The optimal duration of dual antiplatelet therapy after coronary stent implantation: to go too far is as bad as to fall short
Introduction
The association of aspirin and a P2Y12 inhibitor, or dual antiplatelet therapy (DAPT), is one of the most widely used treatments in cardiovascular medicine, with an estimated yearly indication in more than 2 million patients with myocardial infarction (MI) or treated with percutaneous coronary intervention (PCI) (1). DAPT efficiently reduces platelet aggregation, limiting the risk for stent thrombosis or vascular thrombosis at sites distant from the initially stented lesion (2). Yet, by the same mechanism DAPT increases the risk for major bleeding, which have been linked to increased morbidity and mortality (3-6). For this reason the optimal duration of treatment, which maximize efficacy by ischemic events prevention, and minimize the concomitant risks for serious bleeding complications, have been extensively explored during the last 20 years (7,8). During this period a series of technical and pharmacological advancements have been introduced and at the same time treatment decision-making have been informed by subgroup data, suggesting treatment individualization based on various patient’s subsets. A summary of the available evidence from RCTs and specific subgroups will be discussed in this document.
DAPT duration after PCI: summary of the evidence from randomized controlled trials
The optimal duration of DAPT after coronary stenting has been extensively explored in 18 randomized controlled trials (9) with more than 40,000 patients included (Table 1) (7). Most of these studies used as a comparator the initial landmark point proposed by guidelines at that time (i.e., 12 months of treatment) (26-28), while the experimental arm was based on a reduction of treatment duration (e.g., to 3 or 6 months) or on a prolongation of the treatment duration (e.g., 24 or 30 months). Few trials make an exception due to a special design (e.g., 6 vs. 24 months) (19,21,29). For the purpose of a simple classification we divided these studies in two clusters:
- Trials testing reduction of DAPT duration;
- Trials testing prolongation of DAPT duration.
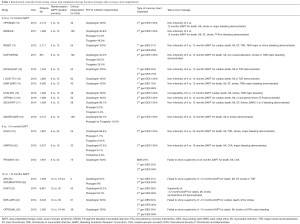
Full table
Reducing the duration of DAPT after coronary stenting (Figure 1)
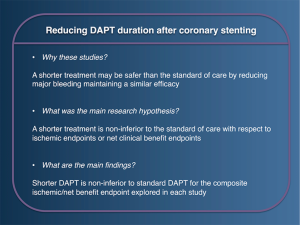
The reduction of DAPT duration from the initial standard proposed of 12 months has been evaluated in eleven RCTs (Table 1) (10-15,17), three tested 3 vs. 12 months of treatment, eight tested 6 vs. 12 months. In general these studies tested the primary hypothesis that a shorter DAPT regimen was non-inferior to the standard of care in terms of ischemic events or net adverse clinical events (NACE) (i.e., ischemic and bleeding events merged in a single composite endpoint). The possibility to reduce safely treatment duration has been often tested in conjunction to specific stent designs, in order to demonstrate their safety in the context of a shorter treatment duration (30-32).
The first study published among this cluster was the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) trial (12). This included 1,443 patients treated with drug-eluting stent (DES) implantation and randomized to 6 vs. 12 months DAPT thereafter (12). Most patients were treated with everolimus-eluting stents and roughly half presented with acute coronary syndrome (ACS). The trial ultimately demonstrated non-inferiority of 6 vs. 12 months of DAPT with respect to the primary end point, a composite of cardiac death, MI, or ischemia-driven target vessel revascularization. TIMI major and minor bleeding, trended higher in the 12-month group, but the difference was not statistically significant [hazard ratio (HR) 0.40; 95% CI: 0.13–1.27; P=0.12) (12).
The Second Generation Drug-Eluting Stent Implantation Followed by Six-Versus Twelve-Month Dual Antiplatelet Therapy (SECURITY) (17) and the Intracoronary Stenting and Antithrombotic Regimen: Safety and Efficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) (14) both tested among patients treated with DES, the non-inferiority of 6 vs. 12 months DAPT for a composite primary endpoint including both ischemic and major bleeding. Both studies were prematurely terminated due to slow enrollment but ultimately reached the pre-specified non-inferiority hypothesis. Major bleeding was rare and similar between the two study arms in both studies.
The Evaluate Safety and Effectiveness of the Tivoli DES and the Firebird DES for Treatment of Coronary Revascularization (I-LOVE-IT 2) trial randomized 1,829 patients across 32 centers in China, to 6 vs. 12 months of DAPT (13). All patients were treated with DES, but the type of stent was also randomized following the factorial 2:2 design of the study, with a balanced mixture of durable-polymer vs. bioresorbable-polymer cobalt-chromium sirolimus eluting stents. The trial ultimately demonstrated the non-inferiority of 6 months DAPT for the primary end point of cardiac death, MI and target lesion revascularization. The rate of major bleeding was similar in the two groups (1.2% vs. 0.7%; P=0.21) (13).
A factorial design was also implemented in the Impact of Intravascular Ultrasound Guidance on Outcomes of XIENCE PRIME Stents in Long Lesions (IVUS-XPL) study (15). In this case the first randomization was based on the implementation of intravascular ultrasound guidance to complete the PCI, while the second randomization was for DAPT duration (6 vs. 12 months) (15). Second-generation everolimus-eluting stent was used in all 1,400 patients included in the study. At 12-months follow-up the composite of Cardiac death, MI, stroke and TIMI major bleeding was similar between patients treated for 6- or 12-month (2.2% vs. 2.1%; P=0.85). Interestingly, at the subgroup analysis for the primary endpoint, patients treated with intravascular ultrasound guided stent implantation benefitted more from a shorter DAPT treatment as compared to those treated with angiographic guidance alone (Pint=0.018) (15).
Similarly in the OPTIMAl duration of Clopidogrel after implantation of second-generation drug-eluting stents (OPTIMA-C) trial 1,368 patients undergoing biolimus-eluting or zotarolimus-eluting stent implantation have been randomized to 6 vs. 12 months DAPT (16). The main study hypothesis was to test non-inferiority of 6 vs. 12 months of DAPT for the composite primary endpoint of cardiac death, MI or ischemia driven target lesion revascularization. The primary endpoint ultimately occurred in 1.2% of patients in the short DAPT arm and 0.6% in the long DAPT arm, with the study formally meeting the pre-specifies non-inferiority margin, which was however largely exceeding the observed absolute risk difference between the two study arms (non-inferiority margin 4%) (16).
More recently two randomized trials tested a shorter DAPT duration of 6 months in patients with ACS or presenting with ST-segment elevated myocardial infarction (STEMI). The first study called SMART-DATE was executed in 31 centers in South Korea, enrolling a total of 2,712 patients undergoing PCI for an ACS (18). According to the study protocol, patients were randomized to a treatment with DAPT for 6 or 12 months. Acute presentation was balanced with roughly one-third of patients presenting with STEMI, one-third presenting with non ST-segment elevated myocardial infarction (NSTEMI) and one-third with unstable angina. The P2Y12 inhibitor most commonly implemented was clopidogrel (~80%), while ticagrelor and prasugrel became available only during the final period of enrollment. After 18 months of post-procedural follow-up the primary endpoint, a composite of all-cause death, MI, or stroke, occurred equally in the two study arms (4.7% vs. 4.2%) meeting the pre-specified non-inferiority hypothesis. Yet, a significant excess of MI was noted in the short DAPT arm (absolute risk difference 1%), that together with a wide non-inferiority margin for the primary study endpoint raised some concerns regarding the safety of a short DAPT regimen in ACS patients.
In line with this study, the more recent DAPT-STEMI trial (NCT01459627), presented at the transcatheter cardiovascular therapeutics congress in 2017, included 870 patients with STEMI treated with primary PCI and second-generation DES that after 6 months of uneventful treatment with DAPT were randomized to continue treatment up to 12 months or to stop P2Y12 inhibitor and continue with aspirin only. The primary study endpoint was a composite of death, MI, revascularization, stroke and major bleeding at 24 months after primary PCI. Short DAPT was found to be non-inferior as compared to the standard 12 months treatment duration (short DAPT 4.8% vs. long DAPT 6.6%; Pnon-inferiority=0.004). Yet, due to the small sample size, the low event rate and the wide non-inferiority margin these results should be interpreted with caution.
Six months of treatment were compared to a more than 12 months treatment duration in two non-inferiority studies: ITALIC and NIPPON (20,29) trial (Table 1), in which patients were randomly allocated to 6 vs. 24 months of DAPT and 6 vs. 18 months of DAPT respectively. Both studies met the prespecified non-inferiority, yet the results from these studies should be interpreted with caution due to the study early termination and the wide non-inferiority margin selected, which exceeded the event rate of the experimental arm (20,29).
Ultimately three randomized studies tested an even shorter DAPT duration, lasting three months after DES implantation (10,11). The Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor ZES Implantation (RESET) and Optimized Duration of Clopidogrel Therapy Following Treatment With the Zotarolimus-Eluting Stent in Real-World Clinical Practice (10) trials randomized 2,117 and 3,119 patients respectively after Endeavour zotarolimus-eluting stent (no longer available in the market) (10,11). In both studies the study population was at low ischemic risk, and a non-inferiority hypothesis for the NACE endpoint was tested. Ultimately both studies reached the pre-specified non-inferiority margin, showing a substantial equipoise for ischemic events in the short vs. long DAPT arm in the population selected.
More recently, the REDUCE trial (NCT02118870), presented at the transcatheter cardiovascular therapeutics congress in 2017, selected a population with a higher baseline ischemic risk to test the non-inferiority of 3 vs. 12 months of DAPT in patients with ACS treated with PCI. The 1,496 patients included in the study have been treated at index procedure exclusively with a bioabsorbable polymer DES with a luminal CD34+ antibody coating (COMBO stent). The primary endpoint was a composite of all cause death, MI, stent thrombosis, stroke, target vessel revascularization or bleeding, with a wide, 5%, non-inferiority margin. The study ultimately reached non-inferiority with an event rate of 8.2% in the short DAPT arm and 8.4% in the long DAPT arm (Pnon-inferiority<0.001). More than half of the included patients have been treated with potent P2Y12 inhibitors. Yet, exploring secondary ischemic endpoints a worrisome, borderline, increase of stent thrombosis (1.2% vs. 0.4%; P=0.08) and all-cause mortality (1.9% vs. 0.8%; P=0.07) in the short DAPT arm raised some concerns regarding the efficacy of a 3-month DAPT duration among patients presenting with ACS, in line with those observed in the SMART-DATE trial.
Finally, the DETECT-OCT study explored the interesting interrelation between stent strut coverage evaluated by optical coherence tomography (OCT) and the impact of DAPT duration (33). This study was not randomized for DAPT duration, but instead assigned treatment (3 vs. 12 months) based on OCT findings, assigning a longer DAPT duration (i.e., 12 months) if >6% of uncovered stent strut were observed at the 3 months invasive follow-up, or a shorter DAPT duration (i.e., 3 months) in case of sufficient stent strut coverage. The composite endpoint of cardiac death, MI, stent thrombosis, and major bleeding was rare and similar in both study arms at 12 months follow-up. Despite these results regard a secondary analysis in a non-randomized study with a small sample size, the concept of an imaging guided DAPT duration is interesting and may deserve to be better explored in appropriately sized prospective studies.
Taken together, these studies were all of medium to small size, and with a relatively low event rate. In addition the relatively low period of divergence between the two treatment arms (i.e., 6 months in studies testing 6 vs. 12 months of DAPT and 9 months in those testing 3 vs. 12 months of DAPT) made these trials largely underpowered to detect differences in treatment effect for rare clinical events, which occurred less often than many had anticipated during trial design. In addition, the slight to moderate differences in inclusion criteria, type of stent used, clinical events definitions and trial design (e.g., timing of randomization could occur at the time of stent implantation or at the time of DAPT divergence) adds to the complex interpretation of the overall study results.
Prolonging the duration of DAPT after coronary stenting (Figure 2)

Four trials compared the standard of care 12 months of DAPT, with a prolonged treatment duration for up to 18–48 months (Table 1) (22-25). These studies globally tested the superiority hypothesis of a longer DAPT duration for ischemic endpoints including very late stent thrombosis, MI and others.
The Dual-Antiplatelet Treatment Beyond 1 Year After Drug-Eluting Stent Implantation (ARCTIC INTERRUPTION) trial (22), extended follow-up of the ARCTIC study (9), based its primary hypothesis on the superiority of ≥18 months DAPT vs. 12 months after stent implantation (22). The study population was highly selected, including only patients undergoing elective PCI. The primary efficacy endpoint occurred in 4% of patients in both study arms, while a significant excess of bleeding was detected in the prolonged DAPT arm (22).
In the Optimal Duration of Clopidogrel Therapy With DES to Reduce Late Coronary Arterial Thrombotic Events (DES LATE) trial 5,045 patients free from adverse events during the first 12 months after DES implantation were included. As was observed in the ARCTIC-INTERRUPTION study, the primary endpoint of cardiac death, MI, or stroke was similar in the two study arms (24). Yet, at difference with the prior study, major bleeding were also similar between the two study arms. Also the more recent Optimal Dual Antiplatelet Therapy (OPTIDUAL) Trial, testing 48 months vs. 12 months of DAPT with clopidogrel after stenting, failed to show superiority of a prolonged treatment with DAPT: in fact, no difference for the primary endpoint of death, MI, stroke, or major hemorrhage was detected between the two study arms (5.8% vs. 7.5%; HR: 0.75; 95% CI: 0.50 to 1.28), and in the same way no difference was noted when major bleeding alone were evaluated (25).
Also designed to test superiority for ischemic events of a longer treatment duration with DAPT, the PROlonging Dual antIplatelet treatment after Grading stent-induced intimal hYperplasia study (PRODIGY) trial, randomly allocated patients to a long DAPT strategy for 24 months vs. a short DAPT for 6 months (21). The PRODIGY trial ultimately found no difference between 6 and 24 months DAPT for the composite primary efficacy endpoint of death, MI, stroke, while it detected a significant excess of BARC 2, 3 or 5 bleeding in patients in the longer DAPT duration arm (21).
Importantly, these three trials taken separately were underpowered to detect a significant difference for more rare ischemic events (e.g., stent thrombosis). Specifically designed with this objective, the large Dual Antiplatelet Therapy (DAPT) study, included 9,961 patients treated with DES during index PCI that after an initial 12 months uneventful period of treatment (study run-in phase) were randomized to an extended treatment duration up to 30 months or to single antiplatelet therapy with aspirin (23). At difference with all previous studies, in the DAPT trial extended DAPT resulted in a 1% absolute reduction in very late stent thrombosis, a 1.6% absolute reduction of major adverse cardiovascular and cerebrovascular events (MACCE). This was driven by a 2% reduction of MI, which were in half of the cases related to a vessel different from the one initially treated during the index procedure. However, the benefit observed on the ischemic side was counterbalanced by an excess of major bleeding, with a 0.9% absolute increase in moderate or severe GUSTO bleeding and 2.6% of BARC 2, 3 or 5 bleeding (23). In addition a signal towards an increase in all-cause mortality was observed among patients treated with longer DAPT. This was later also confirmed in several meta-analyses (34-37), but was ultimately excluded in an internal revision from the American Food and Drug Administration (FDA) (38). At a more thorough evaluation of the single fatal events, most were related to cancer (39), and despite this signal was observed also in other studies (37), the authors attributed this finding to chance.
Current evidence for DAPT duration individualization
In synthesis, results from multiple RCTs suggest that prolonging treatment with DAPT is associated with a reduction of stent or non-stent related ischemic events but to a significant increase of bleeding (2). Importantly both the advantage in term of ischemic event reduction and the excess of bleeding may vary significantly based on the baseline ischemic and bleeding risk of the patient, pushing the trade-off in one direction or in the other based on the intrinsic patient characteristics. For this reason multiple studies have explored the effect on outcomes based on different DAPT duration in several subgroups (2,7,40-45). This may play an important role in treatment decision-making by individualizing the therapeutic strategy on a single patient basis. Taking into account all these variables may seem tricky (Figure 3), but their careful evaluation is the key for optimal treatment individualization.
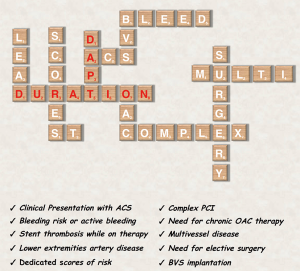
Clinical presentation
The clinical presentation at the time of PCI (e.g., stable CAD or ACS) is a major determinant of the patients’ baseline ischemic risk and for this reason have an important role in the selection of antiplatelet treatment duration (7,46,47). The interaction between clinical presentation and DAPT duration has been studied in a pre-specified analysis of the PRODIGY trial (41). In this study, nearly three-quarters of the patients enrolled presented with ACS and the remaining with stable coronary artery disease (CAD). A significant heterogeneity of the effect of long vs. short DAPT duration was noted for NACE (Pint=0.024), suggesting a significant net harm from a longer DAPT in stable CAD patients (NACE in the 24-month vs. 6-month DAPT arm: 13.3% vs. 5.6%; HR 2.5, 95% CI: 1.35 to 4.69, P=0.004), and a substantial equipoise of a longer treatment duration in the ACS population (16.1% vs. 14.1%; HR 1.15, 95% CI: 0.88 to 1.50; P=0.29) (41).
In the larger DAPT trial, a prolonged DAPT up to 30 months significantly reduced MACCE among patients with (3.9% vs. 6.8%; P<0.001) but not in those without MI (4.4% vs. 5.3%; P=0.08) at presentation, with a positive interaction testing (Pint=0.03) suggesting a higher efficacy of longer DAPT among patients with MI as compared to those without (40). In addition, longer DAPT was associated with a significant increase in all-cause death among patients presenting without MI (2.1% vs. 1.5%; P=0.04) (40,47), but not in those with MI at presentation.
These findings are supported by a meta-analysis from 6 RCTs including 33,435 patients with prior MI, and comparing the effects of 12 vs. >12 months DAPT (48). Extended DAPT was associated with a significant reduction of cardiovascular death, MI or stroke (6.4% vs. 7.5%; ARD =1.1%; P=0.001). This benefit was observed within each component of the primary endpoint separately appraised, including a significant 15% reduction of cardiovascular death (RR 0.85, 95% CI: 0.74 to 0.98) (48). Interestingly, since most patients with MI are nowadays treated with potent P2Y12 inhibitors, it will be important to point out if the benefit of extended DAPT treatment may change based on the type of P2Y12 inhibitor used, as it was suggested in a separate study (49). Clinical guidelines support considering clinical presentation at the time of DAPT duration selection, and generally recommend 6 months of DAPT after PCI in patients with stable CAD and 12 months in patients presenting with ACS.
Risk scores
Clinical risk scores have long been used to guide the indication for oral anticoagulation in atrial fibrillation patients. Recently, specific risk scores have been validated for DAPT duration decision-making in patients undergoing PCI (Figure 4) (2,7,50). The concept of using a risk score to guide DAPT duration decision-making was introduced for the first time in the PRODIGY trial (51). In this analysis, patients stratified according to the CRUSADE bleeding risk score into high (CRUSADE >40) vs. non-high (CRUSADE ≤40) bleeding risk categories, showed a significant interaction with the antiplatelet treatment duration. Patients deemed at high bleeding risk had a significant increase in major bleeding and red blood cell transfusion when treated with 24- versus 6-month DAPT (9.7% versus 3.7%; ARD 6%; 95% CI: 0.4% to 12.3%; P=0.04; number needed to treat to harm =17), whereas those not deemed at high bleeding risk were not exposed to a significant excess of these complications even if treated with longer DAPT duration (2.4% versus 1.6%; ARD 0.8%; 95% CI: 0.6% to 2.2%; P=0.25) (Pint=0.05) (51).
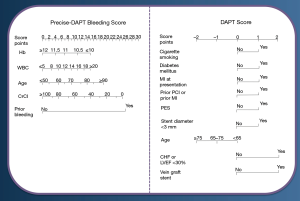
A specific tool for DAPT duration decision-making was developed within the DAPT trial dataset (n=11,648) to identify patients having a net benefit in terms of both ischemia and bleeding from a 30-month as compared to a 12-month treatment (52). After multivariable modeling nine clinical and procedural variables independently associated with ischemia alone or with bleeding alone were selected and included in a clinical risk score (DAPT score—Figure 4). This tool, which score ranges from −2 to +10, has been validated to predict the difference between the anticipated reduction in ischemic events and the anticipated increase in bleeding events with extended DAPT. Ultimately, patients with a score of 2 or more appeared to derive a net benefit from a longer treatment duration, in turn patients with a score of less than 2 did not derive such benefit, and were better addressed with a 12-month treatment duration. Yet, this tool does not allow informing early decision-making at the time of PCI to select shorter treatment durations within the first year from treatment initiation.
This aspect has been addressed by the PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (53) study, which pooled data from eight RCTs including a total of 14,963 patients treated with PCI and subsequent DAPT (53). The authors aimed to explore long-term bleeding predictors while on DAPT, and develop a predicting tool to inform DAPT duration. After multivariable modeling five clinical and laboratory factors emerged as independent predictors of out-of-hospital bleeding and have been included in a novel risk score (PRECISE-DAPT—Figure 4). The PRECISE-DAPT score was validated in two independent external cohorts of 8,595 from the PLATO trial, and 6,172 patients from the BernPCI registry.
Among patients randomly allocated to short (3 or 6 months) or long (12 or 24 months) DAPT duration (n=10,081), patients deemed at high bleeding risk based on a PRECISE DAPT score ≥25 were associated to a significant increase of TIMI major or minor bleeding when treated with long DAPT as compared to short DAPT. Importantly, prolonging DAPT duration in this group was not associated to a benefit in terms of ischemic endpoint, hence driving the net benefit towards a shorter treatment duration. On the contrary, patients not deemed at high bleeding risk, as those with a PRECISE-DAPT score <25, did not derive a significant harm in term of bleeding events from a longer treatment duration, but instead were associated to a significant reduction of the composite ischemic endpoint of MI, definite stent thrombosis, stroke and target vessel revascularization. It is important to emphasize that these risk prediction tools has not been yet prospectively validated in the setting of a randomized clinical trial and further investigation in this field is desirable.
Stent thrombosis
Managing patients with stent thrombosis is challenging given the lack of randomized data in this group of patients. Patients suffering stent thrombosis have a higher risk of stent thrombosis recurrence (54), which appears to persist overtime. Hence, targeting these patients with longer DAPT may be reasonable to reduce the accrued risk of recurrent events overtime, if the treatment can be tolerated. In this context, is of upmost importance to explore and treat correctable causes of stent thrombosis, whether these are clinical [e.g., poor medication adherence (55), unscheduled treatment interruption/cessation (56)], or mechanical (e.g., sub-optimal stent deployment).
Lower extremities artery disease
Peripheral artery disease may often coexist in patients with CAD, and represents an important marker of further higher ischemic risk (57,58). In the CHARISMA trial comparing DAPT with aspirin alone during primary or secondary prevention, 3,096 patients qualified as having peripheral artery disease. In this patients subgroup DAPT up to a median of 28 months was associated with a lower rate of MI and hospitalization for ischemic events as compared to aspirin alone (59). In the PEGASUS trial, patients with a history of peripheral artery disease carried a 60% higher risk of MACE (60). In this subgroup, treatment with ticagrelor 60 mg b.i.d. compared with placebo provided a robust absolute risk reduction of 5.2% at 3 years for the primary ischemic endpoint, with a significant reduction of acute limb ischemic events and a reduction of both cardiovascular and all-cause mortality (60). Similarly in the PRODIGY trial, a history of peripheral artery disease was associated with a higher risk of death and ischemic events (HR 2.80, 95% CI: 2.05 to 3.83; P<0.001), and prolonged vs. short DAPT significantly reduced the incidence of the primary efficacy endpoint in patients with peripheral artery disease (16.1% vs. 27.3%; HR 0.54, 95% CI: 0.31 to 0.95; P=0.03) but not in patients without (9.3% vs. 7.4%; HR 1.28, 95% CI: 0.92 to 1.77; P=0.14), with a positive interaction testing (P=0.01) (61). Longer DAPT provided a consistent benefit in these patients by also reducing definite or probable stent thrombosis and all-cause mortality (61).
Need for long-term oral anticoagulation
Patients with a need for long-term oral anticoagulation have been invariably excluded from randomized clinical trials for DAPT duration. There is a paucity of data to inform the optimal duration of DAPT in these patients (triple therapy), and whether aspirin or a P2Y12 inhibitor should be preferentially be discontinued. The ISAR-TRIPLE study enrolled 614 patients requiring oral anticoagulants (OAC) and undergoing coronary stent implantation (62). Patients were randomly assigned DAPT with aspirin and clopidogrel for 6 weeks or for 6 months, with subsequent withdrawal of clopidogrel and treatment continuation with aspirin and OAC. At 9 months of follow-up the primary endpoint of death, MI, stent thrombosis, ischaemic stroke, or TIMI major bleeding was similar between patients randomized to 6 weeks DAPT + OAC vs. 6 months DAPT + OAC (9.8% vs. 8.8%; HR 1.14, 95% CI: 0.68–1.91; P=0.63) (62). In addition, no difference for TIMI major bleeding (5.3% vs. 4.0%; HR 1.35, 95% CI: 0.64–2.84; P=0.44) was observed. Yet, due to the small sample size this study cannot be considered conclusive. At any rate it appear reasonable to limit as much as possible DAPT duration in patients with OAC, defining based on the competing ischemic and bleeding risk the optimal treatment strategy (7).
Complex PCI
PCI complexity has been consistently considered by interventional cardiologists as a decisive element for decisions upon DAPT duration (63,64). International guidelines have recently endorsed a standardized definition for PCI complexity (7). This is based on criteria selected in a patient-level analysis of more than 9,000 patients randomly allocated to different DAPT durations (≥12 vs. ≤6 months) after coronary stenting (65). The used definition was based on six elements: three vessel PCI, implantation of 3 or more stents, three or more coronary lesions, bifurcation stenting, total stent length >60 mm, treatment of a chronic total occlusion (65). The presence of at least 1 of these features qualified the procedure as a complex PCI. At follow-up, patients undergoing complex PCI had a higher crude rate of major adverse cardiovascular events (MACE) (5.0% vs. 2.5%; P=0.001), and continuing DAPT on a long term (≥12 months) as compared to a short term DAPT (≤6 months) significantly reduced MACE (HR 0.56, 95% CI: 0.35 to 0.89). In addition the magnitude of the benefit was directly related to the number of complex PCI elements accounted at baseline. In turn, patients not qualifying as complex PCI derived no benefit from a longer DAPT course (HR 1.01, 95% CI: 0.75 to 1.35). Yet, irrespective of PCI complexity, longer DAPT was associated with a higher risk of major bleeding (65).
Bioresorbable stent implantation
Current generation of poly-lactic acid based bioresorbable vascular scaffolds (BVS) showed a higher risk of device thrombosis as compared to metallic DESs in various clinical trials and meta-analysis (66-68). While the duration of DAPT suggested in the protocols of these studies was of 12 months, an excess of device related thrombosis was observed also in the very-late period (i.e., >12 months after implantation). The excess of very late scaffold thrombosis may be due to the slow adsorption process and the presence of scaffold remnants up to 4–5 years after implantation, representing a potential trigger for late ischemic events that could potentially be prevented by longer DAPT (69). Yet, no dedicated studies examining the optimal duration of DAPT after implantation of a BVS is currently available. Hence, based on expert consensus, international guidelines suggest extending DAPT duration beyond 12 months after BVS implantation (7).
Other two bioresorbable scaffold technologies are currently marketed in Europe. The DESsolve (Elixir Medical Corporation, Sunnyvale, California) is a poly-lactic acid based BVS with self-correcting properties and an expected adsorption time of 12 months (70). These properties appear promising as may theoretically reduce the risk of very late scaffold thrombosis but no robust clinical data can still support this concept. At difference with the other two, the Magmaris (Biotronik, Berlin, Germany) is a metallic, magnesium-alloy, sirolimus-eluting scaffold with a higher tensile strength and an expected absorption time of 12 months (71). Interestingly, in ex vivo animal studies this scaffold showed lower platelet coverage and thrombus deposition as compared to both first-generation BVS (72) and to an equivalent, stainless steel sirolimus-eluting stent (73). Yet, whether these characteristics translate in a superior safety compared to other BVS remain to be demonstrated, and the clinical experience with this technology is still limited by the low number of patients included in randomized clinical trials (71). Optimal duration of DAPT with this device is unclear and is not supported by solid evidence. Clinical trials (71,74) and registries (NCT02817802) recommended at least 6 months of DAPT after magnesium-allow scaffold implantation, and clinical data up to 24 months based on a limited number of patients (n=184—84% discontinued DAPT before 24 months) did not report episodes of definite or probable scaffold thrombosis (75).
Future perspectives: will we continue treating patients with DAPT?
DAPT is one of the most important treatment options in current cardiology. The field has rapidly modified in the recent years, in fact both European and American cardiology scientific societies published a dedicated focused-update on DAPT duration (Table 2). Yet, many future studies have potential to further change practice.
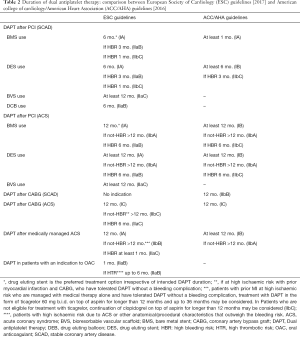
Full table
Aspirin is the cornerstone treatment for patients with secondary prevention of cardiovascular events, and current guidelines recommended its utilization for an indefinite period of time after the index event. Utilization of low-dose aspirin on top of a P2Y12 inhibitor has proven superior to aspirin alone to prevent ischemic events after ACS or stent implantation (3). Yet, all the studies performed so far compared DAPT with single antiplatelet therapy with aspirin after coronary stent implantation, while a comparison with single antiplatelet therapy with a P2Y12 inhibitor alone was never performed. Clopidogrel alone provided superior efficacy and similar safety compared to aspirin alone in patients with atherosclerotic vascular disease in the CAPRIE trial (76). In addition potent P2Y12 inhibitors prasugrel and ticagrelor offers a more consistent and potent inhibition of platelet reactivity as compared to clopidogrel, hence potentially reducing the treatment gap for clopidogrel non-responders (4,5). In the more recent SOCRATES trial 13,199 patients with an ischemic stroke or transient ischemic attack (TIA) were randomly assigned to either ticagrelor or aspirin. The primary end point (stroke, MI, or death within 90 days) occurred in 6.7% of patients treated with ticagrelor and 7.5% of those treated with aspirin (HR, 0.89; 95% CI: 0.78–1.01; P=0.07) (77). Interestingly, there was no excess of major bleeding and intracranial hemorrhage in patients treated with ticagrelor as compared to those treated with aspirin (77).
In the same line, the MATCH trial randomized 7,599 high-risk patients with recent ischemic stroke or TIA and already on treatment with clopidogrel, to low-dose aspirin or placebo (78). Ultimately the study showed that adding aspirin on top of clopidogrel did not result in a significant reduction ischemic event, but only to an excess of major bleeding, including intracranial hemorrhage (78).
For these reasons much attention has been pointed in evaluating the efficacy and safety of a strategy with a single P2Y12 inhibition in spite of DAPT in patients undergoing coronary stent implantation (79). The GLOBAL LEADERS trial has been designed to evaluate in a PCI treated population the effects of 24-month Ticagrelor monotherapy (associated with aspirin only during the first month) compared to 12-month standard DAPT. The primary outcome was a composite of all-cause mortality or non-fatal, new Q-wave MI at 24 months. A total of 15,991 patients were randomly allocated to Ticagrelor 90 mg twice daily for 24 months plus ASA ≤100 mg for one month (Experimental arm) versus DAPT with either Ticagrelor (ACS) or Clopidogrel (stable CAD) for 12 months plus ASA ≤100 mg for 24 months (Control). The key safety endpoint was investigator-reported BARC class 3 or 5 bleeding. At 24 months of follow-up the primary endpoint was not statistically different between the two treatments tested (3.81% vs. 4.37%, RR 0.87, P=0.073), neither when both components of the primary endpoint were separately appraised (all cause death: 2.81% vs. 3.17%, RR 0.88, P=0.182). No difference for investigator-reported BARC 3 or 5 bleeding events was found (2.04% vs. 2.12%, RR 0.97, P=0.766). Nevertheless adherence to the experimental therapy was observed lower than those of the standard of care (78% vs. 93%), which may have in part impaired the statistical power of the study. Other two ongoing trials are currently investigating this strategy and may help giving a cleared picture to the field [TWILIGHT (NCT02270242) and TICO (NCT02494895)].
Conclusions
DAPT has been extensively studied for more than 20 years, opening opportunities for a thorough and evidence based treatment selection. The interpretation of the current evidence suggest that, as a general concept, longer DAPT duration is associated with a reduction of non-fatal ischemic events, but in turn increases major bleeding at a similar extent. For this reason DAPT duration should be individualized on a single patient basis, taking into account the baseline ischemic and bleeding risk status. As recommended by international guidelines clinical presentation and bleeding risk status are the main drivers of DAPT duration, together with several other factors that can further refine the risk assessment towards the maximization of the net clinical benefit.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Population Division. Department of Economic and Social Affairs. United Nations. Revision of World Population Prospects, 2015.
- Costa F, Windecker S, Valgimigli M. Dual Antiplatelet Therapy Duration: Reconciling the Inconsistencies. Drugs 2017;77:1733-54. [Crossref] [PubMed]
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Valgimigli M, Costa F, Lokhnygina Y, et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J 2017;38:804-10. [PubMed]
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213-60. [Crossref] [PubMed]
- Valgimigli M, Ariotti S, Costa F. Duration of dual antiplatelet therapy after drug-eluting stent implantation: will we ever reach a consensus? Eur Heart J 2015;36:1219-22. [Crossref] [PubMed]
- Collet JP, Cuisset T, Range G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100-9. [Crossref] [PubMed]
- Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013;310:2510-22. [PubMed]
- Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60:1340-8. [Crossref] [PubMed]
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012;125:505-13. [Crossref] [PubMed]
- Han Y, Xu B, Xu K, et al. Six Versus 12 Months of Dual Antiplatelet Therapy After Implantation of Biodegradable Polymer Sirolimus-Eluting Stent: Randomized Substudy of the I-LOVE-IT 2 Trial. Circ Cardiovasc Interv 2016;9. [Crossref] [PubMed]
- Schulz-Schüpke S, Byrne RA, Ten Berg JM, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015;36:1252-63. [Crossref] [PubMed]
- Hong SJ, Shin DH, Kim JS, et al. 6-Month Versus 12-Month Dual-Antiplatelet Therapy Following Long Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JACC Cardiovasc Interv 2016;9:1438-46. [Crossref] [PubMed]
- Lee BK, Kim JS, Lee OH, et al. Safety of six-month dual antiplatelet therapy after second-generation drug-eluting stent implantation: OPTIMA-C Randomised Clinical Trial and OCT Substudy. EuroIntervention 2018;13:1923-30. [Crossref] [PubMed]
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014;64:2086-97. [Crossref] [PubMed]
- Hahn JY, Song YB, Oh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet 2018;391:1274-84. [Crossref] [PubMed]
- Gilard M, Barragan P, Noryani AA, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol 2015;65:777-86. [Crossref] [PubMed]
- Nakamura MI, Ako R, Shinke J, et al. 6 months versus 18 months dual antiplatelet treatment for patients underwent bioabsorbable polymer and abluminal coated DES deployment: NIPPON randomized study. European Society of Cardiology Scientific Session, Rome 2016.
- Valgimigli M, Campo G, Monti M, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125:2015-26. [Crossref] [PubMed]
- Collet JP, Silvain J, Barthelemy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014;384:1577-85. [Crossref] [PubMed]
- Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155-66. [Crossref] [PubMed]
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014;129:304-12. [Crossref] [PubMed]
- Helft G, Steg PG, Le Feuvre C, et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 2016;37:365-74. [PubMed]
- Authors/Task Force m, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619.
- Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI), Wijns W, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55. [Crossref] [PubMed]
- Costa F, Ariotti S, Valgimigli M, et al. Perspectives on the 2014 ESC/EACTS Guidelines on Myocardial Revascularization: Fifty Years of Revascularization: Where Are We and Where Are We Heading? J Cardiovasc Transl Res 2015;8:211-20. [Crossref] [PubMed]
- Nakamura M, Iijima R, Ako J, et al. Dual Antiplatelet Therapy for 6 Versus 18 Months After Biodegradable Polymer Drug-Eluting Stent Implantation. JACC Cardiovasc Interv 2017;10:1189-98. [Crossref] [PubMed]
- Ariotti S, Costa F, Valgimigli M. Coronary stent selection and optimal course of dual antiplatelet therapy in patients at high bleeding or thrombotic risk: navigating between limited evidence and clinical concerns. Curr Opin Cardiol 2015;30:325-32. [PubMed]
- Ariotti S, Adamo M, Costa F, et al. Is Bare-Metal Stent Implantation Still Justifiable in High Bleeding Risk Patients Undergoing Percutaneous Coronary Intervention?: A Pre-Specified Analysis From the ZEUS Trial. JACC Cardiovasc Interv 2016;9:426-36. [Crossref] [PubMed]
- Urban P, Meredith IT, Abizaid A, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med 2015;373:2038-47. [Crossref] [PubMed]
- Lee SY, Kim JS, Yoon HJ, et al. Early Strut Coverage in Patients Receiving Drug-Eluting Stents and its Implications for Dual Antiplatelet Therapy: A Randomized Trial. JACC Cardiovasc Imaging 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Navarese EP, Andreotti F, Schulze V, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ 2015;350:h1618. [Crossref] [PubMed]
- Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015;385:2371-82. [Crossref] [PubMed]
- Bittl JA, Baber U, Bradley SM, et al. Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1116-39. [Crossref] [PubMed]
- Costa F, Valgimigli M. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N Engl J Med 2015;373:1271-2. [Crossref] [PubMed]
- US Food and Drug Administration. FDA Drug Safety Communication: FDA review finds long-term treatment with blood-thinning medicine Plavix (clopidogrel) does not change risk of death, 2015.
- Mauri L, Elmariah S, Yeh RW, et al. Causes of late mortality with dual antiplatelet therapy after coronary stents. Eur Heart J 2016;37:378-85. [PubMed]
- Yeh RW, Kereiakes DJ, Steg PG, et al. Benefits and Risks of Extended Duration Dual Antiplatelet Therapy After PCI in Patients With and Without Acute Myocardial Infarction. J Am Coll Cardiol 2015;65:2211-21. [Crossref] [PubMed]
- Costa F, Vranckx P, Leonardi S, et al. Impact of clinical presentation on ischaemic and bleeding outcomes in patients receiving 6- or 24-month duration of dual-antiplatelet therapy after stent implantation: a pre-specified analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia) trial. Eur Heart J 2015;36:1242-51. [Crossref] [PubMed]
- Crimi G, Leonardi S, Costa F, et al. Role of stent type and of duration of dual antiplatelet therapy in patients with chronic kidney disease undergoing percutaneous coronary interventions. Is bare metal stent implantation still a justifiable choice? A post-hoc analysis of the all comer PRODIGY trial. Int J Cardiol 2016;212:110-7. [Crossref] [PubMed]
- Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J 2016;174:95-102. [Crossref] [PubMed]
- Adamo M, Costa F, Vranckx P, et al. Does smoking habit affect the randomized comparison of 6 versus 24-month dual antiplatelet therapy duration? Insights from the PRODIGY trial. Int J Cardiol 2015;190:242-5. [Crossref] [PubMed]
- Costa F, Adamo M, Ariotti S, et al. Left main or proximal left anterior descending coronary artery disease location identifies high-risk patients deriving potentially greater benefit from prolonged dual antiplatelet therapy duration. EuroIntervention 2016;11:e1222-30. [Crossref] [PubMed]
- Costa F, Brugaletta S. Antithrombotic Therapy in Acute Coronary Syndrome: Striking a Happy Medium. Rev Esp Cardiol (Engl Ed) 2018;71:782-6. [Crossref] [PubMed]
- Costa F, Valgimigli M. Impact of Clinical Presentation on Dual Antiplatelet Therapy Duration: Let's Re-Evaluate Our Priorities. J Am Coll Cardiol 2015;66:1203-4. [Crossref] [PubMed]
- Udell JA, Bonaca MP, Collet JP, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J 2016;37:390-9. [PubMed]
- Costa F, Adamo M, Ariotti S, et al. Impact of greater than 12-month dual antiplatelet therapy duration on mortality: Drug-specific or a class-effect? A meta-analysis. Int J Cardiol 2015;201:179-81. [Crossref] [PubMed]
- Andó G, Costa F. Bleeding risk stratification in acute coronary syndromes. Is it still valid in the era of the radial approach? Postepy Kardiol Interwencyjnej 2015;11:170-3. [Crossref] [PubMed]
- Costa F, Tijssen JG, Ariotti S, et al. Incremental Value of the CRUSADE, ACUITY, and HAS-BLED Risk Scores for the Prediction of Hemorrhagic Events After Coronary Stent Implantation in Patients Undergoing Long or Short Duration of Dual Antiplatelet Therapy. J Am Heart Assoc 2015;4. [Crossref] [PubMed]
- Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA 2016;315:1735-49. [Crossref] [PubMed]
- Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025-34. [Crossref] [PubMed]
- Armstrong EJ, Sab S, Singh GD, et al. Predictors and outcomes of recurrent stent thrombosis: results from a multicenter registry. JACC Cardiovasc Interv 2014;7:1105-13. [Crossref] [PubMed]
- Valgimigli M, Garcia Garcia H, Vrijens B, et al. Standardized classification and framework for reporting, interpreting, and analysing medication non-adherence in cardiovascular clinical trials: a consensus report from the Non-adherence Academic Research Consortium (NARC). Eur Heart J 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013;382:1714-22. [Crossref] [PubMed]
- European Stroke Organisation, Tendera M, Aboyans V, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851-906. [Crossref] [PubMed]
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e686-e725. [PubMed]
- Cacoub PP, Bhatt DL, Steg PG, et al. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J 2009;30:192-201. [Crossref] [PubMed]
- Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for Prevention of Ischemic Events After Myocardial Infarction in Patients With Peripheral Artery Disease. J Am Coll Cardiol 2016;67:2719-28. [Crossref] [PubMed]
- Franzone A, Piccolo R, Gargiulo G, et al. Prolonged vs Short Duration of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients With or Without Peripheral Arterial Disease: A Subgroup Analysis of the PRODIGY Randomized Clinical Trial. JAMA Cardiol 2016;1:795-803. [Crossref] [PubMed]
- Fiedler KA, Maeng M, Mehilli J, et al. Duration of Triple Therapy in Patients Requiring Oral Anticoagulation After Drug-Eluting Stent Implantation: The ISAR-TRIPLE Trial. J Am Coll Cardiol 2015;65:1619-29. [Crossref] [PubMed]
- Valgimigli M, Costa F, Byrne R, et al. Dual antiplatelet therapy duration after coronary stenting in clinical practice: results of an EAPCI survey. EuroIntervention 2015;11:68-74. [Crossref] [PubMed]
- Collet JP, Roffi M, Byrne RA, et al. Case-based implementation of the 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease. Eur Heart J 2018;39:e1-e33. [Crossref] [PubMed]
- Giustino G, Chieffo A, Palmerini T, et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J Am Coll Cardiol 2016;68:1851-64. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2016;387:537-44. [Crossref] [PubMed]
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med 2017;376:2319-28. [Crossref] [PubMed]
- Capodanno D, Angiolillo DJ. Antiplatelet Therapy After Implantation of Bioresorbable Vascular Scaffolds: A Review of the Published Data, Practical Recommendations, and Future Directions. JACC Cardiovasc Interv 2017;10:425-37. [Crossref] [PubMed]
- Abizaid A, Costa RA, Schofer J, et al. Serial Multimodality Imaging and 2-Year Clinical Outcomes of the Novel DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold System for the Treatment of Single De Novo Coronary Lesions. JACC Cardiovasc Interv 2016;9:565-74. [Crossref] [PubMed]
- Haude M, Ince H, Abizaid A, et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2016;387:31-9. [Crossref] [PubMed]
- Waksman R, Lipinski MJ, Acampado E, et al. Comparison of Acute Thrombogenicity for Metallic and Polymeric Bioabsorbable Scaffolds: Magmaris Versus Absorb in a Porcine Arteriovenous Shunt Model. Circ Cardiovasc Interv 2017.10. [PubMed]
- Lipinski MJ, Acampado E, Cheng Q, et al. Comparison of Acute Thrombogenicity for Magnesium versus Stainless Steel Stents in a Porcine Arteriovenous Shunt Model. EuroIntervention 2018. [Epub ahead of print]. [PubMed]
- Haude M, Erbel R, Erne P, et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013;381:836-44. [Crossref] [PubMed]
- Haude M, Ince H, Kische S, et al. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention 2017;13:432-9. [Crossref] [PubMed]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348:1329-39. [Crossref] [PubMed]
- Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N Engl J Med 2016;375:35-43. [Crossref] [PubMed]
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331-7. [Crossref] [PubMed]
- Gargiulo G, Windecker S, Vranckx P, et al. A Critical Appraisal of Aspirin in Secondary Prevention: Is Less More? Circulation 2016;134:1881-906. [Crossref] [PubMed]


