
Non-aortic vascular findings on chest CT angiogram: including arch vessels and bronchial arteries
Introduction
CT angiogram (CTA) has become the modality of choice for imaging of thoracic vascular pathologies, involving the aorta and the pulmonary arteries. Apart from showing exquisite details of the aorta and pulmonary artery, CTA provides an accurate picture on the anatomy and pathology of the arch vessels and other aortic branches with far greater information than what we can achieve with ultrasound or plain radiography. The purpose of this article is to describe the common non-aortic and non-pulmonary vascular pathologies a radiologist may come across in a chest CTA.
CT angiography protocol for aortic arch vessels and other aortic branches
The exclusive imaging of arch vessels requires an optimal combination of temporal resolution, contrast opacification and spatial resolution. The ability to achieve the best possible image has significantly improved over the years and we have come to the point of rapid sub-second imaging with the least possible contrast dose using the current generation of dual source CT scanners (1). The intravenous line is usually placed on the asymptomatic upper limb or by default on the right side to avoid streak artefacts across the arch vessels. At our institute, we use iso-osmolar iodinated contrast of concentration 320 mg/mL with a saline chase; the contrast dose being 100 to 120 mL based on the patient’s weight. A flow rate of 4 to 5 mL/second is ideal. A bolus tracking technique (threshold of 150 Hounsfield units) is used to trigger the acquisition with the region of interest placed in the descending aorta just below the level of the carina. We use automated attenuation-based selection of individualized tube parameters to have an optimal radiation exposure. The field of view should cover from the skull base to the upper abdomen based on the anteroposterior and lateral scouts. Finally, in patients with compromised renal function, rapid dual source CT imaging in arterial phase can be made use of to limit the dose of contrast to as low as 40 mL (2).
Anatomy of the arch and great vessels
The most common pattern of aortic arch branching is the trifurcation pattern i.e., the innominate artery, left common carotid artery and the left subclavian artery, which is seen in approximately 70 percent of patients (3). Six pairs of branchial arch arteries develop in the fetus. The common variations of aortic arch can be attributed to abnormal persistence or disappearance of segments of these branchial arch arteries. The common “variant” aortic arch patterns include left common carotid artery origin from innominate artery (Bovine arch) which is seen in 13% of the population, left vertebral artery arising directly from aortic arch (0.5%), aberrant right subclavian artery (0.4% to 2%). Other rare variants include aberrant right vertebral artery, double/right sided aortic arch (4). Identifying these variants in the preprocedural CTA provides valuable information to the interventional radiologist about the access pathway to the cervical, cerebral or upper limb vasculature and will obviate the need of a diagnostic arch aortogram during the interventional procedure.
Aortic arch is classified into three types based on the distance of innominate artery origin from the top of aortic arch, with reference diameter being the diameter of right or left common carotid artery (5). If the innominate origin is at the level of the top of the aortic arch, it is type 1 (Figure 1A). If the innominate origin is inferior to the top of aortic arch by 1.5 to 2 times the diameter of the common carotid artery, then it is type 2; if still lower, then it is type 3 (Figure 1B). Type 2 and type 3 arches pose significant challenges in carotid and neurovascular interventional procedures. Appropriate catheter selection can be planned prior to these procedures anticipating the difficult access.
Pathology of great vessels
The great vessels that originate from the aortic arch can be affected by a wide range of pathologies within the thoracic cage. Commonly encountered ones include aneurysms, trauma, atherosclerotic disease large vessel vasculitis, and thoracic outlet syndrome (TOS).
Aneurysms
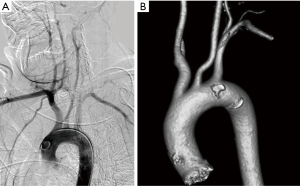
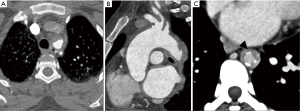
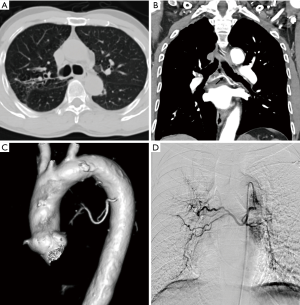
Common causes of aneurysms of supra-aortic arch branches include atherosclerosis, Marfan syndrome and connective tissue disorders, arteritis, mycotic, post traumatic or post-operative states. Isolated aneurysms of distal segment of subclavian arteries are likely to be due to TOS. Aneurysms of the arch vessels comprise 0.4% to 4% of all aneurysms (6). They may be asymptomatic or sometimes present with features of mass effect on vital structures such as dyspnea and hoarseness of voice, rupture (11% risk), distal thrombo-embolic phenomenon (50%) and infection (7) (Figure 2). In one of the largest reported case series involving arch vessel aneurysms, the most commonly affected vessel was the subclavian artery (50%), followed by the common carotid (26%), internal carotid (10%) and innominate arteries (3%) (6). Aneurysms can be fusiform, when there is a circumferential luminal dilatation, or saccular, when there is a focal outpouching, or a combination of the two.
Treatment is indicated for symptomatic aneurysms, saccular aneurysms and asymptomatic aneurysms greater than 3 cm usually by surgical resection, exclusion by stent graft or hybrid procedures (8). Cross sectional imaging is essential to look for status of bilateral vertebral arteries, particularly if endovascular treatment is contemplated. MDCT angiography is essential for diagnosis, characterization and treatment planning (9). It provides the required information for planning endovascular reconstruction of the vessel including the length of the diseased segment, proximal and distal landing zones and side branches in the treatment zone which can serve as endoleaks in future.
Trauma
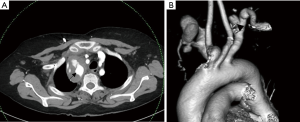
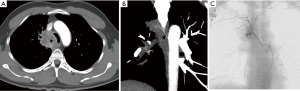
Trauma to the aortic arch branches results from blunt or penetrating trauma to the upper chest or neck. They can either occur along with aortic injury or in isolation. They are clinically occult and require a low threshold of suspicion. Manifestations include traumatic occlusions, dissections, contrast extravasations, pseudo-aneurysms and arterio-venous fistulae. Hematoma in the lower cervical region or superior mediastinum usually indicates brachio-cephalic or proximal subclavian artery injury (Figure 3). Injury to internal mammary artery can be overlooked but may lead to catastrophic effects by impaired right ventricular filling due to mass effect due to the expanding hematoma, particularly on the left side (10). CT is useful for identifying vascular injuries and associated osseous/pulmonary/soft tissue injuries which often coexist and for planning surgical or endovascular treatment. Findings in the CT angiography include irregular vessel margins, filling defects, active contrast extravasation, occlusion of a vessel, and caliber changes in the vessels (11). A negative CTA even in the presence of hematoma obviates the need for diagnostic angiogram (12). One limitation of CTA in the situation of trauma to the great vessels is in assessment of the subclavian artery ipsilateral to the venous contrast inflow; can be avoided by careful evaluation of the plain CT and planning contrast injection accordingly.
Atherosclerosis
Atherosclerosis is the commonest cause of obstructive lesions involving the arch vessels. Patients typically present with clinical features of cerebrovascular and upper limb ischemia or steal phenomenon when it involves the subclavian artery. Atherosclerotic diseases of upper extremities are uncommon compared to lower extremities and may remain asymptomatic due to collaterals. Tandem lesions are common (13) (Figure 4). Symptoms are mostly chronic with acute exacerbations due to distal thrombo-embolic phenomena leading to digital ischemia, or neuro-ocular symptoms. Arm claudication symptoms and blood pressure differences between the arms are commonly noted. In case of proximal subclavian occlusions symptoms of subclavian steal syndrome with Transient ischemic attacks or syncopal attacks predominate. In patients with internal mammary artery grafts, proximal subclavian occlusion may lead to myocardial ischemia and pre-procedure evaluation of subclavian arteries is indicated prior to coronary bypass grafting procedures (14). The left subclavian artery is the most commonly involved vessel and majority of the lesions are prevertebral (15). Patients presenting with signs and symptoms of upper limb or cerebrovascular ischemia are typically evaluated with Duplex ultrasound as the first investigation. However, the utility of duplex ultrasound is limited in the setting of atherosclerotic disease of the intrathoracic arch vessels. Echocardiography performed from the suprasternal notch has been shown to be a valuable tool in screening of the supra-aortic arch vessel pathology. However, CTA is the imaging modality of choice for accessing atherosclerotic disease of the arch vessels as it provides exceptional spatial resolution for assessment as well as treatment planning, i.e., the length and degree of obstruction and presence of tandem lesions. It can also show collateral pathways which maintain limb or cerebral perfusion in chronically symptomatic patients and help in decision making on best medial management or intervention (16). CTA can also quantify the calcific burden of a stenosis or occlusion which has a strong bearing on the choice of intervention, viz., endovascular or surgical.
Vasculitis
Takayasu arteritis is the commonest form of vasculitis affecting the arch vessels. It is a non-specific inflammatory disease of aorta and great vessels predominantly, also involving the pulmonary and coronary circulations with a strong female predominance. It is characterized by concentric wall thickening and fibrosis early on, further leading on to stenosis and aneurysmal dilatations. Diagnosis is based on either Ishikawa/American college of Rheumatology criteria. The disease is classified into 5 types based on the location of involvement, of which types I and II can involve the supra-aortic branches. There is no preference noted in the involvement of the arch vessels. Symptoms are initially constitutional; as the vascular lesions progress and lead to stenotic foci or aneurysms, patients may present with a spectrum of features including, but not limited to, neuro-ocular syndromes, weakened pulses, vascular bruits and renovascular hypertension. Duplex ultrasound can be used as an effective screening tool to demonstrate a long segment, smooth, echogenic, circumferentially thickened vessel wall (macaroni sign) with stenotic flow in the accessible vessels (17). CT/MR angiography is the imaging modality of choice and depicts both luminal and mural changes. Mural changes include concentric wall thickening with venous phase enhancement in the active phase of the disease (at least 3 mm concentric thickening) and intra-mural calcifications in the burnt-out phase (Figure 5). Edematous inner wall and enhancing outer wall—the so called “double ring sign” is also said to be characteristic for the disease and is associated with poor prognosis (18). MRA is advantageous for diagnosis and follow up of these patients as it offers a radiation free demonstration of the findings. The active lesions are FDG-avid in active phase. Luminal changes include stenosis, occlusions aneurysmal dilatations. Mid segment of subclavian/carotid arteries, distal innominate artery and non-ostial proximal renal artery involvement are said to be characteristic. Giant cell arteritis, polyarteritis nodosa, other forms of aortitis and peri-aortitis may also involve the great vessels but can be differentiated clinically and radiologically, although there may be overlap of the spectrum (19).
TOS
TOS includes a complex of neurological or vascular upper extremity symptoms related to extrinsic compression of the neurovascular structures at the point of exit from the thorax. Although classically diagnosed by the clinical signs and symptoms, imaging plays a crucial role in excluding alternative diagnoses and plan treatment. The syndrome can be neurological or vascular; however, considering the scope of this review, vascular TOS is discussed in detail. It can be arterial or venous.
The thoracic outlet can be divided into three spaces, namely the scalene triangle, the costoclavicular space and the subcoracoid pectoralis minor space (20). The thoracic outlet, at all these levels, is a dynamic space; abduction of the ipsilateral arm results in narrowing of the space. Patients with TOS experience compression of the neurovascular structures during this process. Conventional radiography as the preliminary investigation can show presence of cervical rib or anomalous 1st rib as cause of the TOS, particularly in patients who are scheduled for MRI as the confirmatory modality. Cross sectional angiographic imaging, either CT or MRI, forms the cornerstone of imaging in vascular TOS (21). Dynamic maneuvers (abduction of the arms) are performed during the imaging process to elicit the causative vascular event. MRI has a particular advantage in this group of patients who are predominantly young, owing to the need for dynamic imaging which can increase the radiation dose in the case of a CTA.
Neurogenic TOS accounts for 90% of the cases of TOS. Among the vascular cases, venous is more prevalent. The classic site of venous compression is the costoclavicular space, between the first rib, subclavius muscle and the clavicle. Two types of presentations can be seen; acute axillosubclavian venous thrombosis (effort thrombosis or Paget Schroetter syndrome) and chronic intermittent upper extremity swelling without thrombosis (McCleery syndrome). Bony anatomy is often normal. CT or MR angiography, in the venous phase, demonstrates axillosubclavian venous thrombosis, narrowing of the subclavian vein or venous collaterals (21).
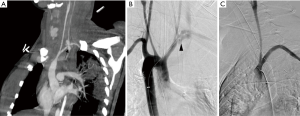
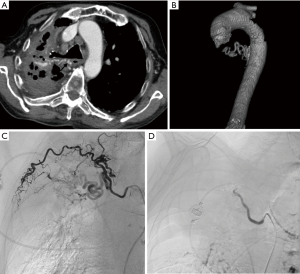
Arterial TOS is the least common of the three subtypes, accounting for 3% of all cases of TOS. The primary damage to the subclavian artery that occurs at the level of the first rib and the resultant distal embolic phenomena are the two main issues in arterial TOS. It is invariably associated with underlying bone abnormalities, such as a cervical rib (Figure 6), anomalous first rib, or a malunited fracture of the first rib or clavicle. CT angiography clearly depicts the tell-tale signs of vascular damage at the compression site, in the form of dissection, aneurysm formation or thrombosis. Distal embolic phenomena can also be identified (Figure 7). Dynamic maneuvers provide supportive evidence to demonstrate the narrowing of the subclavian artery at the thoracic outlet, but standalone, it is not sufficient for the diagnosis of TOS (21).
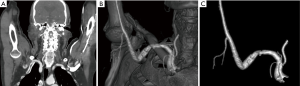
Assessment of post-operative patients of TOS with recurrent symptoms is excellent with CT angiography as it clearly depicts the post-surgical bony alterations, native vessel/vascular graft status and other soft tissue complications.
Bronchial arteries
Introduction
Lung has two vascular systems—pulmonary and bronchial circulations. Bronchial system provides 1% blood supply and carries oxygenated blood in high pressure. It does not participate in gas exchange but provides nourishment to the supporting structures of the lungs, including the pulmonary arteries (22,23). Both systems are interconnected through several microvascular anastomoses at the level of the alveoli and respiratory bronchioles (23).
Anatomy of bronchial artery
Bronchial arteries arise directly from the proximal descending thoracic aorta between superior endplate of T5 vertebra and inferior endplate of T6 vertebra (orthotopic origin). Any other site of origin is termed as ectopic origin (20,24-26). Incidence of ectopic bronchial artery ranges from 8.3–56% (25,27,28). Common ectopic sites of origin include the inferior aortic arch, distal descending thoracic aorta, subclavian artery, brachiocephalic trunk, thyrocervical trunk, and internal mammary artery and even a coronary artery (25). Bronchial arteries which originate from a coronary artery may be asymptomatic or may lead to myocardial infarction or angina due to coronary steal phenomenon (29).
The right bronchial artery commonly shares its origin with an intercostal artery (30) (52% of cases at CT angiography), a finding known as a common intercosto-bronchial trunk (ICBAT). Less commonly, the left and right bronchial arteries share a common origin (32% of right and 36% of left bronchial arteries at CT angiography), known as the common trunk of both bronchial arteries.
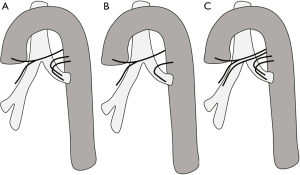
There are 9 types of variations in the origins and number of the bronchial arteries (Figure 8); the most common is type 1 (40.6% of cases), with one right bronchial artery arising from a common ICBAT and two left bronchial arteries. Type 2 is the second most common pattern (21.3%), consisting of a single bronchial artery on each side. Type 3 is the third most common pattern (20.6%), consisting of two bronchial arteries on each side, with one of the right bronchial arteries originating from an ICBAT. Types 4–9 are other rarer variants. Both bronchial arteries usually travel behind the trachea and mainstem bronchi before entering the lung via the hila (30). Normal bronchial arteries are small, measuring less than 2 mm at their origin and 0.5 mm distally as they enter the bronchopulmonary segment. Normal bronchial arteries manifest on CECT as small nodular or linear enhancing structures and are best depicted on multiplanar images, given their undulating course (20,30,31).
The bronchial arteries normally receive only about 1% of the total cardiac output and help maintain airway and lung function (32). They provide systemic blood supply to the tracheobronchial tree, visceral pleura, esophagus, vasa vasorum of the thoracic aorta and pulmonary arteries, nerves, pulmonary veins, and lymph nodes in the thorax (26,33). When there is an inflammatory pathology involving lungs or an increase demand of oxygenated blood to lung tissue, it undergoes smooth muscle wall hypertrophy and dilatation (23,33).
Pathology
When bronchial arteries are dilated, its diameter increases to greater than 2 mm and become tortuous (31). Visualization of dilated bronchial arteries at imaging should alert the radiologist to rule out obstructive disorders affecting the pulmonary circulation, chronic infectious and/or inflammatory processes, chronic thromboembolic disease, and congenital cardiovascular anomalies of the thorax.
The causes of bronchial artery dilatation can be divided into four categories as follows:
- Congenital pulmonary artery anomaly;
- Acquired intrinsic pulmonary artery obstruction;
- Acquired extrinsic pulmonary artery obstruction;
- Acute or chronic inflammation.
Congenital pulmonary artery anomaly
The congenital conditions that are most commonly associated with pulmonary vascular insufficiency leading to bronchial artery dilatation are Tetralogy of Fallot (TOF), proximal interruption of the pulmonary artery and anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA). In TOF, systemic arteries called major aortopulmonary collateral arteries (MAPCA) develop and supply the insufficient pulmonary bed. These may be difficult to differentiate from hypertrophied bronchial arteries (34) and are further discussed in detail under a separate topic. In cases of proximal pulmonary artery interruption, the mediastinal portion of the right or left pulmonary artery is atretic, affected lung is hypoplastic and the distal pulmonic circulation is supplied by systemic collaterals, via subclavian, intercostal and most commonly bronchial arteries (8,35). In ALCAPA, hypertrophied bronchial arteries may supply the left coronary artery territory (36,37).
Acquired intrinsic pulmonary artery obstruction
The causes include chronic pulmonary thromboembolism and arteritis involving the pulmonary circulation (Takayasu arteritis), where the bronchial artery hypertrophy is induced by chronic hypoxemia of the lung tissue (35,38-40).
Acquired extrinsic pulmonary artery obstruction
Fibrosing mediastinitis is a benign but sometimes progressive disorder characterized by infiltrative fibrous tissue in the mediastinum. It can be nongranulomatous or granulomatous (41). It can involve pulmonary arteries (42) causing constriction and obstruction of the pulmonary arteries, generally unilateral (41). Ipsilateral bronchial artery dilatation is seen when fibrous tissue encases and narrows a pulmonary artery as a physiologic response to decreased blood flow to the lung (43).
Acute or chronic inflammation
Inflammatory response of lung parenchyma leads to release of angiogenic growth factors which promote neovascularity as well as hypervascularity via collateral pathways with an increase in bronchopulmonary anastomosis at precapillary and capillary levels (24,44). Causes include bronchiectasis (Figure 9), chronic infection, primary and secondary lung cancers (Figure 10) (45). Such patients may present with hemoptysis, which can be life threatening at times. CECT is useful in demonstrating the cause and site of bleeding and identifying abnormal bronchial arteries or, rarely, active contrast agent extravasation as the source (46).
Bronchial arteriovenous malformation
Bronchial arteriovenous malformation is a rare congenital or acquired disorder which results in a left-to-left or left-to-right extracardiac shunt. Acquired causes are infectious or inflammatory lung diseases, penetrating lung trauma, and tumor-primary/secondary (47). It is characterized by a tortuous and enlarged bronchial artery that communicates with a pulmonary artery or pulmonary vein (47,48). It is more common in men, can occur at any age, and usually affects the right lung. Patients often are asymptomatic, but the condition may result in massive hemoptysis and death. Contrast CT with arterial and venous phases is the noninvasive investigation of choice. It manifests as a tortuous and dilated bronchial artery, often with focal aneurysms and anomalous communication with a pulmonary artery or vein. Digital subtraction bronchial angiography confirms the diagnosis. It can be treated endovascularly via coils or vascular plugs.
Hemoptysis and bronchial artery intervention
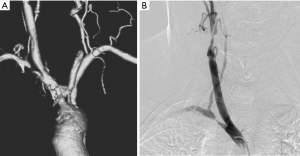
Massive hemoptysis is generally defined as expectoration of greater than 300 mL of blood in 24 hours or any amount of blood which causes a 2 gm% drop in hemoglobin from baseline or hemodynamic instability. In 90% of cases, the source of hemoptysis is a hypertrophied bronchial artery (22,26). Most cases of clinically significant hemoptysis are treated with bronchial artery embolization (BAE). Contrast CT images are useful in the pre-embolization assessment of patients with hemoptysis as it allows identification of dilated bronchial arteries, their origins as well as the disease process causing hemoptysis (Figure 11). CT imaging is fast and provides a detailed vascular road map for the interventional radiologist before the procedure (20,22). The use of CECT before angiography reduces the rate of catheterization failures and the number of patients needing surgical intervention (49).
Recent advances in clinical application of bronchial arteries
Chronic rejection is one of the main causes of death following lung transplantation and it manifest as bronchiolitis obliterans (50). There is an inflammation affecting smaller airways-bronchioles which manifests with wall thickening, eventually obliteration with reduction in airflow, pulmonary artery perfusion and resultant airway ischemia. Transplant surgeons usually revascularize only the pulmonary blood flow after lung transplantation but airways derive 50% of their blood supply from the bronchial arteries and 50% from the pulmonary arteries. Hence emerging evidences advocate bronchial artery revascularization along with pulmonary artery in an attempt to avert or delay the development of bronchiolitis obliterans syndrome (50). Hence accurate mapping of the degree and location of the bronchial arteries by CTA will become a necessity before lung transplantation in future.
Miscellaneous conditions
MAPCAs
MAPCAs are branches of the descending thoracic aorta, exclusive of the bronchial arteries, which collateralise the pulmonary bed in patients with a spectrum of pulmonary artery insufficiency. They represent persistent intersegmental arteries which fail to regress due to demand supply of the suboptimal pulmonary blood flow. The two congenital heart diseases associated with MAPCAs are pulmonary atresia with ventricular septal defect and Fallot’s tetralogy (51).
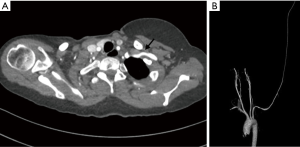
MDCT angiography assessment of the MAPCAs, along with the native pulmonary arteries, forms a cornerstone of management of pediatric and adult patients with these congenital heart conditions (52). It accurately depicts the number, origin, course and intrapulmonary connections of the MAPCAs (Figure 12). These vessels may also show pathological segments of aneurysmal dilatation or stenosis. Communication between MAPCAs and native pulmonary arteries may be central (at the hilum or major lobar branches), or peripheral (at segmental or subsegmental levels). Knowledge of the degree of pulmonary artery stenosis and the number and size of the MAPCAs is essential in planning the definitive treatment of these patients.
Differentiation of MAPCAs from bronchial arteries may pose a diagnostic challenge. Unlike bronchial arteries, MAPCAs do not have mediastinal branches, connect to larger pulmonary artery branches and never anastomose with intercostal arteries.
Broncho-pulmonary sequestration
Pulmonary sequestration is a condition, either congenital or acquired, in which there is an abnormal mass of lung tissue which has lost communication with the tracheobronchial tree. It is typically characterised by systemic arterial supply which usually enters through the inferior pulmonary ligament (53). Pulmonary sequestration can be intralobar or extralobar.
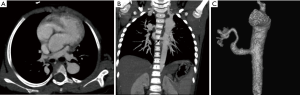
Intralobar sequestration (ILS), which is the commonest of the two, presents in childhood and adolescence with repeated respiratory infections. It is most common in lower lobes (left > right), shares the pleura with adjacent lung, and drains into a pulmonary vein (Figure 13). Extralobar sequestration presents in neonatal period with respiratory distress, has a separate pleural cover, and drains into a systemic vein.
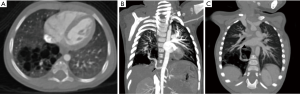
The systemic arterial supply, being a striking feature in both types, is most apparent in the CT angiography of the thorax, imaged in the arterial and venous phases (54). The systemic arterial supply takes origin from the descending thoracic aorta in most instances (70–80%); in the rest of the patients it originates from abdominal aorta, celiac axis, or splenic artery, intercostal artery, and rarely from the subclavian, internal thoracic, and pericardiacophrenic arteries. The anomalous venous drainage is generally via the pulmonary veins in ILS, and through the azygos vein/hemiazygos system, portal vein, right atrium, or IVC in ELS.
Conclusions
With the widespread availability and ease of procedure, CTA has replaced diagnostic angiography as modality of choice for evaluation of thoracic vascular pathologies. Similar to evaluation of aorta, CT angiography also plays a significant role in diagnosing, characterization and pre-treatment work up of non-aortic vascular pathologies of the chest. With advances in CT technology the acquisition, interpretation and clinical applications of CT angiography will continue to grow in the years to come.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Boroto K, Remy-Jardin M, Flohr T, et al. Thoracic applications of dual-source CT technology. Eur J Radiol 2008;68:375-84. [Crossref] [PubMed]
- Vasconcelos R, Vrtiska TJ, Foley TA, et al. Reducing Iodine Contrast Volume in CT Angiography of the Abdominal Aorta Using Integrated Tube Potential Selection and Weight-Based Method Without Compromising Image Quality. AJR Am J Roentgenol 2017;208:552-63. [Crossref] [PubMed]
- Lippert H, Pabst R. Arterial Variations in Man: Classification and Frequency. Munich, Germany JF Bergmann Verlag 1985;VI:122.
- Lale P, Toprak U, Yagız G, et al. Variations in the Branching Pattern of the Aortic Arch Detected with Computerized Tomography Angiography. Adv Radiol 2014;2014:969728. [Crossref]
- Dumont TM, Mokin M, Wach MM, et al. Understanding risk factors for perioperative ischemic events with carotid stenting: Is patient age over 80 years or is unfavorable arch anatomy to blame? J Neurointerv Surg 2014;6:219-24. [Crossref] [PubMed]
- Cury M, Greenberg RK, Morales JP, et al. Supra-aortic vessels aneurysms: Diagnosis and prompt intervention. J Vasc Surg 2009;49:4-10. [Crossref] [PubMed]
- Cherry K, Zelenock GB, Huber TS, et al. Treatment of extracranial, carotid, innominate, subclavian and axillary aneurysms. Mastery Vasc Endovasc Surg An Illus Rev 2006;79:84.
- Kieffer E, Chiche L, Koskas F, et al. Aneurysms of the innominate artery: Surgical treatment of 27 patients. J Vasc Surg 2001;34:222-8. [Crossref] [PubMed]
- Schoder M, Cejna M, Hölzenbein T, et al. Elective and emergent endovascular treatment of subclavian artery aneurysms and injuries. J Endovasc Ther 2003;10:58-65. [Crossref] [PubMed]
- McPherson SJ. Thoracic aortic and great vessel trauma and its management. Semin Intervent Radiol 2007;24:180-96. [Crossref] [PubMed]
- Rivas LA, Fishman JE, Múnera F, et al. Multislice CT in thoracic trauma. Radiol Clin North Am 2003;41:599-616. [Crossref] [PubMed]
- Sammer M, Wang E, Blackmore CC, et al. Indeterminate CT angiography in blunt thoracic trauma: Is CT angiography enough? AJR Am J Roentgenol 2007;189:603-8. [Crossref] [PubMed]
- Reul GJ, Jacobs MJ, Gregoric ID, et al. Innominate artery occlusive disease: surgical approach and long-term results. J Vasc Surg 1991;14:405-12. [Crossref] [PubMed]
- Angle JF, Matsumoto AH, McGraw JK, et al. Percutaneous Angioplasty and Stenting of Left Subclavian Artery Stenosis in Patients with Left Internal Mammary-Coronary Bypass Grafts: Clinical Experience and Long-Term Follow-up. Vasc Endovascular Surg 2003;37:89-97. [Crossref] [PubMed]
- Ruegg WR, Vandis FJ, Feldman HJ, et al. Aortic arch vessel disease and rationale for echocardiographic screening. J Am Soc Echocardiogr 2013;26:114-25. [Crossref] [PubMed]
- Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2-clinical applications beyond trauma patients. AJR Am J Roentgenol 2013;201:753-63. [Crossref] [PubMed]
- Chaubal N, Dighe M, Shah M. Sonographic and color Doppler findings in aortoarteritis (Takayasu arteritis). J Ultrasound Med 2004;23:937-44. [Crossref] [PubMed]
- Hayashi H, Katayama N, Takagi R, et al. CT analysis of vascular wall during the active phase of Takayasu’s aortitis. Eur Radiol 1991;1:S239.
- Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Takayasu Arteritis and Giant Cell Arteritis. Medicine (Baltimore) 2009;88:221-6. [Crossref] [PubMed]
- Remy-Jardin M, Doyen J, Remy J, et al. Functional anatomy of the thoracic outlet: evaluation with spiral CT. Radiology 1997;205:843-51. [Crossref] [PubMed]
- Raptis CA, Sridhar S, Thompson RW, et al. Imaging of the Patient with Thoracic Outlet Syndrome. Radiographics 2016;36:984-1000. [Crossref] [PubMed]
- Bruzzi JF, Rémy-Jardin M, Delhaye D, et al. Multi–Detector Row CT of Hemoptysis. Radiographics 2006;26:3-22. [Crossref] [PubMed]
- Pump KK. Distribution of bronchial arteries in the human lung. Chest 1972;62:447-51. [Crossref] [PubMed]
- Osiro S, Wear C, Hudson R, et al. A friend to the airways: A review of the emerging clinical importance of the bronchial arterial circulation. Surg Radiol Anat 2012;34:791-8. [Crossref] [PubMed]
- Hartmann IJC, Remy-Jardin M, Menchini L, et al. Ectopic origin of bronchial arteries: Assessment with multidetector helical CT angiography. Eur Radiol 2007;17:1943-53. [Crossref] [PubMed]
- Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395-409. [Crossref] [PubMed]
- Sancho C, Escalante E, Domínguez J, et al. Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol 1998;21:300-4. [Crossref] [PubMed]
- Cohen AM, Doershuk CF, Stern RC. Bronchial artery embolization to control hemoptysis in cystic fibrosis. Radiology 1990;175:401-5. [Crossref] [PubMed]
- Aupetit JF, Gallet M, Boutarin J. Coronary-to-bronchial artery anastomosis complicated with myocardial infarction. Int J Cardiol 1988;18:93-7. [Crossref] [PubMed]
- Morita Y, Takase K, Ichikawa H, et al. Bronchial Artery Anatomy: Preoperative 3D Simulation with Multidetector CT. Radiology 2010;255:934-43. [Crossref] [PubMed]
- Furuse M, Saito K, Kunieda E, et al. Bronchial arteries: CT demonstration with arteriographic correlation. Radiology 1987;162:393-8. [Crossref] [PubMed]
- McCullagh A, Rosenthal M, Wanner A, et al. The bronchial circulation--worth a closer look: a review of the relationship between the bronchial vasculature and airway inflammation. Pediatr Pulmonol 2010;45:1-13. [Crossref] [PubMed]
- Pump KK. The bronchial arteries and their anastomoses in the human lung. Dis Chest 1963;43:245-55. [Crossref] [PubMed]
- Nørgaard MA, Alphonso N, Cochrane AD, et al. Major aorto-pulmonary collateral arteries of patients with pulmonary atresia and ventricular septal defect are dilated bronchial arteries. Eur J Cardiothorac Surg 2006;29:653-8. [Crossref] [PubMed]
- Castañer E, Alguersuari A, Gallardo X, et al. When to suspect pulmonary vasculitis: radiologic and clinical clues. Radiographics 2010;30:33-53. [Crossref] [PubMed]
- Peña E, Nguyen ET, Merchant N, et al. ALCAPA Syndrome: Not Just a Pediatric Disease. Radiographics 2009;29:553-65. [Crossref] [PubMed]
- Karolczak MA, Wieteska J, Bȩc L, et al. Anomalous origin of the left coronary artery (LCA) from pulmonary trunk (Bland-White-Garland syndrome) with systemic collateral supply. Med Sci Monit 2001;7:755-8. [PubMed]
- Pengo V, Lensing AW, Prins MH, et al. Incidence of Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Embolism. N Engl J Med 2004;350:2257-64. [Crossref] [PubMed]
- Kasai T, Chiba S. Macroscopic anatomy of the bronchial arteries. Anat Anz 1979;145:166-81. [PubMed]
- Matsunaga N, Hayashi K, Sakamoto I, et al. Takayasu arteritis: protean radiologic manifestations and diagnosis. Radiographics 1997;17:579-94. [Crossref] [PubMed]
- McNeeley MF, Chung JH, Bhalla S, et al. Imaging of granulomatous fibrosing mediastinitis. AJR Am J Roentgenol 2012;199:319-27. [Crossref] [PubMed]
- Rossi SE, McAdams HP, Rosado-de-Christenson ML, et al. Fibrosing mediastinitis. Radiographics 2001;21:737-57. [Crossref] [PubMed]
- Denlinger CE, Fernandez FG, Patterson GA, et al. Fibrosing Mediastinitis Associated With Complete Occlusion of the Left Main Pulmonary Artery. Ann Thorac Surg 2009;87:323. [Crossref] [PubMed]
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med 2001;164:S39-45. [Crossref] [PubMed]
- Panos RJ, Barr LF, Walsh TJ., et al. Factors Associated with Fatal Hemoptysis in Cancer Patients. Chest 1988;94:1008-13. [Crossref] [PubMed]
- Katabathina VS, Restrepo CS, Betancourt Cuellar SL, et al. Imaging of Oncologic Emergencies: What Every Radiologist Should Know. Radiographics 2013;33:1533-53. [Crossref] [PubMed]
- Yon JR, Ravenel JG. Congenital brochial artery-pulmonary artery fistula in an adult. J Comput Assist Tomogr 2010;34:418-20. [Crossref] [PubMed]
- Nugent Z, Oliveira V, Maclusky I, et al. Bronchial artery-pulmonary artery malformation as a cause of cryptogenic hemoptysis. Pediatr Pulmonol 2013;48:930-3. [Crossref] [PubMed]
- Khalil A, Fartoukh M, Parrot A, et al. Impact of MDCT angiography on the management of patients with hemoptysis. AJR Am J Roentgenol 2010;195:772-8. [Crossref] [PubMed]
- Pettersson GB, Karam K, Thuita L, et al. Comparative study of bronchial artery revascularization in lung transplantation. J Thorac Cardiovasc Surg 2013;146:894-900.e3. [Crossref] [PubMed]
- Chandrasekan S, Ramadhas K. Mdct Assessment of Major Aorto-Pulmonarycollaterals. Available online: http://www.iosrjournals.org/iosr-jdms/papers/Vol16-issue1/Version-4/G1601044448.pdf
- Rajeshkannan R, Moorthy S, Sreekumar KP, et al. Role of 64-MDCT in evaluation of pulmonary atresia with ventricular septal defect. AJR Am J Roentgenol 2010;194:110-8. [Crossref] [PubMed]
- Berrocal T, Madrid C, Novo S, et al. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics 2004;24:e17. [Crossref] [PubMed]
- Accogli S, Gabelloni M, Faggioni L, et al. Imaging of pulmonary sequestration: what the radiologist needs to know. European Congress of Radiology 2016. Available online: https://posterng.netkey.at/esr/viewing/index.php?module=viewing_poster&task=&pi=133676


