Mesenteric ischemia: what the radiologist needs to know
Introduction
Mesenteric ischemia is an umbrella term encompassing a broad array of disorders causing insufficient blood flow to the abdominal viscera and has both acute and chronic variations (1-3). Though uncommon, accounting for 0.09% to 0.2% of acute surgical admissions (4), it remains a highly morbid condition, with reported mortality rates ranging from 30% to 90% (1). Diagnosis is challenging secondary to the rarity of the disease, its nonspecific clinical presentation, and often subtle or non-specific imaging findings (5,6). Consequently, a high index of suspicion on the part of the clinician and the radiologist, as well as familiarity with the spectrum of imaging findings associated with mesenteric ischemia, is required to ensure prompt recognition of the disease.
Clinical features
Acute mesenteric ischemia (AMI)
Conventionally, the defining clinical feature of AMI has been described as “pain out of proportion to the exam” (2), though the most common clinical manifestations are vague and include nausea and vomiting, bloating, and diarrhea (7). The classic triad of pain out of proportion to exam findings, bowel emptying (vomiting and diarrhea), and a potential embolic source are inconsistent findings (8). The presence of peritonitis on physical examination is an ominous prognostic indicator suggestive of irreversible ischemia and bowel necrosis (4).
Chronic mesenteric ischemia
The signs and symptoms of chronic mesenteric ischemia are more insidious and include early satiety, weight loss, postprandial abdominal pain, and “food fear” (2,4).
Laboratory evaluation is nonspecific but may demonstrate leukocytosis with left shift or elevated serum amylase (9). A recent preliminary study suggests that D-dimer and other biomarkers, such as intestinal fatty acid binding protein (I-FABP), serum alpha-glutathione S-transferase (alpha-GST), and cobalt-albumin binding assay (CABA), may play a confirmatory role in the diagnosis of mesenteric ischemia (4). However, none of these markers are currently used as a part of standard clinical practice (10), and further research is needed to assess their reliability.
Classification
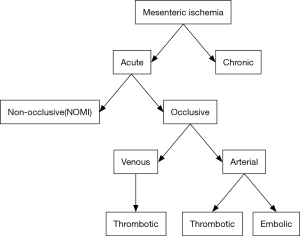
Mesenteric ischemia can be acute or chronic and is further subcategorized as either occlusive or non-occlusive (Figure 1). Occlusive mesenteric ischemia can affect either arteries or veins (11,12). Embolic arterial obstruction is the most common cause of AMI, accounting for approximately 40–50% of cases (2,5,9,13). The superior mesenteric artery (SMA) is most commonly affected, likely secondary to its high flow rate and narrow angle of takeoff from the abdominal aorta. Most emboli lodge anywhere from 3 to 10 cm distal to the vessel’s origin, typically beyond the branch point of the middle colic artery, which results in sparing of the duodenum and transverse colon (7,9,12-14). Only about 15% of emboli occur at the origin of the SMA (9).
Thrombotic arterial mesenteric ischemia, responsible for 15–30% of cases, has the worst prognosis of any form of mesenteric ischemia (1,13). This is because it typically occurs in the setting of preexisting atherosclerosis and affects the ostia of mesenteric vessels; after critical stenosis occurs, a period of low flow may cause vascular thrombosis and vessel occlusion, resulting in a large territory of ischemic bowel (1,9).
Mesenteric venous thrombosis accounts for 5–15% of cases and may be due to clotting diatheses such as the factor V Leiden mutation; deficiencies in protein S, protein C, or antithrombin III; or antiphospholipid antibody syndrome. The superior mesenteric (70–95%), inferior mesenteric, and portal veins are most commonly involved (2,9,13).
Non-occlusive mesenteric ischemia (NOMI) is caused by intestinal hypoperfusion in the absence of vascular occlusion. NOMI constitutes 5–15% of cases of mesenteric ischemia (2) and is associated with the highest rates of in-hospital mortality (9). These patients frequently have profound, debilitating comorbid conditions, and diagnosis may be delayed due to patient obtundation (9).
Conventional vascular anatomy
The celiac axis (CA), SMA, and inferior mesenteric artery (IMA) constitute the arterial supply to the bowel and arise directly from the aorta. The CA gives rise to the common hepatic, splenic, and left gastric arteries, which provide blood flow to the stomach, spleen, and pancreas. The common hepatic artery (CA) gives rise to the gastroduodenal artery (GDA) as its first branch, usually located at the superior aspect of the pancreas, and continues on as the proper hepatic artery before bifurcating into its terminal branches, the right and left hepatic arteries (9,13-15).
The SMA originates 1 cm below the CA and gives rise to jejunal and ileal branches, the inferior pancreaticoduodenal artery, the ileocolic artery, and the right and middle colic arteries. These vessels supply the pancreas, portions of the duodenum, the entirety of the jejunum, ileum, and ascending colon, and the transverse colon to the splenic flexure (9,13-15). The SMA will occasionally have a common trunk with the celiac axis or give rise to a replaced (often right) hepatic artery.
The most diminutive of the mesenteric arteries, the IMA arises above the aortic bifurcation and gives rise to the left colic artery and sigmoidal branches before terminating as the superior rectal artery. These branches supply the descending and sigmoid colon and rectum. The proximal vascular territory of the IMA overlaps the distal SMA territory, creating a watershed zone of perfusion susceptible to ischemia (9,13-15).
Numerous collateral pathways allow for redundant flow to mesenteric vascular territories in the event of arterial occlusion (15). The CA and SMA collateralize primarily via the gastroduodenal and pancreaticoduodenal arteries (13,14) and less commonly through the arc of Barkow (an anastomosis between the left and right gastroepiploic arteries) or arc of Buhler (a persistent communication between embryonic ventral segmental arteries) (15,16). The SMA and IMA collateralize via the arc of Riolan (a connection between the middle and left colic arteries) and the marginal artery of Drummond, which consists of branches of the ileocolic and right, left, and middle colic arteries (13,15).
The superior and inferior mesenteric veins receive blood from the veins within their respective arterial territories. The superior mesenteric vein drains directly into the portal vein at the portosplenic confluence, while the inferior mesenteric vein first drains into the splenic or superior mesenteric vein or their confluence (13).
Diagnosis
Per the American College of Radiology (ACR) Appropriateness Criteria, computed tomographic angiography (CTA) is the first-line diagnostic modality for mesenteric ischemia (17). Rapid, widely available, and relatively inexpensive, CTA has a sensitivity of 96% and a specificity of 94% in the diagnosis of both the acute and chronic forms of mesenteric ischemia (17), while a recent analysis of CTA in the setting of AMI documented a sensitivity of 89%, a specificity of 99%, and positive and negative predictive values of 97% (18). CTA facilitates evaluation of both vascular and bowel abnormalities and may be useful in documenting potential alternative causes of abdominal pain (17,18). Given the potentially catastrophic consequences of a missed diagnosis, elevated creatinine or impaired renal function should not preclude administration of intravenous contrast material and performance of CTA (8).
Once considered the gold standard for diagnosis of mesenteric ischemia, catheter-based angiography has been relegated to a second-line modality (17) given its invasive nature and lack of availability at some centers (7,13). Its primary advantage over CTA is that it allows for diagnosis and treatment in a single stage (7,17).
Due to its longer image-acquisition times and limited spatial resolution, magnetic resonance angiography (MRA) is best suited for assessment of chronic mesenteric ischemia, for which it has been shown to have high sensitivity and specificity (7,13,17).
Doppler ultrasound can be used to evaluate for chronic mesenteric ischemia, with peak systolic velocities above 275 cm/s in the SMA and 200 cm/s in the CA correlating with 70% stenosis in these vessels (9,17). However, the accuracy of US is heavily operator dependent, and the presence of gas within the bowel lumen or large patient size can impede visualization of the mesenteric vessels and their distal course. For these reasons, US is not routinely used to diagnose AMI (17).
Conventional abdominal radiographs are neither sensitive nor specific for assessment of mesenteric ischemia and may not be abnormal until bowel infarction has already occurred (13,17).
Recent analyses suggest that use of dual-energy CT (DECT) may reduce radiation exposure and increase the accuracy of mesenteric ischemia diagnosis (19-24). The creation of iodine-selective, virtual noncontrast, and low keV monoenergetic images using dual-energy post-processing techniques enhances the conspicuity of differential bowel wall enhancement and identifies bowel wall hemorrhage, aiding in the recognition of ischemic segments (21,22).
CT protocol
Early investigations into mesenteric ischemia advocated the use of both non-contrast and contrast-enhanced imaging to maximize the sensitivity of detection (25), though more contemporary authors (5,13,14,19,26) have determined that diagnostic accuracy can be maintained when non-contrast images are omitted. While non-contrast images do not aid in vascular luminal evaluation, they can facilitate identification of vascular calcification and hyperdense mural hemorrhage (25). Neutral oral contrast agents (e.g., water) can be administered to distend the bowel lumen, allowing for characterization of the degree of mucosal enhancement and enabling visualization of the vascular tree with multiplanar thick maximum intensity projections or volume renderings (13,14). Biphasic imaging assesses both the mesenteric arterial and venous systems and improves visualization of bowel wall enhancement (14); use of the portal venous phase acquisition alone results in lower rates of diagnosis, so is not recommended (26).
At our institution, noncontrast CT is performed at the discretion of the radiologist. Acquisition of CTA images is performed after a preset aortic threshold (usually 150 HU with monitoring performed using 120 kVp) is reached using bolus tracking software, with portal phase images acquired at 60 seconds.
CTA images are reconstructed in three planes using a narrow and overlapping slice thickness (e.g., 2 mm slice thickness, 1 mm reconstruction interval). Additional thick maximum intensity projections in the sagittal and coronal planes are helpful to visualize the mesenteric artery origins and SMA distribution. Coronal thick maximum intensity projection images (e.g., 8 mm thick, 5 mm reconstruction interval) are reconstructed from the portal phase acquisition to display the mesenteric venous arcade. Interactive 3D rendering/reformatting can be performed according to local availability and expertise, but can improve and facilitate rapid diagnosis, especially linking vascular and bowel imaging findings (27).
Imaging features
Evaluation of the bowel
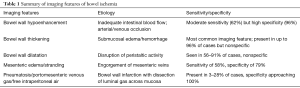
Regardless of the etiology of mesenteric ischemia, the affected organ is the bowel, with bowel ischemia resulting in a spectrum of imaging findings, which are summarized in Table 1. Critically, vascular findings manifest prior to intestinal findings in cases of mesenteric ischemia (8,28). Thus, CT diagnosis of mesenteric ischemia is predicated on scrutiny of both the mesenteric vasculature and the bowel and its surrounding mesentery, with particular attention paid to bowel wall thickening, luminal diameter, mural enhancement, and the presence of ascites.
Full table
Depending on the etiology, mesenteric ischemia may result in either thickening or thinning of the bowel wall and either mural hypo- or hyperenhancement. In cases of venous occlusion, infiltration of the perienteric mesentery may be an important clue to diagnosis (19,29).
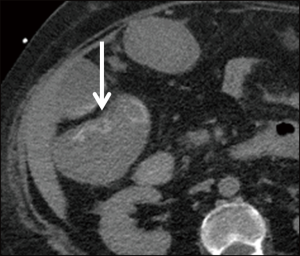
Normally 3–5 mm thick, the bowel wall can reach up to 15 mm in thickness in cases of acute ischemia secondary to mural hemorrhage or edema (25,29) (Figure 2). Bowel wall thickening is the most sensitive indicator of ischemia, though this also happens to be the least specific, as wall thickening can also be observed in infectious or inflammatory states (25,29).
Another frequent but nonspecific finding in bowel ischemia is luminal dilation, which commonly occurs when transmural infarction results in interruption of peristalsis (25,30). Luminal distention is seen more commonly in cases of ischemia due to bowel obstruction and is less frequently observed in ischemia of arterial origin (29).
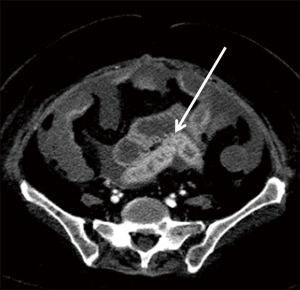
Normal perfusion to loops of small and large bowel results in uniform, homogeneous mural enhancement, especially during the portal venous phase of imaging. Disruption of arterial flow causes decreased or absent mural enhancement (6,29), a finding referred to as “pale ischemia” (5). Prolonged interruption of arterial blood supply can result in microvascular capillary damage (7) with the destruction of intramural vessels and tissue and subsequent loss of muscular tone yielding a “paper thin” appearance of the bowel wall (5,29). With the restoration of perfusion, bowel wall hyperemia may ensue, causing mural hyperenhancement (Figure 3), similar to that seen with shock bowel (5).
Elevated mesenteric venous pressure, typically caused by strangulating obstruction or venous occlusion, can result in infiltration of fluid into the mesentery, resulting in perienteric stranding or ascites (29).
End-stage ischemia of any cause may ultimately result in pneumatosis intestinalis, or the presence of air within the bowel wall (Figure 4). When seen in conjunction with portomesenteric gas, the specificity for bowel ischemia is close to 100% (5). One early investigation into the subject found that “band-like” pneumatosis is frequently seen with transmural bowel infarction, while “bubble-like” pneumatosis more commonly occurs in cases of partial mural ischemia (31).
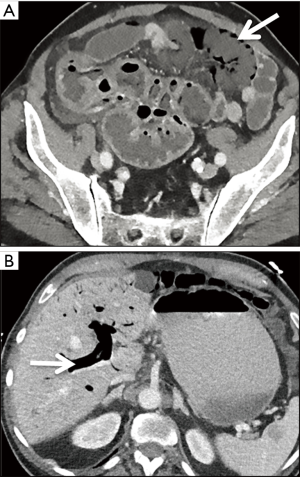
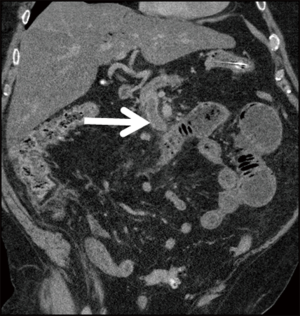
Arterial occlusion
Causes of arterial occlusion include emboli to the mesenteric vasculature, which commonly originate from the left atrium, left ventricle, or left-sided cardiac valves (13), and arterial thrombosis. Recognition of clot within the mesenteric vasculature, especially in the proximal SMA (Figure 5), should prompt the radiologist to scrutinize the heart to attempt to identify the etiology. Moreover, up to 20% of patients with mesenteric emboli may have concurrent emboli to organs such as the spleen and kidneys (13), a finding that may help further clarify the diagnosis. Mesenteric emboli of cardiac origin occur commonly in atrial fibrillation. CT may demonstrate mesenteric stranding and ascites, though focal bowel dilatation and mural stratification can also occur (32).
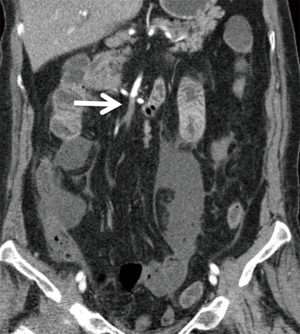
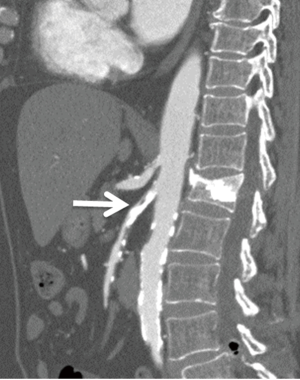
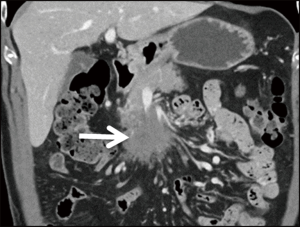
Arterial thrombosis most often occurs in the setting of severe atheromatous disease (13) (Figure 6). An uncommon cause of arterial thrombosis is SMA dissection, which can occur as a continuation of aortic dissection or in isolation (Figure 7). Intestinal ischemia may result if the SMA arises from the false lumen of an aortic dissection (33). Alternatively, cystic medial necrosis, trauma, or fibrous dysplasia may rarely predispose to isolated SMA dissection with resultant mesenteric hypoperfusion and thinning of the small bowel wall (30).
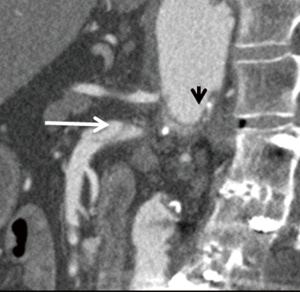
Segmental arterial mediolysis (SAM) is a disorder characterized by destruction of the outer arterial media and most commonly affects the splanchnic vessels. Dissecting aneurysms can result, yielding sudden intraabdominal or retroperitoneal hemorrhage. The classic manifestation is “abdominal apoplexy,” the hallmarks of which include abdominal pain, a rapid fall in hematocrit, and hypovolemic shock. Ischemic bowel can develop. Imaging most commonly shows “beading” of the arterial wall with alternating stenoses and aneurysms (34).
Venous occlusion
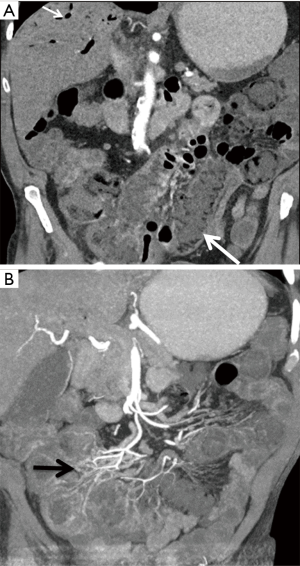
In contrast to the paper-thin appearance of the bowel wall seen with isolated arterial occlusion, mesenteric venous occlusion (Figure 8) most commonly results in circumferential bowel wall thickening (30). Arterial inflow continues unabated despite outflow obstruction secondary to venous thrombosis, resulting in elevated intramural hydrostatic pressure (5). Hypodense edema in the submucosal layer of the bowel interleaved between the enhancing mucosa and muscularis propria causes a “target” or “halo” appearance (13). Normally 2–3 mm in diameter, the small bowel wall may expand up to 1.5 cm (30). Mesenteric fat stranding occurs due to extravasation of fluid into the mesentery. Conversely, mesenteric fluid is less common with mesenteric ischemia secondary to arterial occlusion (5,29). Secondary causes of venous occlusion include inflammatory and infectious conditions such as diverticulitis, appendicitis, inflammatory bowel disease, and pancreatitis or neoplasia (13) (Figure 9).
NOMI
In low-flow states caused by conditions such as cardiogenic or hemorrhagic shock, sepsis, or arrhythmia, reflex splanchnic vasoconstriction occurs to divert blood flow to critical organs such as the heart and brain (5,13). Subsequent intestinal hypoperfusion leads to increased vascular permeability, with seepage of plasma and red blood cells into the bowel wall and mesentery (29). Characteristic CT findings include small bowel mural thickening and hyperenhancement with sparing of the colon, luminal dilatation, a flat inferior vena cava (IVC), and ascites, a constellation of features known as “shock bowel” (5,7). Hyperenhancement of the adrenal glands may be seen (7). NOMI can be challenging to diagnose on CT, as its imaging appearance may be similar to infectious, inflammatory, or traumatic forms of enteritis (5,29); however, it is a diffuse small bowel process. Clinical history may be integral to radiologic diagnosis, and catheter/CT angiography may be a helpful adjunctive measure in equivocal cases. Typical angiographic findings include narrowing and irregularity of SMA branches, decreased filling of intramural vessels, and spasm of mesenteric arcades (7) (Figure 10). Findings that may provide clues as to the underlying cause of ischemia are summarized in Table 2.

Full table
Obstruction
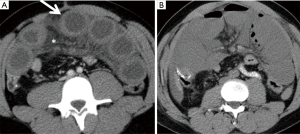
Bowel obstruction resulting in ischemia is termed “strangulation” (Figure 11), and this phenomenon occurs most frequently with closed-loop obstruction (29), which occurs when two points along the bowel are obstructed at the same site (35). This often results in a C- or U-shaped configuration of the bowel, with potential compromise of the intervening mesenteric vessels by the volvulus, internal hernia or adhesion causing the closed loop (36). Closed-loop obstructions often involve the mesentery and mesenteric vessels and can result from or predispose to volvulus, indicated by the convergence of the mesenteric vasculature to a single point or “whirling” of mesenteric vessels (3,29). Additional imaging features described in closed loop obstruction include a radial distribution of bowel loops; the presence of two closely apposed, collapsed loops; and the beak or triangular loop signs, which refer to fusiform tapering of fluid-filled bowel loops (35). Specific CT features of ischemia in patients with bowel obstruction include decreased bowel wall enhancement, the small-bowel feces sign, and increased bowel wall attenuation on unenhanced images, likely secondary to intramural hemorrhage (6,25,36). Common but less specific findings of ischemia in the context of obstruction are similar to those seen with venous occlusion and include bowel wall thickening, the “target” or “halo” signs, and mesenteric edema or ascites (29).
Vasculitis/vascular disorders
Classified by the size of the vessel predominantly involved in the disease process (small, medium, or large), the vasculitides cause vascular inflammation and necrosis. When the disease involves the mesenteric circulation, mesenteric ischemia can ensue (30,37). The most common of the vasculitides to affect the gastrointestinal tract is polyarteritis nodosa, a medium-vessel vasculitis (3). Other common vasculitides to involve the small bowel include Henoch-Schonlein purpura, systemic lupus erythematosus (SLE), and Behcet’s disease (30). Imaging features are nonspecific and include mural thickening, mucosal ulceration, hemorrhage, and stricture formation (3,30). Relatively unique features include involvement of the duodenum (Figure 12) or concurrent involvement of both the jejunum and ileum or the small bowel and colon. Concomitant involvement of the genitourinary system may further suggest vasculitis as the etiology of the ischemia (3).
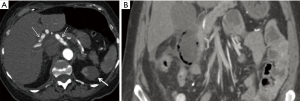
Polyarteritis nodosa produces inflammation that can result in segmental weakening of the vessel wall with characteristic formation of multiple small aneurysms. The mesenteric, renal, and hepatic vessels are most commonly affected, and the aneurysms are typically small, measuring 1 cm in diameter or less (37). Uncommonly, mural thrombus or embolus due to SMA aneurysm can result in mesenteric ischemia (33).
Trauma
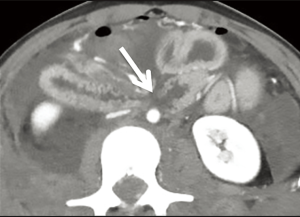
Both penetrating and blunt trauma can result in mesenteric ischemia. Penetrating injury can cause direct vascular damage with extensive hemoperitoneum and resultant mesenteric ischemia, while blunt trauma can cause disruption of blood flow due to a tear at the mesenteric vascular attachment (Figure 13). Over time, post-traumatic strictures can develop (3).
Post-procedural
Mesenteric ischemia can occur in the aftermath of invasive procedures, particularly in patients with severe atherosclerosis or poor cardiopulmonary function (38). Emboli can become dislodged during coronary, abdominal, or cerebral angiography; intestinal hypoperfusion can result from elevated intraabdominal pressure during laparoscopic surgery; and the combination of decreased intravascular volume from colon preparation and reduction in vascular tone caused by medications used for conscious sedation can cause low-flow states during colonoscopy (10,38). As a result, abdominal pain of unclear etiology following any invasive procedure should precipitate investigation of mesenteric ischemia (10).
Sickle cell disease
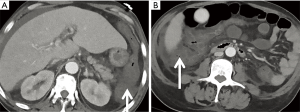
A relatively rare cause of bowel ischemia, acute gastrointestinal vaso-occlusive ischemia (GVOI) should be considered in patients with sickle cell disease who present with acute abdominal pain. On CT, these patients demonstrate diffuse or segmental bowel wall thickening preferentially involving the colon, particularly the ascending portion (Figure 14). The duodenum and rectum are generally spared. Bowel wall enhancement is preserved (39).
Radiation enteritis
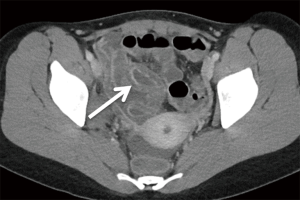
A complication of abdominal radiation, radiation enteritis develops secondary to obliterative arteritis affecting the microvascular circulation in the small bowel mucosa and submucosa. A dose of at least 4,500–5,000 cGy is required for symptoms to manifest, and the distal small bowel is most commonly affected (3,40). Acute radiation enteritis occurs within several days or weeks following radiation therapy. Imaging findings include bowel wall thickening with adjacent mesenteric inflammatory stranding and an asymmetric submucosal halo (40) (Figure 15). Chronic bowel changes evolve over the course of 1–2 years and progress predominantly to stricture formation with loss of the normal mucosal fold pattern and hyperenhancement at imaging, but sinus tracts, fistulae, adhesions, and ulcerations can also be seen. Clinical history is essential for diagnosis, as the bowel changes remain isolated to the bowel contained within the radiation port (3,40).
Treatment
The treatment of mesenteric ischemia depends on its acuity, the patient’s clinical presentation, and preoperative comorbidities.
Initial medical management strategies in the treatment of AMI focus on preservation of hemodynamic status (4). Aggressive fluid resuscitation and avoidance of oral intake may be helpful in preventing exacerbation of intestinal ischemia. Bowel infarction can result in hyperkalemia and metabolic acidosis, which may precipitate a systemic inflammatory response or sepsis (2). The early initiation of heparin, antibiotic therapy, and, if needed, vasodilators is also a vital component of care (2,4).
The most important prognostic factor in patients with AMI is intestinal viability (4). Those with peritonitis and clear signs of bowel infarction or perforation should undergo emergent open laparotomy, which allows for direct visualization of the bowel, reestablishment of blood flow to areas of ischemic bowel, and resection of all regions of non-viable intestine (4). Revascularization methods include embolectomy, angioplasty, and bypass (4). Because bowel viability can be challenging to assess in the perioperative period, planned re-exploration or “second-look laparotomy” is becoming an increasingly accepted technique in patients who undergo open surgery (4). In patients without frank evidence of bowel necrosis, endovascular treatment may allow for restoration of mesenteric blood flow with lower rates of in-hospital mortality. The primary disadvantage of this approach is that it does not allow for direct inspection of the bowel and assessment of viability (4,9,41). A hybrid technique, consisting of open surgery followed by endovascular SMA revascularization, has shown promise, as it allows for direct bowel evaluation and prompt restoration of mesenteric flow (41). However, experience with this technique remains limited, and its use is relatively rare (2,41).
In patients with chronic mesenteric ischemia, endovascular repair is utilized more commonly than open repair. Endovascular repair is associated with fewer complications and shorter hospital stays, though at the expense of lower long-term patency and earlier recurrence of symptoms (2).
Treatment of NOMI is predicated on correction of the underlying cause of reduced intestinal perfusion (2,9). Mortality in these patients, who often have extensive comorbidity and poor overall protoplasm, is high, ranging from 50% to 83% (2). Intravascular infusion of vasodilators such as papaverine hydrochloride may be beneficial in some patients (2,9).
In most patients with mesenteric venous thrombosis, the only treatment necessary is long-term anticoagulation, typically in the form heparin in the acute setting followed by a transition to oral medication over 24–48 hours (2,9).
Pitfalls
Mesenteric ischemia presents with a vast array of imaging features, with varying degrees of specificity (40). Diseases that can mimic ischemia on imaging are summarized in Table 3. Bowel wall thickening, for example, has been described with neoplastic, infectious, and inflammatory processes and can be seen with generalized edema. Furthermore, lack of luminal distention can result in apparent bowel wall thickening and generate false-positive results (33). Bowel dilatation is another non-specific feature that can be seen with other pathologies, such as obstruction or ileus (42). Searching for additional findings associated with mesenteric ischemia, such as bowel wall hypoenhancement or mesenteric stranding, may help improve specificity (33).

Full table
The “halo” sign, caused by hypodense submucosal edema interdigitated between enhancing mucosa and muscularis propria (13), has been described as a characteristic feature of acute ischemia (11,30). But this finding is nonspecific, and submucosal edema or fat deposition in inflammatory conditions such as Crohn’s disease, radiation or neutropenic enteritis, angioedema, and graft-versus-host disease may simulate ischemia (40).
In ischemic disease, pneumatosis and portomesenteric gas are ominous prognostic indicators commonly associated with transmural bowel infarction (31). However, many benign causes of pneumatosis have been described (Figure 16), including connective tissue disease, emphysema, asthma, and intraabdominal procedures (33). In these cases, the bowel wall may otherwise be unremarkable, and portomesenteric gas is less likely to result. In addition, patients are often clinically asymptomatic (43).
Future directions
As previously described, the use of DECT is becoming more commonplace in the evaluation of patients with suspected mesenteric ischemia, as it allows for the creation of iodine-selective, virtual non-contrast, and virtual monoenergetic images that enhance the conspicuity of differential bowel wall enhancement (21,22).
Frequency selective nonlinear blending is a relatively novel post-processing technique that permits the separation of image information into high and low frequencies, where the high frequencies comprise image noise and the low frequencies contain information about image contrast (44). This enables selective augmentation of a range of HUs within an image without enhancing image noise in a manner that is not dependent on image acquisition technique (45). Utilization of this technique in a recent study (46) yielded promising results when compared to standard biphasic CT, with increased sensitivity for the diagnosis of mesenteric ischemia due to increased attenuation differences between ischemic and non-ischemic segments of bowel. Because this post-processing tool does not depend on image acquisition method, it may be useful in centers in which DECT is not available (45).
Jo and colleagues (47) recently described a split-bolus technique in which images are acquired in a single phase following the injection of two discrete contrast boluses, separated by an interval of 22–33 seconds. At the expense of a slightly higher volume of iodinated contrast, they demonstrated high diagnostic accuracy and confidence with a statistically significant reduction in effective radiation dose. In addition, the split-bolus protocol generated fewer images to review, which may decrease radiologist mental fatigue and missed findings.
Acknowledgments
None.
Footnote
Conflicts of Interest: JG Fletcher: Research Grant Siemens Healthcare. The other authors have no conflicts of interest to declare.
References
- Herbert GS, Steele SR. Acute and chronic mesenteric ischemia. Surg Clin North Am 2007;87:1115-34. ix. [Crossref] [PubMed]
- Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med 2016;374:959-68. [Crossref] [PubMed]
- Rha SE, Ha HK, Lee SH, et al. CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics 2000;20:29-42. [Crossref] [PubMed]
- Bala M, Kashuk J, Moore EE, et al. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg 2017;12:38. [Crossref] [PubMed]
- Dhatt HS, Behr SC, Miracle A, et al. Radiological Evaluation of Bowel Ischemia. Radiol Clin North Am 2015;53:1241-54. [Crossref] [PubMed]
- Sheedy SP, Earnest F 4th, Fletcher JG, et al. CT of small-bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology 2006;241:729-36. [Crossref] [PubMed]
- Gore RM, Yaghmai V, Thakrar KH, et al. Imaging in intestinal ischemic disorders. Radiol Clin North Am 2008;46:845-75. v. [Crossref] [PubMed]
- Bjorck M, Koelemay M, Acosta S, et al. Editor's Choice - Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2017;53:460-510. [Crossref] [PubMed]
- Bobadilla JL. Mesenteric ischemia. Surg Clin North Am 2013;93:925-40. ix. [Crossref] [PubMed]
- Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg 2016;42:253-70. [Crossref] [PubMed]
- Levy AD. Mesenteric ischemia. Radiol Clin North Am 2007;45:593-9. x. [Crossref] [PubMed]
- Mastoraki A, Mastoraki S, Tziava E, et al. Mesenteric ischemia: Pathogenesis and challenging diagnostic and therapeutic modalities. World J Gastrointest Pathophysiol 2016;7:125-30. [Crossref] [PubMed]
- Costa AF, Chidambaram V, Lee JJ, et al. Multidetector computed tomography of mesenteric ischaemia. Insights Imaging 2014;5:657-66. [Crossref] [PubMed]
- Horton KM, Fishman EK. CT angiography of the mesenteric circulation. Radiol Clin North Am 2010;48:331-45. viii. [Crossref] [PubMed]
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997;77:289-306. [Crossref] [PubMed]
- DiPoce J, Jimenez G, Weintraub J. Historical perspective: eponyms of vascular radiology. Radiographics 2014;34:1120-40. [Crossref] [PubMed]
- Oliva IB, Davarpanah AH, Rybicki FJ, et al. ACR Appropriateness Criteria (R) imaging of mesenteric ischemia. Abdom Imaging 2013;38:714-9. [Crossref] [PubMed]
- Henes FO, Pickhardt PJ, Herzyk A, et al. CT angiography in the setting of suspected acute mesenteric ischemia: prevalence of ischemic and alternative diagnoses. Abdom Radiol (NY) 2017;42:1152-61. [Crossref] [PubMed]
- Moschetta M, Telegrafo M, Rella L, et al. Multi-detector CT features of acute intestinal ischemia and their prognostic correlations. World J Radiol 2014;6:130-8. [Crossref] [PubMed]
- Vlahos I, Chung R, Nair A, et al. Dual-energy CT: vascular applications. AJR Am J Roentgenol 2012;199:S87-97. [Crossref] [PubMed]
- Potretzke TA, Brace CL, Lubner MG, et al. Early small-bowel ischemia: dual-energy CT improves conspicuity compared with conventional CT in a swine model. Radiology 2015;275:119-26. [Crossref] [PubMed]
- Fulwadhva UP, Wortman JR, Sodickson AD. Use of Dual-Energy CT and Iodine Maps in Evaluation of Bowel Disease. Radiographics 2016;36:393-406. [Crossref] [PubMed]
- Darras KE, McLaughlin PD, Kang H, et al. Virtual monoenergetic reconstruction of contrast-enhanced dual energy CT at 70keV maximizes mural enhancement in acute small bowel obstruction. Eur J Radiol 2016;85:950-6. [Crossref] [PubMed]
- Machida H, Tanaka I, Fukui R, et al. Dual-Energy Spectral CT: Various Clinical Vascular Applications. Radiographics 2016;36:1215-32. [Crossref] [PubMed]
- Wiesner W, Khurana B, Ji H, et al. CT of acute bowel ischemia. Radiology 2003;226:635-50. [Crossref] [PubMed]
- Schieda N, Fasih N, Shabana W. Triphasic CT in the diagnosis of acute mesenteric ischaemia. Eur Radiol 2013;23:1891-900. [Crossref] [PubMed]
- Horton KM, Fishman EK. Multi-detector row CT of mesenteric ischemia: can it be done? Radiographics 2001;21:1463-73. [Crossref] [PubMed]
- Wadman M, Block T, Ekberg O, et al. Impact of MDCT with intravenous contrast on the survival in patients with acute superior mesenteric artery occlusion. Emerg Radiol 2010;17:171-8. [Crossref] [PubMed]
- Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol 2009;192:408-16. [Crossref] [PubMed]
- Horton KM, Fishman EK. Multidetector CT angiography in the diagnosis of mesenteric ischemia. Radiol Clin North Am 2007;45:275-88. [Crossref] [PubMed]
- Wiesner W, Mortele KJ, Glickman JN, et al. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol 2001;177:1319-23. [Crossref] [PubMed]
- Barajas RF Jr, Yeh BM, Webb EM, et al. Spectrum of CT findings in patients with atrial fibrillation and nontraumatic acute abdomen. AJR Am J Roentgenol 2009;193:485-92. [Crossref] [PubMed]
- Wasnik A, Kaza RK, Al-Hawary MM, et al. Multidetector CT imaging in mesenteric ischemia--pearls and pitfalls. Emerg Radiol 2011;18:145-56. [Crossref] [PubMed]
- Chao CP. Segmental arterial mediolysis. Semin Intervent Radiol 2009;26:224-32. [Crossref] [PubMed]
- Balthazar EJ, Birnbaum BA, Megibow AJ, et al. Closed-loop and strangulating intestinal obstruction: CT signs. Radiology 1992;185:769-75. [Crossref] [PubMed]
- Geffroy Y, Boulay-Coletta I, Julles MC, et al. Increased unenhanced bowel-wall attenuation at multidetector CT is highly specific of ischemia complicating small-bowel obstruction. Radiology 2014;270:159-67. [Crossref] [PubMed]
- Ha HK, Lee SH, Rha SE, et al. Radiologic features of vasculitis involving the gastrointestinal tract. Radiographics 2000;20:779-94. [Crossref] [PubMed]
- Al-Khyatt W, Thomas JD, Humes DJ, et al. Intestinal ischemia following laparoscopic surgery: a case series. J Med Case Rep 2013;7:25. [Crossref] [PubMed]
- Gardner CS, Jaffe TA. Acute gastrointestinal vaso-occlusive ischemia in sickle cell disease: CT imaging features and clinical outcome. Abdom Radiol (NY) 2016;41:466-75. [Crossref] [PubMed]
- Umphrey H, Canon CL, Lockhart ME. Differential diagnosis of small bowel ischemia. Radiol Clin North Am 2008;46:943-52. vi-vii. [Crossref] [PubMed]
- Zhao Y, Yin H, Yao C, et al. Management of Acute Mesenteric Ischemia: A Critical Review and Treatment Algorithm. Vasc Endovascular Surg 2016;50:183-92. [Crossref] [PubMed]
- Aschoff AJ, Stuber G, Becker BW, et al. Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging 2009;34:345-57. [Crossref] [PubMed]
- Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol 2007;188:1604-13. [Crossref] [PubMed]
- Bier G, Bongers MN, Ditt H, et al. Accuracy of Non-Enhanced CT in Detecting Early Ischemic Edema Using Frequency Selective Non-Linear Blending. PLoS One 2016;11:e0147378. [Crossref] [PubMed]
- Bongers MN, Bier G, Marcus R, et al. Image Quality of a Novel Frequency Selective Nonlinear Blending Algorithm: An Ex Vivo Phantom Study in Comparison to Single-Energy Acquisitions and Dual-Energy Acquisitions With Monoenergetic Reconstructions. Invest Radiol 2016;51:647-54. [Crossref] [PubMed]
- Schneeweiss S, Esser M, Thaiss W, et al. Improved CT-detection of acute bowel ischemia using frequency selective non-linear image blending. Acta Radiol Open 2017;6:2058460117718224. [Crossref] [PubMed]
- Jo PC, Cabral FC, Sahin A, et al. Split-bolus single scan CTA for evaluation of mesenteric ischemia. Abdom Radiol (NY) 2018;43:1368-78. [Crossref] [PubMed]