
Significance of oral health in adult patients with congenital heart disease
Introduction
A few decades ago, congenital heart disease has primarily been considered as pediatric entity since specifically patients with severe defects rarely survived until reaching adulthood. Since then significant advances in the diagnosis and treatment, particularly noninvasive imaging and surgical repair, allowed for a continuously improved long term survival of the affected patients. Yet more than 90% of patients with congenital heart disease reach adulthood (1,2). At the same time, congenital heart disease associated mortality shows a significant decline of more than 30% within two decades (3). The median age of patients has now increased to 40 years (4). As a consequence, the number of adult patients with congenital heart disease is constantly growing and was estimated to reach almost 2.3 million patients in Europe and at least 1.4 million patients in the United States (5,6). Modern treatment approaches are considerably life prolonging but often not curative. Hence, in many cases chronic and complex cardiac co-morbidities, e.g., arrhythmia, pulmonary hypertension, or thromboembolism might develop (1,7). Due to life-long cardiovascular anomalies, e.g., valvular lesions and residual shunts or the presence of implantable prostheses and conduits, patients with congenital heart disease, in addition, show an elevated susceptibility for the development of infective endocarditis. Although uncommon, infective endocarditis is a potentially fatal event that, upon manifestation, causes a very high morbidity and mortality. In samples of the general population about 50% of patients with infective endocarditis need surgical interventions for therapy and the mortality reaches 30% at 1 year (8). The manifestation of infective endocarditis in patients with congenital heart disease seems to elevate the risk for a fatal outcome within the first year following diagnosis more than 30-fold as compared to congenital heart disease patients without infective endocarditis (9). Among patients with congenital heart disease infective endocarditis manifests up to 140 times more as compared to healthy controls (10).
The oral cavity harbors a complex commensal microflora and is affected by some of the most prevalent human infectious diseases, i.e., dental caries and periodontitis (11,12). Since oral bacteria are often responsible for the manifestation of infective endocarditis (13) the oral cavity is considered as a highly relevant source of bacteremia. The present review addresses the issue of oral health in adult patients with congenital heart disease and the potential interrelations between them.
Oral health and disease
The healthy oral cavity is colonized by a complex microflora, among which various Streptococcus species and Actinomyces species are most frequent (14). Two odontogenic diseases, i.e., dental caries and periodontitis, represent highly prevalent human diseases (11,12,15,16). Both entities are primarily caused by dysbiotic bacterial infections of the tooth surface and/or the gingival sulcus (17-19). Dysbiosis describes a shift in the relative amounts of the single members of the commensal bacterial community clinically leading to transition from health to disease. In odontogenic diseases, dybiosis commonly occurs as a result of insufficient or completely lacking regular tooth cleaning measures including the interdental spaces (18).
Dental caries
Although accurate and actual epidemiological evidence on the frequency of dental caries is only insufficiently available, it has been estimated that this entity is the most prevalent condition showing a global prevalence of untreated lesions of 35% affecting 2.4 billion people worldwide (20,21). Among adults the prevalence reaches peaks at the age of 25 years and later in life around 70 years. Dental caries is primarily considered as an infectious disease caused by the colonization of the tooth surface by a pathogenic bacterial biofilm (15). A shift of the biofilm from a state being compatible with health to an acidic environment favors the proliferation and probably phenotypic adaption of acid tolerant bacteria ultimately amplifying the acidic potential of the biofilm (22,23). Mainly Lactobacillus sp. (species) and Streptococcus sp., namely Streptococcus mutans, have been suggested to be responsible for the acidification of the biofilm (24,25). More recent etiologic models take into account that many other endogenous bacteria that are present within the dental biofilm might also contribute to the acidification whereas other species are capable of base formation and thus to counteract acidification (24,26,27). The formation of a dental biofilm is an essential step for the formation of caries but it is commonly accepted that dental caries has a complex multifactorial etiology and requires the interactions between various impacts, i.e., the biofilm, salivary functions, the tooth structure, different genetic and behavioral factors (28). The latter specifically refers to the diet and the oral hygiene efforts of the patient. The frequent ingestion of fermentable carbohydrates, i.e., sugars, can induce a prolonged acidification of the mature biofilm (15). Due to an intensified production of mostly weak organic acids the pH drops below a critical value resulting in decomposition of the hydroxyapatite which comprises the mineral phase of the tooth (29,30). Upon progression the demineralization results in the collapse of the tooth surface and leads to the manifestation of a clinically detectable caries lesion, including discoloration and cavitation (31) (Figure 1).
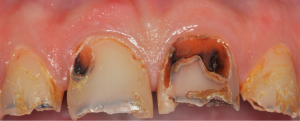
Periapical periodontitis
When continuing, the caries lesion expands and destructs growing parts of the clinical tooth crown. Finally it penetrates into the pulp chamber thereby allowing oral endogenous bacteria to colonize this soft tissue compartment. The perforating defect induces an irreversible inflammatory process inevitably resulting in the necrosis of the entire pulpal soft tissue. As a consequence bacteria immediately colonize the necrotic tissue and then enter the periapical tissue, i.e., the apical parts of the periodontal ligament and the surrounding alveolar bone, passing through the physiological foramen at the tip of the root (Figure 2). The type and structure of the bacterial community depends whether the affected tooth has already received endodontic root canal treatment. In previously untreated cases commonly a complex bacterial community of 10–20 different, predominantly gram-negative species can be found (32,33). On the contrary, unsuccessful treated root canals show a reduced bacterial diversity of 3–6 different gram-positive and gram-negative species, including Staphylococcus sp. (34). Moreover, the recurrence and/or persistence of Enterococcus faecalis seems to be responsible for endodontic treatment failure and is likely also involved in the periapical inflammation in these cases (35). The bacterial infection habitually results in an inflammatory process within the periapical tissue, that commonly presents with a chronic course, is only rarely associated with clinical symptoms, i.e., pain or swelling and thus remains unrecognized by the patient for long time periods. The prevalence of apical periodontitis at previously endodontically untreated teeth was found to reach even 83% (36-38). Following root canal treatment the prevalence of periapical inflammation still shows prevalence rates of 30–60% (39-41).
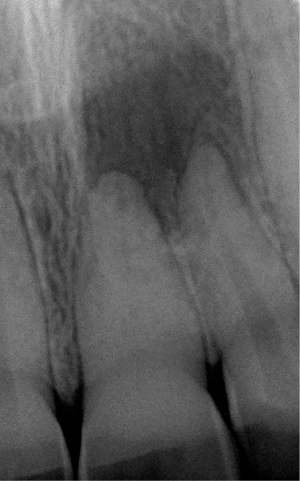
Periodontitis
Periodontitis is a biofilm-associated chronic inflammatory disease affecting the tooth supporting periodontal tissue at the gingival margin leading to the destruction of the periodontal ligament and the alveolar bone (Figure 3) (42). As a result the tissue loss causes an increasing depth of the gingival sulcus thereby transforming into a periodontal pocket (Figure 4), the loosening of the tooth and finally the loss of the entire tooth. The periodontitis-associated biofilm is typically a community of 100–300 endogenous microorganisms that results from a shift of the microbial community within the gingival pocket from a symbiotic to a dysbiotic state (17,43,44). Despite different co-factors, i.e., smoking or diabetes mellitus, seem to promote this transition it is primarily caused by inconstant and incomplete regular tooth cleaning measures (45). For many years the prevailing hypothesis suggested that several specific gram-negative anaerobe bacteria, i.e., Prophyromonas gingivalis, Treponema denticola, Tannerella forsythia and Aggregatibacter actinomycetemcomitans are centrally involved into the pathogenesis of periodontitis (46). More recent metagenomic analysis, however, revealed that the role of these bacteria might have been overestimated in earlier studies. Instead, current data suggested that the relative abundance of many bacterial species within the periodontal biofilm is different at diseased as compared to healthy sites and results in changes of the crosstalk between bacteria and the host (17). Also periodontitis shows a high prevalence since, for example, in the United States >40% of the adults present with periodontitis (47). In addition, more than 10% of the global population is even affected by severe periodontitis (48).
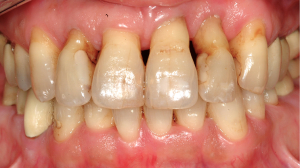
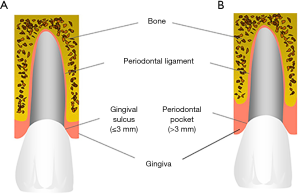
Systemic effects of oral infectious diseases
Despite a decline of infective endocarditis due to infection with bacteria of the viridans group, streptococci together with staphylococci are responsible for approximately 80% of the cases with infective endocarditis (13). Streptococci represent the most important group of causative bacteria in Europe and still the second most group of infective bacteria in the United States. Enterococci comprise the third leading cause of infective endocarditis. Specifically, the viridans streptococci are considered as oral commensals (49). Moreover, although only rarely involved into the pathogenesis of infective endocarditis a recent study in New Zealand observed a strong association between bacteremia with the HACEK group of bacteria and infective endocarditis (50). This group of gram-negative bacteria preferentially colonizes the oropharynx and the upper respiratory tract and encompasses Haemophilus species, Aggregatibacter actinomycetemcomitans, Aggregatibacter aphrophilus, Aggregatibacter paraprophilus, Cardiobacterium sp. Eikenella corrodens and Kingella sp. (51). Two of these bacteria, namely Aggregatibacter actinomycetemcomitans and Eikenella corrodens are strongly associated with periodontitis and are typically found in high numbers in periodontal pockets (52-54).
When considering the interrelationship between oral infectious disease and infective endocarditis the source of entry of oral bacteria into the bloodstream has to be identified. Regarding periodontitis particularly the dentogingival surface area, i.e., the surface of the periodontal pocket, has been proposed to allow oral and specifically periodontal bacteria to penetrate both, the junctional epithelium and the connective tissue barrier of the lamina propria ultimately leading to bacterial infiltration of the capillaries and, thus, the systemic bloodstream (55). It has been estimated that the surface area of the periodontal pocket might reach a size of 50 to even 200 cm2 (56,57). More recent detailed analysis revealed that the surface area of the gingival part of the periodontal pocket in fact amounts to 44 cm2 and, in addition, a considerable increase of the pocket surface area is associated with periodontitis (58). The incidence of bacteraemia due to the manifestation of apical periodontitis has yet not been extensively studied. Interestingly, one of the most important bacteria causing persistent apical periodontitis Enterococcus faecalis has been isolated already in the year 1899 from a patient with infective endocarditis (59). During endodontic root canal treatment bacteremia occurs in approximately one third of the cases (60,61). The incidence of bacteremia due to the presence of asymptomatic periapical lesions during routine activities has not been determined so far. However, chronic periapical bacterial infection was proposed as source of severe systemic infections, mostly thromboembolic events and cerebral abscesses, in several previous reports (62-64).
It is commonly accepted that bacteremia is detectible after minor mucosal trauma, including almost all standard dental diagnostic and therapeutic procedures (65,66). More recent studies report an incidence of 15–25% for the induction of bacteremia following diagnostic probing or subgingival cleaning of periodontal pockets (67). However, apart from dental procedures bacteremia might also occur during common daily activities, i.e., mastication or teeth brushing (68). The incidence of periodontitis associated bacteremia caused by these activities seems to correlate with the severity of periodontal tissue defects as represented by the depth of periodontal pockets (69).
Taken together, both, professional dental care and the manifestation of chronic infectious oral diseases are associated with a significant risk for the induction of bacteremia and, consequently, for the development of infective endocarditis (Figure 5). Comparing both eventualities for bacterial entry into the bloodstream the chronic persistence of oral diseases provoking highly recurrent bacteremia is associated with a 1,000-fold higher risk for infective endocarditis than occasional professional dental care (70).
Effects of congenital heart disease on oral health
Obviously there seems to exist a two way interrelationship between oral health and congenital heart disease, that causes additional elevation of the tooth related risk for infective endocarditis. According to several previous reports, the manifestation of many severe chronic conditions exerts direct and/or indirect effects on oral and dental health. For example among patients with severe organ failure needing organ transplantation or heart valve replacement, more than two thirds had septic oral infections whereas only 15% of healthy controls showed chronic oral and tooth related infections (71). When focussing on patients with congenital heart disease there were found significant higher numbers of active caries lesions and an increased caries prevalence in several pediatric populations as compared to healthy controls (72-75). However, different from the primary dentition many studies observed similar caries experience in the permanent dentition among patients with congenital heart disease and healthy controls (76-78). The higher rate of caries defects might be attributed at least partially to the coincidence of developmental defects of the dentition and congenital heart defects (79). In a pediatric population almost 50% of 39 children with congenital heart disease had enamel hypoplasia whereas only 23% of healthy controls presented with such defects (74). Various reasons might be responsible for the higher rate of developmental dental defects in patients with congenital heart disease. Apart from genetic mutations coincidentally inducing developmental defects of both, the heart and the teeth, particularly the impairment of the developing tissue by the systemic effects of the heart disease. For example impaired oxygen perfusion might contribute to an increased occurrence of malformed teeth. Specifically ameloblasts have been proposed to be highly susceptible to metabolic disturbances which might cause enamel hypomineralization subsequently inducing an elevated risk for the manifestation of caries (80,81). Moreover, some of the medicines often used in cardiac treatment are acidic or contain significant amounts of cariogenic carbohydrates (82,83) that might additionally enhance the predisposition for defects of the mineralized dental tissues, i.e., caries and erosions. Particularly digoxin and thiazide diuretics are still administered as sucrose containing suspensions (81,84). A strong correlation has been found between the duration of digoxin medication and the prevalence of caries in a pediatric population (77). In addition medication induced hyposalivation has been presumed in children with congenital heart disease (73,80). Especially the treatment with thiazide diuretics was suggested to be associated with salivary flow reductions (80).
Although data on the incidence of periapical infection in individuals with congenital heart disease are only scarcely available, several reports give evidence of a more intensive affection of these groups of patients. In a pediatric sample of patients the number of endodontically treated teeth was more than twice as high than in healthy controls, indicating that at least in some of the patient temporary infection of the periapical tissue has manifested (74). Also regarding gingival and periodontal disease there are only insufficient data available. According to the observations made on a sample of 60 children with severe congenital heart defects nearly twice as many of the teeth presented with signs of bacteria induced gingivitis as compared to sex and age matched healthy controls (85). This result was confirmed in two very recent surveys that found a higher gingival index among children with congenital heart disease (86,87). Moreover, in one of these studies more than two thirds of children presented with signs of gingival and/or periodontal inflammation (86).
Prevention of oral disease in patients with congenital heart defects
Based on the assumption that the diagnosis and treatment of oral diseases causes significant bacteremia with high frequency, it is commonly accepted to use systemic antibiotics prior to professional dental care for the prevention of infective endocarditis at least in selected patients with underlying cardiac disease including individuals with congenital heart disease (88). The recommendation for a prophylactic antibiotic therapy has come into question in the past years since the revised British NICE guidelines advised against antibiotic prophylaxis. Reassuring follow up data two years after implementation of the NICE guidelines revealed no significant increase in the number of cases of infective endocarditis (89). However, analysis of the 5-year follow-up data showed that the abandonment of antibiotic prophylactic therapy caused 35 additional cases of infective endocarditis per month than would have been expected under the use of antibiotics (90). This epidemiologic observation is in line with a recent clinical study that determined the presence of bacteria in the bloodstream following dental care with and without preventive use of antibiotics. Under prophylactic antibiotic therapy no viable bacteria were detectable using a culture technique for determination. Since analysis of the same blood samples using PCR revealed strong bloodstream infection the antibiotic treatment did not seem to prevent the bacteremia itself but lead to an very effective inactivation of bacteria that potentially might have elicited infective endocarditis (91). Amongst adults with CHD, particularly adults with complex congenital heart anomalies, ventricular septal defects, prosthetic valves, and with left sided heart disease, the incidence of infective endicarditis is even higher compared to the general population (92,93). Therefore, the new recommendations for antibiotic prophylaxis are viewed exceedingly controversial in the area of CHD and at least some experienced clinicians even see a wider spectrum of indications (94).
Transient bacteremia caused by chronic dental infections is frequently associated with routine daily activities. Hence, prophylaxis against this type of bacteremia must be directed towards the treatment and prevention of the odontogenic disease itself. Maintenance of oral health is commonly based on regular oral hygiene measures, i.e., brushing and flossing of teeth, topical use of fluoride, low cariogenic nutrition, and routine dental care (15). Assuming that congenital heart disease is associated with a higher prevalence of oral diseases, systematic oral maintenance care is effective in preventing risks for oral infections as shown in a recent Swedish study. Allocating children with congenital heart disease to a special preventive program at 1 year of age has led to a significant lower rate of new approximal caries lesions between 13 and 19 years of age than in the general population that was not referred to a specialist in pediatric dentistry for preventive care (95). Patients with congenital heart disease receiving specialist preventive treatment had only one fifth of newly developed caries lesions compared to healthy controls. In line with these observations both, the development of plaque and gingival inflammation was significantly reduced in a Norwegian sample of children with congenital heart disease up to 5 years when enrolled into a systematic prevention program (77). In this study also the rate of caries seemed to be reduced due to preventive dental care. Without adhesion to a preventive program significantly less patients make use of regular dental maintenance care (85). A considerably high proportion of young people with congenital heart disease aged 14–18 years did not have a dental visit during the past year and even more than 40% never flossed their teeth indicating insufficient control of potentially infective bacterial deposits (96). Yet, it remains to be elucidated if adult patients with congenital heart disease in fact have a higher risk for caries, gingivitis or periodontitis. There exists some evidence, however, that patients surviving childhood show poorer adhesion to dental maintenance care including oral hygiene measures since a more intensive manifestation of dental deposits, i.e., plaque and plaque induced gingival inflammation can be found in these individuals (86,87). It is commonly accepted that the incidence of caries is high among children and decreases in adults. On the contrary, periodontal disease is rarely seen in young age but manifests in high prevalence in adulthood (97). Based on these considerations it might be assumed that adult patients with congenital heart disease and poor dental maintenance might develop periodontitis with higher incidence than healthy individuals. In consequence periodontitis affected patients with congenital heart disease might be exposed to an additional risk for infective endocarditis.
Conclusions
Taken together patients with congenital heart disease are exposed to an elevated risk for infective endocarditis caused by oral infections. The cardiac conditions might have significant impact on the oral health additionally enhancing the risk for the manifestation of chronic infectious oral diseases, i.e., periodontitis, apical periodontitis and caries at least in younger patients with congenital heart disease. Yet, there exists only insufficient evidence on the interrelationship between congenital heart disease and oral health in adult patients. Several studies did not confirm a higher prevalence of caries in the permanent dentition, but showed a higher frequency and amount of bacterial deposits, i.e., plaque, and gingival inflammation. Moreover, adult patients often show only poor adherence to preventive oral hygiene measures and dental maintenance care. Although different bacteria are involved in the pathogenesis of caries and periodontitis, insufficient routine control of infectious dental deposits is commonly considered as the primary reason for both entities. Regarding the higher susceptibility for the manifestation of plaque in adolescence and adult patients with congenital heart disease it can, thus, be assumed that adult patients might have a higher prevalence of periodontitis compared to healthy controls.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Neidenbach RC, Lummert E, Vigl M, et al. Non-cardiac comorbidities in adults with inherited and CHD. Cardiovasc Diagn Ther 2018;8:423-31. [Crossref] [PubMed]
- Moons P, Bovijn L, Budts W, et al. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010;122:2264-72. [Crossref] [PubMed]
- Khairy P, Ionescu-Ittu R, Mackie AS, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol 2010;56:1149-57. [Crossref] [PubMed]
- Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007;115:163-72. [Crossref] [PubMed]
- Moons P, Meijboom FJ, Baumgartner H, et al. Structure and activities of adult congenital heart disease programs in Europe. Eur Heart J 2010;31:1305-10. [Crossref] [PubMed]
- Gilboa SM, Devine OJ, Kucik JE, et al. Congenital Heart Defects in the United States: Estimating the Magnitude of the Affected Population in 2010. Circulation 2016;134:101-9. [Crossref] [PubMed]
- Mylotte D, Pilote L, Ionescu-Ittu R, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation 2014;129:1804-12. [Crossref] [PubMed]
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol 2017;69:325-44. [Crossref] [PubMed]
- Mylotte D, Rushani D, Therrien J, et al. Incidence, Predictors, and Mortality of Infective Endocarditis in Adults With Congenital Heart Disease Without Prosthetic Valves. Am J Cardiol 2017;120:2278-83. [Crossref] [PubMed]
- Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009):the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009;30:2369-413. [Crossref] [PubMed]
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [Crossref] [PubMed]
- Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000 2017;75:7-23. [Crossref] [PubMed]
- Holland TL, Baddour LM, Bayer AS, et al. Infective endocarditis. Nat Rev Dis Primers 2016;2:16059. [Crossref] [PubMed]
- Yokoyama S, Takeuchi K, Shibata Y, et al. Characterization of oral microbiota and acetaldehyde production. J Oral Microbiol 2018;10:1492316. [Crossref] [PubMed]
- Pitts NB, Zero DT, Marsh PD, et al. Dental caries. Nat Rev Dis Primers 2017;3:17030. [Crossref] [PubMed]
- Doméjean S, Banerjee A, Featherstone JDB. Caries risk/susceptibility assessment:its value in minimum intervention oral healthcare. Br Dent J 2017;223:191-7. [Crossref] [PubMed]
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 2014;35:3-11. [Crossref] [PubMed]
- Rosier BT, De Jager M, Zaura E, et al. Historical and contemporary hypotheses on the development of oral diseases: are we there yet? Front Cell Infect Microbiol 2014;4:92. [Crossref] [PubMed]
- Maske TT, van de Sande FH, Arthur RA, et al. In vitro biofilm models to study dental caries: a systematic review. Biofouling 2017;33:661-75. [Crossref] [PubMed]
- Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990-2010:a systematic analysis. J Dent Res 2013;92:592-7. [Crossref] [PubMed]
- Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of untreated caries: a systematic review and metaregression. J Dent Res 2015;94:650-8. [Crossref] [PubMed]
- Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 1994;8:263-71. [Crossref] [PubMed]
- Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res 2008;42:409-18. [Crossref] [PubMed]
- Simón-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol 2015;23:76-82. [Crossref] [PubMed]
- Bowen WH, Burne RA, Wu H, et al. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol 2018;26:229-42. [Crossref] [PubMed]
- Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 2002;13:108-25. [Crossref] [PubMed]
- Arif N, Sheehy EC, Do T, et al. Diversity of Veillonella spp. from sound and carious sites in children. J Dent Res 2008;87:278-82. [Crossref] [PubMed]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369:51-9. [Crossref] [PubMed]
- Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc 2000;131:887-99. [Crossref] [PubMed]
- Featherstone JD. The continuum of dental caries—evidence for a dynamic disease process. J Dent Res 2004;83 Spec. No. C:C39-42.
- Pitts NB. Modern concepts of caries measurement. J Dent Res 2004;83 Spec No C:C43-7.
- Siqueira JF Jr, Rôças IN. Exploiting molecular methods to explore endodontic infections: Part 2--Redefining the endodontic microbiota. J Endod 2005;31:488-98. [Crossref] [PubMed]
- Siqueira JF Jr, Antunes HS, Rôças IN, et al. Microbiome in the Apical Root Canal System of Teeth with Post-Treatment Apical Periodontitis. PLoS One 2016;11:e0162887. [Crossref] [PubMed]
- Pinheiro ET, Gomes BP, Ferraz CC, et al. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 2003;36:1-11. [Crossref] [PubMed]
- Siqueira JF Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 2008;34:1291-1301.e3. [Crossref] [PubMed]
- Gulsahi K, Gulsahi A, Ungor M, et al. Frequency of root-filled teeth and prevalence of apical periodontitis in an adult Turkish population. Int Endod J 2008;41:78-85. [PubMed]
- Sidaravicius B, Aleksejuniene J, Eriksen HM. Endodontic treatment and prevalence of apical periodontitis in an adult population of Vilnius, Lithuania. Endod Dent Traumatol 1999;15:210-5. [Crossref] [PubMed]
- Al-Omari MA, Hazaa A, Haddad F. Frequency and distribution of root filled teeth and apical periodontitis in a Jordanian subpopulation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;111:e59-e65. [Crossref] [PubMed]
- Kirkevang LL, Hörsted-Bindslev P, Ørstavik D, et al. Frequency and distribution of endodontically treated teeth and apical periodontitis in an urban Danish population. Int Endod J 2001;34:198-205. [Crossref] [PubMed]
- Georgopoulou MK, Spanaki-Voreadi AP, Pantazis N, et al. Frequency and distribution of root filled teeth and apical periodontitis in a Greek population. Int Endod J 2005;38:105-11. [Crossref] [PubMed]
- López-López J, Jané-Salas E, Estrugo-Devesa A, et al. Frequency and distribution of root-filled teeth and apical periodontitis in an adult population of Barcelona, Spain. Int Dent J 2012;62:40-6. [Crossref] [PubMed]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:1809-20. [Crossref] [PubMed]
- Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J 2012;6:1176-85. [Crossref] [PubMed]
- Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol 2011;38 Suppl 11:7-16. [Crossref] [PubMed]
- Mombelli A. Microbial colonization of the periodontal pocket and ist significance for periodontal therapy. Periodontol 2000 2018;76:85-96. [Crossref] [PubMed]
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol 1998;25:134-44. [Crossref] [PubMed]
- Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc 2018;149:576-588.e6. [Crossref] [PubMed]
- Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010:a systematic review and meta-regression. J Dent Res 2014;93:1045-53. [Crossref] [PubMed]
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73. [Crossref] [PubMed]
- Yew HS, Chambers ST, Roberts SA, et al. Association between HACEK bacteraemia and endocarditis. J Med Microbiol 2014;63:892-5. [Crossref] [PubMed]
- Geraci JE, Wilson WR. Symposium on infective endocarditis. III. Endocarditis due to gram-negative bacteria. Report of 56 cases. Mayo Clin Proc 1982;57:145-8. [PubMed]
- Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000 1994;5:78-111. [Crossref] [PubMed]
- Position Paper AAP. Systemic antibiotics in periodontitis. J Periodontol 2004;75:1553-65. [Crossref]
- Teles R, Teles F, Frias-Lopez J, et al. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000 2013;62:95-162. [Crossref] [PubMed]
- Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontol 1998;3:108-20. [Crossref] [PubMed]
- Offenbacher S, Katz V, Fertik G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 1996;67:1103-13. [Crossref]
- Scannapieco FA. Position paper of The American Academy of Periodontology: periodontal disease as a potential risk factor for systemic diseases. J Periodontol 1998;69:841-50. [PubMed]
- Hujoel PP, White BA, García RI, et al. The dentogingival epithelial surface area revisited. J Periodontal Res 2001;36:48-55. [Crossref] [PubMed]
- Maccallum WG, Hastings TW. A case of acute endocarditis caused by Micrococcus zymogenes (Nov. Spec.), with a description of the microorganism. J Exp Med 1899;4:521-34. [Crossref] [PubMed]
- Debelian GJ, Olsen I, Tronstad L. Bacteremia in conjunction with endodontic therapy. Endod Dent Traumatol 1995;11:142-9. [Crossref] [PubMed]
- Savarrio L, Mackenzie D, Riggio M, et al. Detection of bacteraemias during non-surgicalroot canal treatment. J Dent 2005;33:293-303. [Crossref] [PubMed]
- Nair PN. Apical periodontitis: a dynamic encounter between root canal infection and host response. Periodontol 2000 1997;13:121-48. [Crossref] [PubMed]
- Mylonas AI, Tzerbos FH, Mihalaki M, et al. Cerebral abscess of odontogenic origin. J Craniomaxillofac Surg 2007;35:63-7. [Crossref] [PubMed]
- Somma F, Castagnola R, Bollino D, et al. Oral inflammatory process and general health. Part 2: How does the periapical inflammatory process compromise general health? Eur Rev Med Pharmacol Sci 2011;15:35-51. [PubMed]
- Lockhart PB, Durack DT. Oral microflora as a cause of endocarditis and other distant site infections. Infect Dis Clin North Am 1999;13:833-50. [Crossref] [PubMed]
- Lockhart PB. The risk for endocarditis in dental practice. Periodontol 2000 2000;23:127-35. [Crossref] [PubMed]
- Kinane DF, Riggio MP, Walker KF, et al. Bacteraemia following periodontal procedures. J Clin Periodontol 2005;32:708-13. [Crossref] [PubMed]
- Forner L, Larsen T, Kilian M, et al. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 2006;33:401-7. [Crossref] [PubMed]
- Geerts SO, Nys M, De MP, et al. Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J Periodontol 2002;73:73-8. [Crossref] [PubMed]
- Guntheroth WG. How important are dental procedures as a cause of infective endocarditis? Am J Cardiol 1984;54:797-801. [Crossref] [PubMed]
- Rustemeyer J, Bremerich A. Necessity of surgical dental foci treatment prior to organ transplantation and heart valve replacement. Clin Oral Investig 2007;11:171-4. [Crossref] [PubMed]
- Balmer R, Bu’Lock FA. The experiences with oral health and dental prevention of children with congenital heart disease. Cardiol Young 2003;13:439-43. [Crossref] [PubMed]
- Pollard MA, Curzon ME. Dental health and salivary Streptococcus mutans levels in a group of children with heart defects. Int J Paediatr Dent 1992;2:81-5. [Crossref] [PubMed]
- Hallett KB, Radford DJ, Seow WK. Oral health of children with congenital cardiac diseases: a controlled study. Pediatr Dent 1992;14:224-30. [PubMed]
- da Fonseca MA, Evans M, Teske D, et al. The impact of oral health on the quality of life of young patients with congenital cardiac disease. Cardiol Young 2009;19:252-6. [Crossref] [PubMed]
- Balmer R, Booras G, Parsons J. The oral health of children considered very high risk for infective endocarditis. Int J Paediatr Dent 2010;20:173-8. [Crossref] [PubMed]
- Stecksén-Blicks C, Rydberg A, Nyman L, et al. Dental caries experience in children with congenital heart disease: a case-control study. Int J Paediatr Dent 2004;14:94-100. [Crossref] [PubMed]
- Tasioula V, Balmer R, Parsons J. Dental health and treatment in a group of children with congenital heart disease. Pediatr Dent 2008;30:323-8. [PubMed]
- Pimentel EL, Azevedo VM, Castro Rde A, et al. Caries experience in young children with congenital heart disease in a developing country. Braz Oral Res 2013;27:103-8. [Crossref] [PubMed]
- Suga S. Enamel hypomineralization viewed from the pattern of progressive mineralization of human and monkey developing enamel. Adv Dent Res 1989;3:188-98. [Crossref] [PubMed]
- Massignan C, Ximenes M, da Silva Pereira C, et al. Prevalence of enamel defects and association with dental caries in preschool children. Eur Arch Paediatr Dent 2016;17:461-6. [Crossref] [PubMed]
- Rosén L, Rydberg A, Sjöström I, et al. Saliva profiles in children using heart failure medication: a pilot study. Eur Arch Paediatr Dent 2010;11:187-91. [Crossref] [PubMed]
- Moursi AM, Fernandez JB, Daronch M, et al. Nutrition and oral health considerations in children with special health care needs: implications for oral health care providers. Pediatr Dent 2010;32:333-42. [PubMed]
- Sivertsen TB, Åstrøm AN, Greve G, et al. Effectiveness of an oral health intervention program for children with congenital heart defects. BMC Oral Health 2018;18:50. [Crossref] [PubMed]
- Franco E, Saunders CP, Roberts GJ, et al. Dental disease, caries related microflora and salivary IgA of children with severe congenital cardiac disease: an epidemiological and oral microbial survey. Pediatr Dent 1996;18:228-35. [PubMed]
- Pourmoghaddas Z, Meskin M, Sabri M, et al. Dental Caries and Gingival Evaluation in Children with Congenital Heart Disease. Int J Prev Med 2018;9:52. [Crossref] [PubMed]
- Ali HM, Mustafa M, Hasabalrasol S, et al. Presence of plaque, gingivitis and caries in Sudanese children with congenital heart defects. Clin Oral Investig 2017;21:1299-307. [Crossref] [PubMed]
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on American Heart Journal July 2017 Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;116:1736-54. [Crossref] [PubMed]
- Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ 2011 3;342:d2392.
- Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2015 28;385:1
- Reis LC, Rôças IN, Siqueira JF Jr, et al. Bacteremia after supragingival scaling and dental extraction: Culture and molecular analyses. Oral Dis 2018;24:657-63. [Crossref] [PubMed]
- Montanaro C, Dimopoulos K, Shore DF. Infective endocarditis in patients with congenital heart disease: When, where and how. Int J Cardiol 2017;249:171-2. [Crossref] [PubMed]
- Moore B, Cao J, Kotchetkova I, et al. Incidence, predictors and outcomes of infective endocarditis in a contemporary adult congenital heart disease population. Int J Cardiol 2017;249:161-5. [Crossref] [PubMed]
- Neidenbach R, Niwa K, Oto O, et al. Improving medical care and prevention 758 in adults with congenital heart disease (ACHD) - 759 reflections on a global problem Part II: infective 760 endocarditis, pulmonary hypertension, pulmonary arterial 761 hypertension and aortopathy. Cardiovasc Diagn Ther 2018. In Press. [Crossref]
- Julihn A, Jansson P, Regnstrand T, et al. Is congenital malformation a risk factor for caries development in Swedish adolescents? Acta Odontol Scand 2013;71:1636-44. [Crossref] [PubMed]
- Janssens A, Goossens E, Luyckx K, et al. Exploring the relationship between disease-related knowledge and health risk behaviours in young people with congenital heart disease. Eur J Cardiovasc Nurs 2016;15:231-40. [Crossref] [PubMed]
- Papapanou PN, Susin C. Periodontitis epidemiology: is periodontitis under-recognized, over-diagnosed, or both? Periodontol 2000 2017;75:45-51. [Crossref] [PubMed]


