonary artery aneurysms: diagnosis & endovascular therapy
Introduction
Aneurysms and pseudoaneurysms are rare abnormalities of the pulmonary arteries. While their incidence is low, they represent potentially life-threatening conditions and can present a challenge for prompt diagnosis and treatment. An aneurysm of the pulmonary artery is defined as focal dilatation beyond maximum normal diameter (1). On computed tomography, a normal adult main pulmonary artery measures up to 29 mm in diameter and an interlobar pulmonary artery, 17 mm. A true aneurysm is defined as focal dilatation of an artery involving all three layers of the vascular wall—tunica intima, tunica media, and tunica adventitia. A pseudoaneurysm, by contrast, does not involve all three layers and thus poses a higher risk of rupture. A pulmonary artery pseudoaneurysm (PAPA) is a rare and potentially life-threatening disease characterized by focal saccular outpouching of a pulmonary artery representing a contained rupture of that artery (2). The mortality rate associated with the rupture of a pulmonary artery aneurysm (PAA) or PAPA has been reported from 50–100%; death is secondary to aspiration and asphyxia after intrapulmonary hemorrhage (3-6). PAA can also lead to dissection of the pulmonary artery and sudden cardiac death (2,7). Therefore, early diagnosis and treatment are critical for patient survival and optimal outcomes.
Clinical manifestations
Clinical manifestations for both PAA and PAPA are nonspecific and can be seen in many other conditions. In addition, patients with PAA and PAPA may remain asymptomatic (8). Reported symptoms include hemoptysis, shortness of breath, chest pain, palpitations or syncopal episodes (7-10). Additional symptoms attributed to extrinsic bronchial compression by a large PAA or PAPA may include cough, worsening dyspnea, cyanosis or pneumonia (10-12).
Etiology
PAA may be congenital or acquired. In an early autopsy study, the incidence of PAA was reported to be 1:14,000 with most occurring in the main pulmonary artery (13). PAA are more commonly associated with congenital anomalies and PAPA are more commonly associated with acquired etiologies. Overall, most PAA and PAPA are caused by acquired etiologies such as trauma, iatrogenic injury, infection and Behcet’s disease (1). Congenital conditions associated with PAA and PAPA are listed in Table 1.
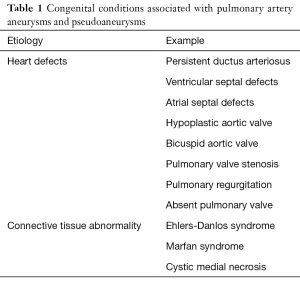
Full table
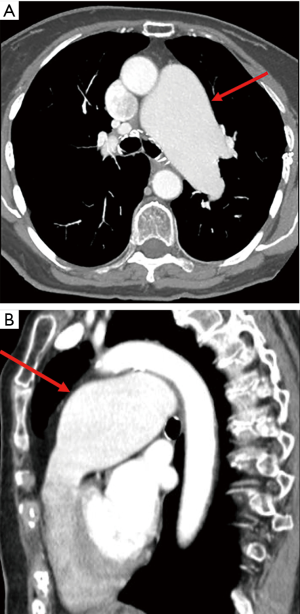
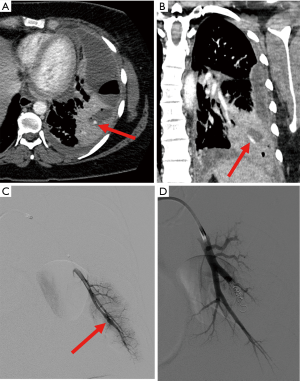
Congenital heart disease is the most common congenital anomaly associated with PAA (13-15). The postulated pathophysiology involves altered flow dynamics and increased hemodynamic sheer stress on the vessel wall, most commonly caused by left-to-right shunts (13-15). In decreasing order, the most common congenital cardiac anomalies associated with PAA include patent ductus arteriosus, ventricular septal defect, atrial septal defect, hypoplastic aortic valve and bicuspid aortic valve (13-15). Other congenital heart defects or valvular deficiencies such as pulmonary valve stenosis (Figure 1) have also been associated with PAA (1).
Another category of congenital conditions associated with PAA include diseases affecting connective tissue and vessels. Such conditions include Ehlers-Danlos syndrome, Marfan syndrome and cystic medial necrosis (9,16). As in arterial aneurysms throughout the body, pulmonary arteries and aneurysms are also subject to LaPlace’s law which states that arterial wall tension is proportional to the vessel radius at a given blood pressure. This suggests that, particularly in weakened arterial walls, larger aneurysmal arteries experience increased wall tension as they approach rupture.
As stated earlier, acquired etiologies of PAA and PAPA are more common than congenital etiologies. Acquired conditions of PAA and PAPA are listed in Table 2.

Full table
Acquired bacterial or fungal infections can cause mycotic pseudoaneurysms and less commonly aneurysms due to their ability to destroy or alter vessel walls. In patients with advanced syphilis, aneurysm formation most commonly occurs in the large pulmonary arteries with destruction occurring at the level of the vasa vasorum (17). Patients with advanced tuberculosis (TB) are at high risk of pseudoaneurysm formation in the intraparenchymal pulmonary arteries (18). These aneurysms known as Rasmussen aneurysms are peripheral PAAs which occur due to erosion of a peripheral pulmonary artery branch (19). These Rasmussen aneurysms typically involve the upper lobes in the setting of reactivation tuberculosis (1). In the post-antibiotic era, syphilis and TB, once problematic and more prevalent pathogens, have seen a dramatic decrease in incidence (8) whereas the more common infectious agents are now taking the spotlight.
Other infectious entities which can lead to PAA or PAPA include pyogenic bacteria (1) and less commonly viral (20) and fungal infections. In the setting of pneumonia, destruction of the vessel wall occurs from the outer wall to inner lumen as a result of invasion from the neighboring consolidation (21). In endocarditis, typically seen in the setting of IV drug abuse, the patients are at risk for septic emboli which can cause aneurysms both centrally and peripherally (22)—a key finding on CT to aid diagnosis. The proposed mechanism by which aneurysms form in endocarditis is endovascular seeding of the lumen by septic emboli (14,22). It is therefore suggested that a PAPA secondary to endocarditis is not necessarily related to an adjacent lung consolidation, which may also be present concomitantly (8).
Pulmonary artery hypertension (PAH) has been widely implicated as a cause of PAA (9). The mechanism of aneurysm formation is similar to other areas of the body involving increased arterial wall stress (14). Numerous etiologies of PAH exist which can ultimately result in aneurysm formation. International consensus by the fifth World Symposium on Pulmonary Hypertension has classified these into five groups (23). These groups are listed in Table 3.
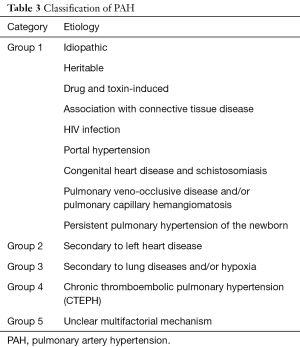
Full table
Pulmonary thromboembolic disease has been implicated as a cause of PAA or PAPA and two mechanisms have been proposed. A pulmonary embolus may cause direct local pulmonary arterial wall injury leading to aneurysm formation (8,24). A second proposed mechanism involves post-stenotic arterial dilatation related to the thromboembolic event (8). Chronic pulmonary embolism is another relatively common cause of PAA. Aneurysms which arise from this condition tend to be associated with mural thickening, webs or intramural thrombi which can calcify (1).
PAPAs may develop as a result of primary lung cancer or pulmonary metastatic disease. Though this phenomenon is rare, multiple cases have been reported (1,8,25,26). The proposed mechanism of PAPA formation involves direct tumoral invasion and vessel wall erosion (25-27).
Another less common cause of PAA or PAPA is vasculitis. Of the vasculitides, Behcet’s disease, has been reported to be the most frequent cause of pulmonary aneurysms (28). Behcet’s disease is a chronic multisystem vasculitis hallmarked by oral and genital ulcers and uveitis (29). It is most prevalent in the Middle East and Asia and may result in aneurysms typically of the right lower lobe with recurrent thrombosis and inflammation (30). It has been reported that the apparent PAAs that arise from Behcet’s disease are in fact PAPA and caused by complications of vasculitis and transmural necrosis (31). Hughes-Stovin syndrome is a rare condition which presents as a combination of systemic venous thrombosis and pulmonary aneurysms (32) and is suggested to be a variant of Bechet’s disease (32,33). As in Behcet’s disease, arterial aneurysms form secondary to obliterative endoarteritis of the vasa vasorum thereby compromising the integrity of the vessel wall (32). Therefore, both of these vasculitides are associated with PAPA rather than PAA (32).
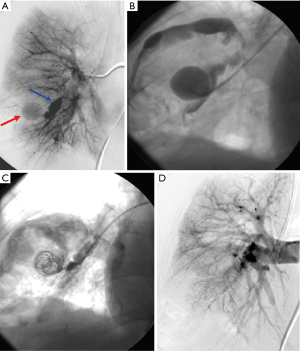
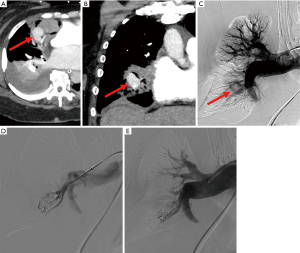
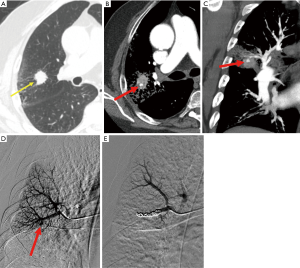
Iatrogenic and traumatic causes have increased in incidence as a source of PAA and PAPA (Figures 2,3). Numerous cases have been reported citing such causes as Swan-Ganz catheter placement, cardiothoracic surgery, chest tube placement, catheter-directed angiography, lung biopsies and even percutaneous ablation (1,3,8,34-36). The resulting pulmonary vascular injuries would more likely form PAPA. PAPA formation has also been reported in cases of penetrating trauma such as stab or gun-shot wounds (8,37).
Several other less common causes of PAA or PAPA have been described. PAA or PAPAs have developed as a result of acute or chronic inflammatory lung disease (38). In their retrospective review, Chen et al observed one patient who was noted to have a PAPA associated with traction bronchiectasis in the setting of pulmonary fibrosis (8). The exact mechanism of vascular injury has not been described. Atherosclerosis has also been reported as a rare cause of PAA as a degenerative vascular disease (14).
Idiopathic PAA is a rare and enigmatic diagnosis in the absence of other causes. In order to better characterize this classification, four pathological criteria have been defined: (I) simple dilatation of the pulmonary trunk, with or without involvement of the rest of the arterial tree; (II) absence of abnormal intra- or extracardiac shunts; (III) absence of chronic cardiac or pulmonary disease, either clinically or at autopsy; (IV) absence of arterial disease, such as syphilis or more than minimal atheromatosis or arteriolar sclerosis (39).
Methods of diagnosis
The mainstay of imaging for both detection and follow-up of PAA and PAPA remains computed tomography angiography (CTA). Given the nonspecific symptoms of PAA and PAPA, CTA focus can be placed on either the pulmonary arterial system or the aorta and bronchial artery system. Multi-detector row CTA performed with bolus tracking over the descending aorta, along with coronal and sagittal multiplanar and maximum-intensity projection (MIP) reformatted images, has been reported as an imaging protocol for hemoptysis (40). Other CTA imaging protocols call for bolus tracking over the main pulmonary artery (41) to optimize pulmonary artery opacification. The advantage of CTA is that it allows for the assessment of presence, size, location and characteristics including saccular or fusiform aneurysm type (42). Equally as important, it can provide information regarding the underlying etiology of an aneurysm in that additional clues may be found in the lungs, heart or mediastinal structures. Where available, three-dimensional and advanced post-image processing software such as AquariusNET (TeraRecon, Foster City, CA, USA) can prove to be extremely valuable in both detection as well as treatment planning. For follow-up post-treatment imaging, a non-contrast CT scan of the chest may immediately precede a CTA to differentiate calcifications or embolic materials from persistent contrast opacification. Dual-energy CT (DECT) is an advanced CT technique that allows for better material differentiation, such as differentiating iodine from other hyperdense materials and can also overcome some artifacts thus improving CT pulmonary angiogram scan quality at relatively reduced contrast and radiation dose (43).
Catheter-directed angiography has been considered the gold-standard for diagnosis of PAA and PAPA. This allows for the determination of the extent of vascular involvement and an assessment of right-sided cardiac pressures (44). In addition, simultaneous endovascular treatments can be performed. Aside from being invasive, a major limitation of pulmonary angiography is that it does not yield information regarding extra-luminal structures, which can be essential to determine etiology. One solution may be cone-beam CT, which can provide additional benefits to the imaging arm and can be a very useful tool for planning appropriate endovascular therapy. The role of non-invasive imaging modalities such as CT and MRI, however, is expanding as the technology for image gathering and reconstruction continues to evolve (42).
Magnetic resonance imaging (MRI), while not as commonly used as CTA for evaluating the pulmonary arteries, is a viable alternative where CTA cannot be employed (i.e., allergy to iodinated contrast material, renal insufficiency). Use of MRI to evaluate the pulmonary arterial system has been documented and described (45,46). T1-weighted images have been shown to adequately demonstrate pseudoaneurysms. Fast spin echo (FSE) and gradient echo imaging are useful in the morphologic evaluation of pulmonary vasculature from the main pulmonary trunk to the subsegmental level (45). When possible, gadolinium-enhanced magnetic resonance angiography improves visualization and evaluation of the subsegmental pulmonary artery branches (47). With regards to the pulmonary arterial system, one distinct advantage of MRI over CTA is that it can identify and elaborate on arterial wall thickening in connective tissue disease. Furthermore, it can provide information regarding blood flow direction in cases of post-stenotic dilatation due to pulmonary valve disease, which is important for early preventative action or treatment (1).
Imaging findings
CT appearances of PAA and PAPA can vary greatly in their number, location, morphology and associated findings. Differentiating PAA and PAPA may not be possible on imaging and requires correlation with clinical history and laboratory findings.
PAA or PAPA can be seen anywhere along the pulmonary arterial tree. Depending on the etiology, PAA or PAPA may have a predilection for central or peripheral arteries. In one of the largest retrospective studies reviewing CTAs in patients with hemoptysis and PAPA, PAPAs showed a strong predilection for the peripheral pulmonary artery branches with 63% occurring in the periphery at the subsegmental pulmonary arterial level (8). The authors also noted that 83% of the PAPA discovered were observed to be solitary occurrences (8). Additional findings associated with PAA or PAPA largely depend on the underlying etiology. As suggested by Chen et al., hemorrhage surrounding a PAPA is a strong predictor of acute trauma (8). Multiple PAA or PAPA, were typically observed in the setting of endocarditis or lung metastases (8). In the pulmonary sequelae of endocarditis, a cavitary lesion may be identified adjacent to the PAPA or more peripherally. In pulmonary metastatic disease, a consolidation or mass would likely accompany a PAPA. Given that the majority of tumor emboli are microscopic, PAPAs typically involve the subsegmental pulmonary arteries and arterioles (42).
Treatment
Once a PAA or PAPA is diagnosed, the next challenge involves determining the appropriate treatment. As highlighted earlier, multiple etiologies exist for both entities and treatment must be tailored to the underlying cause or causes as well as choosing the least invasive procedure while achieving durable results.
Neither a consensus to treat PAA based on size criteria nor treatment guidelines delineating the roles of medical or procedural disciplines currently exist. PAA or PAPA can occur at various locations and take on different forms. In non-urgent, asymptomatic patients, conservative management of a PAA can be considered to address treatable underlying etiologies; the treatment of which is beyond the scope of this review. Medical treatment alone in a more complex case may be inadequate in preventing growth or rupture of a PAA or PAPA and therefore endovascular or surgical therapies may be indicated.
Several surgical techniques and procedures exist to treat PAAs. These include aneurysmorrhaphy, lobectomy, bilobectomy, aneurysmectomy and pneumonectomy (5). Surgical resection, however, carries high risks, especially in patients with severe pulmonary hypertension (48). When feasible, endovascular treatment can be offered as the first line therapy with the advantage of less morbidity and mortality. Although specific guidelines do not exist for endovascular versus surgical treatment of PAA, many case reports support first line consideration of endovascular treatment when feasible. Endovascular therapy may best serve saccular PAA or PAPA, both in the central and peripheral pulmonary arteries as suggested by published case reports. Alternatively, fusiform aneurysms of the peripheral pulmonary arteries may be treated endovascularly where pulmonary function and adequate reserve is available, similar to peripheral pulmonary arteriovenous malformations. Central fusiform aneurysms, however, require surgical management.
In treating PAA and PAPA, one important variable that should be addressed is a bronchopulmonary artery shunt with retrograde flow. In this event, a PAA or PAPA may not be demonstrated on pulmonary angiography due to systemic artery-to-pulmonary artery shunting but can be seen on bronchial or non-bronchial systemic arteriograms (38). In these cases, embolization proximal to the PAA or PAPA from the pulmonary arteries may be insufficient.
Coil embolization of arterial aneurysms or pseudoaneurysms throughout the body is a widely accepted minimally invasive therapy (14,26,49). Intra-saccular embolization with coils has the advantage of preserving the pulmonary arteries distal to a PAA or PAPA while sparing pulmonary function (50) but should be performed with great caution due to the risk of rupture, especially in the case of PAPA. When intra-saccular embolization either carries an increased risk of rupture or is not feasible/incomplete, embolization proximal and distal to the PAA or PAPA neck can be performed (Figures 2-5). As mentioned earlier, proximal embolization alone may still allow perfusion of the aneurysm sac via bronchopulmonary anastomoses distal to a proximal embolization (51). For this reason, when embolizing the pulmonary artery supplying the PAA or PAPA, it is imperative to best rule-out a shunting phenomenon.
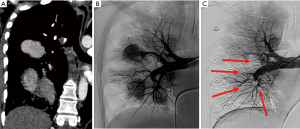
As an alternative to coils, the use of vascular plugs (Figure 6) in rare cases have be described (52,53). As with coil embolization, a bronchopulmonary shunt should be ruled out prior to performing a pulmonary artery embolization proximal to a PAA or PAPA.
Stent-assisted coil embolization is a modified form of coil embolization whereby embolization of an aneurysm is performed through the interstice of a bare-metal stent to maintain vascular patency. Though this technique has not been described in the pulmonary arterial system, it is a viable treatment option which has been described and accepted in other areas of the body for wide-necked aneurysms (54-56) and in many cases, can be applied safely in the pulmonary arteries. A distinct advantage of this technique is the preservation of flow distal to the aneurysm sac.
Although uncommon, the use of stent grafts in the pulmonary arteries has been reported; effectively excluding a pseudoaneurysm sac (57-59) while maintaining vascular patency.
Rare cases of successful glue embolization of PAPA with n-butyl cyanoacrylate (NBCA) have also been described in the literature (31,60,61). A novel technique of transcatheter embolization via the pulmonary artery with an NBCA and iodized oil mixture while employing balloon occlusion has been described to address the presence of a bronchopulmonary shunt (38).
A single earlier case of balloon embolization of a mycotic PAA has also been reported (62).
Although endovascular treatments of PAA and PAPA carry fewer inherent risks compared to surgery, they maintain similar risks to other endovascular embolization procedures throughout the body. These risks include contrast induced nephropathy, non-target embolization, arterial dissection, arterial thrombosis and partial or complete end-organ infarction.
Conclusions
Aneurysms and pseudoaneurysms of the pulmonary arteries are rare entities and often not considered in many clinical situations. CT pulmonary angiogram is the imaging modality of choice for diagnosis of PAAs and pseudoaneurysms. Currently, there is no large study comparing endovascular and surgical treatments. Additionally, consensus guidelines for treatment by either means have also not been established. As many case reports demonstrate, however, when indicated, endovascular therapy is the favored first-line treatment considering its decreased risk profile where treatment by coil embolization or other embolic devices is feasible. Before any treatment is performed, however, managing these patients should be a collaborative multidisciplinary effort between the intensivist, pulmonologist, interventional radiologist, thoracic surgeon, and when applicable, the anesthesiologist. Given the lack of consensus treatment guidelines, a multidisciplinary plan of action is necessary to increase survival while minimizing procedure-related morbidities and mortalities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nguyen ET, Silva CI, Seely JM, et al. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. AJR Am J Roentgenol 2007;188. [Crossref] [PubMed]
- Senbaklavaci O, Kaneko Y, Bartunek A, et al. Rupture and dissection in pulmonary artery aneurysms: incidence, cause, and treatment: review and case report. J Thorac Cardiovasc Surg 2001;121:1006-8. [Crossref] [PubMed]
- Poplausky MR, Rozenblit G, Rundback JH, et al. Swan-Ganz catheter-induced pulmonary artery pseudoaneurysm formation: three case reports and a review of the literature. Chest 2001;120:2105-11. [Crossref] [PubMed]
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000;28:1642-7. [Crossref] [PubMed]
- Ungaro R, Saab S, Almond CH, et al. Solitary peripheral pulmonary artery aneurysms. Pathogenesis and surgical treatment. J Thorac Cardiovasc Surg 1976;71:566-71. [PubMed]
- DeLima LG, Wynands JE, Bourke ME, et al. Catheter-induced pulmonary artery false aneurysm and rupture: case report and review. J Cardiothorac Vasc Anesth 1994;8:70-5. [Crossref] [PubMed]
- Seguchi M, Wada H, Sakakura K, et al. Idiopathic pulmonary artery aneurysm. Circulation 2011;124:e369-70. [Crossref] [PubMed]
- Chen Y, Gilman MD, Humphrey KL, et al. Pulmonary Artery Pseudoaneurysms: Clinical Features and CT Findings. AJR Am J Roentgenol 2017;208:84-91. [Crossref] [PubMed]
- Veldtman GR, Dearani JA, Warnes CA. Low pressure giant pulmonary artery aneurysms in the adult: natural history and management strategies. Heart 2003;89:1067-70. [Crossref] [PubMed]
- Butto F, Lucas RV, Edwards JE. Pulmonary Arterial Aneurysm. Chest 1987;91:237-41. [Crossref] [PubMed]
- Arango Tomás E, Cerezo Madueno F, Salvatierra Velazquez A. Bronchiectasis due to pulmonary artery aneurysm. Interact Cardiovasc Thorac Surg 2013;17:176-8. [Crossref] [PubMed]
- Tsui EY CY, Chow L, Chau LF, et al. Idiopathic pulmonary artery aneurysm: digital subtraction pulmonary angiography grossly underestimates the size of the aneurysm. Clin Imaging 2001;25:178-80. [Crossref] [PubMed]
- Deterling RA Jr, Clagett OT. Aneurysm of the pulmonary artery: review of the literature and report of a case. Am Heart J 1947;34:471-99. [Crossref] [PubMed]
- Bartter T, Irwin RS, Nash G. Aneurysms of the Pulmonary Arteries. Chest 1988;94:1065-75. [Crossref] [PubMed]
- Blades B, Ford W, Clark P. Pulmonary artery aneurysms: report of a case treated by surgical intervention. Circulation 1950;2:565-71. [Crossref] [PubMed]
- Ting P, Jugdutt BI, Le Tan J. Large pulmonary artery aneurysm associated with Marfan syndrome. Int J Angiol 2010;19:e48-50. [Crossref] [PubMed]
- Warthin AS. Syphilis of the pulmonary artery: syphilitic aneurysm of the left upper division: demonstration of spirochete pallida in wall of artery and aneurysmal sac. Am J Syph 1917;1:693-711.
- Plessinger VA, Jolly PN. Rasmussen's aneurysms and fatal hemorrhage in pulmonary tuberculosis. Am Rev Tuberc 1949;60:589-603. [PubMed]
- Remy J, Smith M, Lemaitre L, et al. Treatment of massive hemoptysis by occlusion of a Rasmussen aneurysm. AJR Am J Roentgenol 1980;135:605-6. [Crossref] [PubMed]
- Lee JC, Walters DL, Slaughter RE. Angioembolisation of pulmonary artery pseudoaneurysm arising in H1N1 influenza viral pneumonia. Heart Lung Circ 2011;20:599-601. [Crossref] [PubMed]
- Dransfield MT, Johnson JE. A Mycotic Pulmonary Artery Aneurysm Presenting as an Endobronchial Mass. Chest 2003;124:1610-2.
- Navarro C, Dickinson PC, Kondlapoodi P, et al. Mycotic aneurysms of the pulmonary arteries in intravenous drug addicts. Am J Med 1984;76:1124-31. [Crossref] [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- Sevitt S. Arterial wall lesions after pulmonary embolism, especially ruptures and aneurysms. J Clin Pathol 1976;29:665-74. [Crossref] [PubMed]
- Camargo Jde J, Camargo SM, Machuca TN, et al. Large pulmonary artery pseudoaneurysm due to lung carcinoma: pulmonary artery pseudoaneurysm. J Thorac Imaging 2010;25:W4-5. [PubMed]
- Gomez-Jorge J, Mitchell SE. Embolization of a Pulmonary Artery Pseudoaneurysm Due to Squamous Cell Carcinoma of the Lung. J Vasc Interv Radiol 1999;10:1127-30. [Crossref] [PubMed]
- Hoffmann RT, Spelsberg F, Reiser MF. Lung bleeding caused by tumoral infiltration into the pulmonary artery--minimally invasive repair using microcoils. Cardiovasc Intervent Radiol 2007;30:1282-5. [Crossref] [PubMed]
- Hiller N, Lieberman S, Chajek-Shaul T, et al. Thoracic manifestations of Behçet disease at CT. RadioGraphics 2004;24:801-8. [Crossref] [PubMed]
- O'Duffy JD, Carney JA, Deodhar S. Behçet's Disease: Report of 10 Cases, 3 with New Manifestations. Ann Intern Med 1971;75:561-70. [Crossref] [PubMed]
- Erkan F, Gül A, Tasali E. Pulmonary manifestations of Behçet’s disease. Thorax 2001;56:572-8. [Crossref] [PubMed]
- Cantasdemir M, Kantarci F, Mihmanli I, et al. Emergency endovascular management of pulmonary artery aneurysms in Behcet's disease: report of two cases and a review of the literature. Cardiovasc Intervent Radiol 2002;25:533-7. [Crossref] [PubMed]
- Erkan D, Yazici Y, Sanders A, et al. Is Hughes-Stovin syndrome Behçet's disease? Clin Exp Rheumatol 2004;22:S64-8. [PubMed]
- Durieux P, Bletry O, Huchon G, et al. Multiple pulmonary arterial aneurysms in Behcet's disease and Hughes-Stovin syndrome. Am J Med 1981;71:736-41. [Crossref] [PubMed]
- Borghol S, Alberti N, Frulio N, et al. Pulmonary artery pseudoaneurysm after radiofrequency ablation: Report of two cases. Int J Hyperthermia 2015;31:1-4. [Crossref] [PubMed]
- Sakurai J, Mimura H, Gobara H, et al. Pulmonary artery pseudoaneurysm related to radiofrequency ablation of lung tumor. Cardiovasc Intervent Radiol 2010;33:413-6. [Crossref] [PubMed]
- Yamakado K, Takaki H, Takao M, et al. Massive hemoptysis from pulmonary artery pseudoaneurysm caused by lung radiofrequency ablation: successful treatment by coil embolization. Cardiovasc Intervent Radiol 2010;33:410-2. [Crossref] [PubMed]
- Savage C, Zwischenberger JB, Ventura KC, et al. Hemoptysis secondary to pulmonary pseudoaneurysm 30 years after a gunshot wound. Ann Thorac Surg 2001;71:1021-3. [Crossref] [PubMed]
- Tanahashi Y, Kondo H, Osawa M, et al. Transcatheter embolization of a Rasmussen aneurysm via pulmonary artery with n-butyl cyanoacrylate and iodized oil mixture injection with balloon occlusion. J Vasc Surg Cases Innov Tech 2016;2:161-4. [Crossref]
- Greene DG, Baldwin ED, et al. Pure congenital pulmonary stenosis and idiopathic congenital dilatation of the pulmonary artery. Am J Med 1949;6:24-40. [Crossref] [PubMed]
- Bruzzi JF, Rémy-Jardin M, Delhaye D, et al. Multi–Detector Row CT of Hemoptysis. Radiographics 2006;26:3-22. [Crossref] [PubMed]
- Khadir MM, Chaturvedi A, Nguyen MS, et al. Looking beyond the thrombus: essentials of pulmonary artery imaging on CT. Insights Imaging 2014;5:493-506. [Crossref] [PubMed]
- Castañer E, Gallardo X, Rimola J, et al. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. Radiographics 2006;26:349-71. [Crossref] [PubMed]
- Kröger JR, Hickethier T, Pahn G, et al. Influence of spectral detector CT based monoenergetic images on the computer-aided detection of pulmonary artery embolism. Eur J Radiol 2017;95:242-8. [Crossref] [PubMed]
- Garcia A, Byrne JG, Bueno R, et al. Aneurysm of the Main Pulmonary Artery. Ann Thorac Cardiovasc Surg 2008;14:399-401. [PubMed]
- Ugolini P, Mousseaux E, Sadou Y, et al. Idiopathic dilatation of the pulmonary artery: Report of four cases. Magn Reson Imaging 1999;17:933-7. [Crossref] [PubMed]
- Meaney JF, Weg JG, Chenevert TL, et al. Diagnosis of pulmonary embolism with Magnetic Resonance Angiography. N Engl J Med 1997;336:1422-7. [Crossref] [PubMed]
- Carcano C, Martinez F, Stadtlander K. Iatrogenic pulmonary artery pseudoaneurysm. Appl Radiol 2013.26-9.
- Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 2005;45:1691-9. [Crossref] [PubMed]
- Davidoff AB, Udoff EJ, Schonfeld SA. lntraaneurysmal Embolization of a Pulmonary Artery Aneurysm for Control of Hemoptysis. AJR Am J Roentgenol 1984;142:1019-20. [Crossref] [PubMed]
- Ghaye B, Trotteur G, Dondelinger RF. Multiple pulmonary artery pseudoaneurysms: intrasaccular embolization. Eur Radiol 1997;7:176-9. [Crossref] [PubMed]
- Sbano H, Mitchell AW, Ind PW, et al. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005;184:1253-9. [Crossref] [PubMed]
- Ahmad M, Vatish J, Willis A, et al. Embolisation of an acute inflammatory pulmonary artery aneurysm using an Amplatzer® vascular plug. J Surg Case Rep 2012;2012:15. [Crossref] [PubMed]
- Tzilalis VD, Vourliotakis G, Tsironis IA, et al. Use of an amplatzer vascular plug in embolization of a pulmonary artery aneurysm in a case of hughes-stovin syndrome: a case report. J Med Case Rep 2011;5:425. [Crossref] [PubMed]
- Chalouhi N, Jabbour P, Singhal S, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013;44:1348-53. [Crossref] [PubMed]
- Elaassar O, Auriol J, Marquez R, et al. Endovascular techniques for the treatment of renal artery aneurysms. Cardiovasc Intervent Radiol 2011;34:926-35. [Crossref] [PubMed]
- Schlunz-Hendann M, Wetter A, Landwehr P, et al. Stent-assisted coil embolization of a traumatic wide-necked renal segmental artery pseudoaneurysm. Cardiovasc Intervent Radiol 2011;34:1065-8. [Crossref] [PubMed]
- Park A, Cwikiel W. Endovascular treatment of a pulmonary artery pseudoaneurysm with a stent graft: report of two cases. Acta Radiol 2007;48:45-7. [Crossref] [PubMed]
- Keymel S, Merx MW, Zeus T, et al. Stenting as a rescue treatment of a pulmonary artery false aneurysm caused by swan-ganz catheterization. Case Rep Pulmonol 2014;2014. [Crossref] [PubMed]
- Wilson N, McLeod K, Hallworth D. Exclusion of a pulmonary artery aneurysm using a covered stent. Heart 2000;83:438. [Crossref] [PubMed]
- Cil BE, Geyik S, Akmangit I, et al. Embolization of a giant pulmonary artery aneurysm from Behcet disease with use of cyanoacrylate and the "bubble technique". J Vasc Interv Radiol 2005;16:1545-9. [Crossref] [PubMed]
- Chatterjee K, Colaco B, Colaco C, et al. Rasmussen's aneurysm: A forgotten scourge. Respir Med Case Rep 2015;16:74-6. [Crossref] [PubMed]
- Renie WA, Rodeheffer RJ, Mitchell S, et al. Balloon embolization of a mycotic pulmonary artery aneurysm. Am Rev Respir Dis 1982;126:1107-10. [PubMed]


