
Associations of ABCG1-mediated cholesterol efflux capacity with coronary artery lipid content assessed by near-infrared spectroscopy
Introduction
Epidemiological studies have found inverse associations between high-density lipoprotein cholesterol (HDL-C) and atherosclerotic cardiovascular disease (ASCVD) risk (1). Nonetheless, HDL-C-raising clinical trials have largely shown no benefit on cardiovascular outcome (1). Recent population-based studies have reported that extremely high HDL-C levels were associated with high cardiovascular mortality (2). Furthermore, genetic evidence has also demonstrated that changes in alleles that lead to high HDL-C levels do not associate with the risk of myocardial infarction (3). These inconsistent findings have led to a shift in focus from HDL-C concentrations to HDL functionality as a novel target for preventing ASCVD.
HDL is thought to be atheroprotective, in part, via its ability to efflux cholesterol from lipid-laden macrophages in atherosclerotic plaques (1). Recently, the YELLOW (Reduction in Yellow Plaque by Aggressive Lipid-Lowering Therapy) II trial demonstrated an association between fibrous cap thickness and HDL-mediated cholesterol efflux capacity (CEC), but only the ATP-binding cassette transporter A1 (ABCA1) pathway was assessed (4). HDL-mediated cholesterol efflux occurs via multiple pathways including aqueous diffusion and efflux mediated by three cell-surface proteins: ABCA1, the ATP-binding cassette transporter G1 (ABCG1), and the scavenger receptor B type I (SR-BI). The impact of each individual efflux pathway on coronary plaques has never been investigated.
The importance of coronary lipid-rich plaques has been established in relation to cardiovascular events. Near-infrared spectroscopy (NIRS), a catheter-based imaging modality, has the capability to detect lipid burden in the coronary artery wall. In the current study, we sought to examine the association of ABCA1, ABCG1 and SR-B1-mediated CEC with coronary artery lipid content in patients who underwent NIRS imaging.
Methods
Study population
Seventy four adult patients, who had blood samples collected prior to diagnostic coronary angiography for acute coronary syndrome (ACS) or stable ischemic symptoms at the Royal Adelaide Hospital (RAH) from April 2013 to July 2016, were enrolled. The target vessel to be imaged was selected according to operators’ discretion. The following patients were excluded from the current study: patients who had moderate or severe congestive heart failure (New York Heart Association Class III or IV), those who had significant valvular heart disease or left ventricular systolic dysfunction (left ventricular ejection fraction <35%), patients who had any history of coronary artery spasm, renal impairment (creatinine clearance <60 mL/min), and patients with acute/recent ST-elevation myocardial infarction or blood pressure at rest ≥180 mmHg. This study was approved by the institutional review boards of the RAH (ACTRN12612000594820), and all patients provided written informed consent.
NIRS imaging and its analysis
NIRS imaging was conducted to evaluate coronary lipid burden as previously described (5). Briefly, after intracoronary administration of nitroglycerine (100–200 µg), a coronary catheter-based imaging system: the Lipiscan Coronary Imaging System (InfraReDx, Burlington, MA, USA) was advanced into the distal site of the target coronary artery, and automatic pullback was performed (0.5 mm/s) until reaching the aorta. NIRS Imaging was performed within the same coronary artery at baseline and at follow-up. Treatment during the follow-up period was at the discretion of the treating physician. Acquired NIRS images were analyzed offline using LipiScan analyzer software (LipiScan, InfraReDx, Burlington, MA, USA) by an independent imaging cardiologist as previously described (5,6).
Measurement of laboratory parameters
Fasting blood samples at baseline were centrifuged and stored in aliquots at −80 °C until required. Serum lipid levels were measured by the routine laboratory measurements using enzymatic methods.
Assessment of HDL-mediated CEC
We measured ABCA1, ABCG1, and SR-BI-mediated CEC using apolipoprotein B (apoB)-depleted serum. ApoB-containing lipoproteins were depleted with polyethylene glycol (Sigma, St. Louis, MO, USA) as previously described (7). ABCA1, ABCG1, and SR-BI-mediated CEC were determined using RAW 264.7 macrophages, Chinese hamster ovary (CHO)/CHO-K1 cells, and Fu5AH rat hepatoma cells, respectively (8). RAW 264.7 macrophages were incubated with cAMP to induce ABCA1 expression (8,9). CHO cells were transfected to express human ABCG1 constitutively, kindly provided by Dr. W. Jessup (University of Sydney) (8). The rat hepatoma cell line Fu5AH is used for SR-BI-mediated cholesterol efflux as it expresses high levels of SR-BI (8,10). In brief, cells were plated and labelled with BODIPY-cholesterol (Avanti Polar Lipids, Alabaster, AL, USA) for the ABCA1-mediated assay or [3H] cholesterol for the ABCG1/SR-BI-mediated assays. The assay using BODIPY-cholesterol has been reported to reflect more ABCA1-specifc efflux than that with [3H] cholesterol (7). Cells were incubated with apoB-depleted serum for 4 h. The fluorescence intensity (FI) of the cell media and cell lysates was measured at Ex/Em 485/535 nm using a Perkin Elmer Victor 3 plate reader (Perkin-Elmer, Wellesley, MA, USA) in the ABCA1-mediated efflux assay. Liquid scintigraphy (Perkin Elmer Analytical Sciences, Wellesley, MA, USA) was conducted for the quantification
ABCA1-mediated CEC was calculated as the difference between the percent efflux in cAMP treated cells and that of cAMP non-treated cells. ABCG1-mediated CEC was calculated as the difference between the percent efflux in CHO cells and that of CHO-K1 cells. SR-BI-mediated CEC was calculated as the difference in the percent efflux between the presence and absence of the acceptor. All assays were performed at least in duplicate. To adjust for the inter-assay variation across plates and measuring date, a pooled serum from eight normolipidemic volunteers was included on each plate.
Statistical analysis
Given the small sample size, we performed all analyses using non-parametric statistics, except for the multivariable analyses, where we used linear regression models, after checking for the assumptions. Continuous variables were expressed as median (interquartile range: IQR), and compared using Kolmogorov-Smirnov test. Categorical variables were expressed as frequency and percentage, and compared using Fisher’s exact test. Relationships of HDL-mediated CEC with other variables, including lipid core burden index (LCBI), were assessed by Spearman’s correlation coefficients. Multivariable linear regression analyses were performed to test the associations between baseline variables and change in LCBI. Baseline LCBI was included as a priori, then variables with P value less than 0.20 after adjusting for baseline LCBI were included in the multivariable model. Coronary lipid burden has been demonstrated to be relevant to both clinical presentation of coronary artery disease (CAD); therefore, we examined the interaction effect between clinical presentation of CAD and baseline ABCG1-mediated CEC on change in LCBI, considering our small sample size, the following insignificant interaction effect of statins and baseline ABCG1-mediated CEC on change in LCBI, and the undermentioned consistent associations of coronary lipid burden using NIRS imaging with clinical presentation of CAD (11-13), but not consistent those with statins (5,14,15). A two-sided P value of less than 0.05 was considered significant. All statistical analyses were conducted using Stata 14.2 version (StataCorp., College Station, TX, USA).
Results
Patient characteristics
The characteristics of baseline patients (n=74) are summarized in Table 1. Median age was 60 years of which 27% were female and 32% of the patients presented with ACS. Statins were used by 77% of patients. The frequency of ACS was similar between the two groups of ≤ median versus > median CEC (ABCA1: P=0.21, ABCG1: P=0.46, SR-BI: P=1.00). Forty seven of the 74 patients (64%) completed coronary angiogram and NIRS imaging at follow-up. The median follow-up period was 11.6 months. No percutaneous coronary intervention was performed in the target coronary artery during the follow-up period. Patient characteristics of the 47 patients (subgroup) were generally comparable with the whole cohort (Table S1). In addition, we compared patient characteristics between patients undergoing follow-up NIRS imaging and those without the procedure to test the selection bias (Table S2). No differences in these parameters were observed except for the presence of diabetes. Patients receiving follow-up NIRS showed a higher prevalence of diabetes compared to those without the procedure (30% vs. 7%; P=0.04). However, in the current study, diabetes was not associated with coronary lipid burden and baseline HDL-mediated CEC (Table S3).
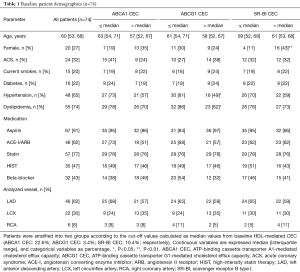
Full table
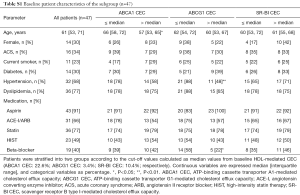
Full table
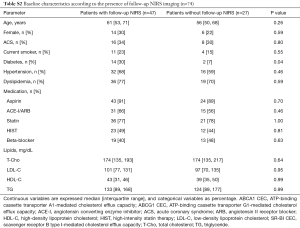
Full table

Full table
Lipid parameters and HDL-mediated CEC at baseline
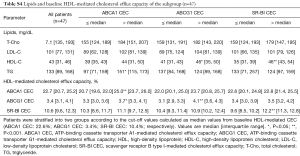
Each efflux pathway involved in HDL-mediated CEC at baseline is shown in Table 2. The assay variabilities were as follows: intra-, inter-assay % coefficient of variability; ABCA1: 6.1%, 13.5%; ABCG1: 5.5%, 12.1%; SR-BI: 5.1%, 10.0%, respectively. Triglyceride levels associated with baseline ABCA1-mediated CEC {97 [71, 159] vs. 151 [115, 177] mg/dL; P=0.02}, whereas HDL-C level associated with both ABCG1 and SR-BI-mediated CEC at baseline {ABCG1: 35 [31, 43] vs. 46 [39, 50] mg/dL; P=0.01, SR-BI: 35 [31, 39] vs. 46 [43, 54] mg/dL; P<0.001}. Lipid parameters and HDL-mediated CEC of the 47 patients were generally comparable with the whole cohort (Table S4).
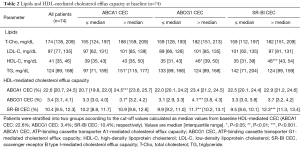
Full table
Full table
Effects of coronary risk factors and medications on baseline LCBI
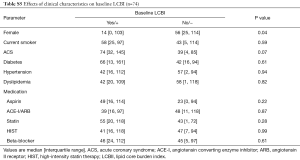
Baseline LCBI values ranged from 0 to 277, with a median [IQR] of 45 [16, 109] (data not shown). Effects of coronary risk factors and medications on baseline LCBI are summarized in Table S5. Males showed a higher baseline LCBI {56 [25, 114] vs. 14 [0, 103]; P=0.04}. A trend towards a higher baseline LCBI was observed in patients with ACS, although this did not meet statistical significance (P=0.07). Of interest, baseline LCBI was similar notwithstanding concomitant use of statin (P=0.28) or high-intensity statin therapy (HIST, P=0.99).
Full table
The impact of degree of baseline HDL-mediated CEC on coronary lipid burden
Patients with a greater baseline ABCG1-mediated CEC showed a greater baseline LCBI {32 [5, 66] vs. 74 [20, 128]; P=0.04, Figure 1}. In contrast, baseline LCBI was similar according to baseline ABCA1 or SR-BI-mediated CEC (ABCA1: P=0.52, SR-BI: P=0.72). Consistent with the whole cohort, patients with baseline ABCG1-mediated CEC >median showed a larger baseline LCBI in the subgroup {31 [3, 91] vs. 84 [47, 190]; P=0.02, Figure S1}. Furthermore, there was a significant difference of change in LCBI between lower and higher groups of baseline ABCG1-mediated CEC {−3 [−16, 0] vs. −30 [−89, 0]; P=0.048, Figure 2}, whereas baseline ABCA1 or SR-BI-mediated CEC were not related to change in LCBI (ABCA1: P=0.85, SR-BI: P=0.39, Figure 2).



Associations of baseline variables with change in LCBI
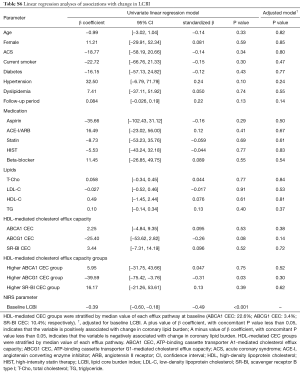
Baseline LCBI (standardized β=−0.49, P<0.001) and higher baseline ABCG1-mediated CEC group (standardized β=−0.31, P=0.03) were significantly associated with change in LCBI (Table S6). Effect of ABCG1-mediated CEC on change in LCBI using multivariable model is shown in Table 3. Baseline LCBI was negatively associated with change in LCBI (standardized β=−0.31, P=0.02). The clinical presentation of CAD was significantly associated with change in LCBI (P=0.003). The interaction effect between clinical presentation of CAD and baseline ABCG1-mediated CEC was significant (P<0.001): the baseline ABCG1-mediated CEC was inversely associated with change in LCBI in ACS patients (standardized β=−0.79, P=0.003), but not in those with stable ischemic symptoms (standardized β=0.11, P=0.52). This multivariable model explained 43% of the variation in change in LCBI.
Full table

Full table
Effect of patient characteristics’ differences between stable ischemic symptoms and ACS
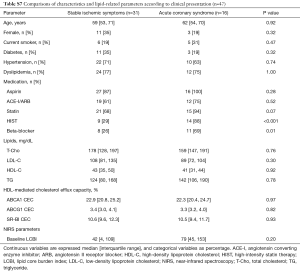
ACS patients showed higher frequencies of HIST and beta-blocker whereas any other coronary risk factors were not different between stable ischemic symptoms and ACS groups (HIST: 29% vs. 88%; P<0.001, beta-blocker: 26% vs. 69%; P=0.01, respectively, Table S7). The frequency of statin was also more likely to be lower in patients with stable ischemic symptoms (68% vs. 94%; P=0.07). On the other hand, no differences in each efflux pathway among this two different clinical presentation groups were observed (ABCA1: P=0.97, ABCG1: P=0.82, SR-BI: P=0.93, respectively). However, there was no significant interaction effect between the differences in the aforementioned medications and clinical presentation of CAD on change in LCBI (statin: P=0.78, HIST: P=0.47, beta-blocker: P=0.38, respectively, data not shown).
Full table
Discussion
The role of HDL in ASCVD is not fully understood, although previous studies have reported inverse associations between HDL-mediated CEC and ASCVD (1). In the current study, CAD patients with a higher baseline ABCG1-mediated CEC showed greater reduction in coronary artery lipid content. Multivariable analyses suggest a potential role of ABCG1-mediated CEC in the formation of coronary artery atheroma, especially in CAD patients with more unstable clinical presentation.
A validation study with histology showed that NIRS is a superior tool for identifying lipid-rich atherosclerotic plaques with a sensitivity and a specificity for the lipid pool of 90% and 93%, respectively (16). While NIRS-derived LCBI has been reported to associate with cardiovascular events (12,17), previous studies have demonstrated inconsistent associations between statin therapy and NIRS-derived LCBI. The YELLOW trial showed the beneficial effect of intensive statin therapy on reducing LCBI (14). In contrast, the IBIS-3 (Integrated Biomarker and Imaging Study 3) study failed to demonstrate a significant reduction in LCBI under intensive statin therapy for 1 year (15). Our previous study also did not show the beneficial effect of statins, including HIST, on regression of LCBI (5). The discrepancy between the results in effects of lowering LDL-C on LCBI suggests the need for further investigation.
While HDL has atheroprotective properties, acute phase response in ACS has been reported to unfavorably remodel HDL composition and function (18). However, the association of clinical presentation of CAD with the specific pathway of HDL-mediated CEC remains discordant (18,19). In addition, little is still known about the relationship of HDL functionality with coronary plaques. The ERASE (Effect of rHDL on Atherosclerosis-Safety and Efficacy) study demonstrated that CSL-111 (reconstituted HDL) improved the plaque characterization index compared to placebo (20). The aforementioned association in the YELLOW II trial was assessed using an ABCA1-upregulated cellular system (4) whereas, in this study, the ABCG1 pathway was associated with regression of the coronary lipid core in ACS patients although the sample size is small. Our findings could support that HDL still has potential to improve plaque composition as demonstrated in the ERASE study.
An increase in the cellular content of oxysterols, including 7-ketocholestrol (7-KC), is shown to induce the transcription of ABCA1 and ABCG1 by activating LXR (21-23). In contrast, 7-KC, which is the major oxysterol present in human atherosclerotic plaques in cholesterol-loaded macrophages, suppressed the transcription of SR-BI (24). In addition, ABCG1 overexpression preserved endothelial cell function in mice exposed to a high-cholesterol diet by promoting the efflux of 7-KC from endothelial cells (25). Indeed, the association between endothelial dysfunction and a larger NIRS-derived lipid content was observed in patients with early atherosclerosis (26). Furthermore, ABCG1 was reported to promote efflux of cholesterol and 7-KC from macrophages, whereas ABCA1 did not (25,27). Cholesterol enrichment enhanced cholesterol efflux via ABCG1, increasing the contribution of ABCG1 to total efflux from 10% to 25% (28). These studies could support our observed beneficial effect of ABCG1 in ACS patients, with a trend towards a higher baseline LCBI for ACS. Consistent with our findings, a previous study demonstrated that regression of lipid-rich plaques was observed mainly in plaques with a larger coronary lipid burden at baseline (29). We observed an association between ABCG1-mediated cholesterol efflux and the regression of coronary lipid burden in ACS patients, but not for patients with stable ischemic symptoms, despite the small number of subjects in this study. Our findings indicate that ABCG1 may play a role in the regression of high-risk populations by changing plaque characterization.
Several caveats should be noted. This is a single center study involving a small sample size. Also a significant number (36%) of the baseline subjects failed to complete the serial NIRS imaging. Blood samples were only available at baseline. Accordingly, we were not able to evaluate the relationship between the change in CEC and LCBI. In addition, although there were no significant associations between the follow-up period and the change in LCBI, it remains unclear whether the heterogeneity of the follow-up period has affected our findings. The primary finding suggests a potential relationship between the ABCG1 pathway and changes in plaque lipid content. Given the possibility of being underpowered, this study cannot exclude the involvements of ABCA1 and SR-BI pathway in regression of coronary lipid burden. Thus future larger prospective studies are needed to elucidate the association of HDL-mediated CEC with regression of coronary lipid burden assessed by NIRS imaging. Unlike ABCA1, the relationship between ABCG1 mediated CEC and cardiovascular outcomes remains unknown.
Although the frequency of diabetes was significantly different between patients with and without the follow-up IVUS, the potential effects of diabetes on our findings is uncertain. All patients had presented for a clinically indicated coronary angiogram. As a result, it is unknown whether the findings would translate to an asymptomatic cohort. Circulating inflammatory markers were not measured, and it is unknown how these may have influenced the findings.
In conclusion, the ABCG1 pathway was associated with the regression of coronary lipid burden. The beneficial effects of ABCG1-mediated cholesterol efflux were observed in patients with more unstable clinical presentation. Our findings imply the need for further studies to elucidate the role of ABCG1 pathway in atherogenesis.
Acknowledgments
We are grateful to Dr. Wendy Jessup (University of Sydney) for her kind gift of cells. This work was supported by the Medicines Company.
Footnote
Conflicts of Interest: K Takata reports grants from the Clinical Research Promotion Foundation. PJ Psaltis has received research funding from the National Heart Foundation of Australia, National Health and Medical Research Council of Australia and Abbott Vascular, and speaker honoraria from AstraZeneca, Merck and Bayer. SJ Nicholls has received speaking honoraria from Amgen, AstraZeneca, Pfizer, Merck Schering-Plough and Takeda, consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, CSL Behring, Eli Lilly, Merck Schering-Plough, NovoNordisk, Omthera, Pfizer, Resverlogix, Roche, Sanofi-Regeneron and Takeda, and research support from Amgen, AstraZeneca, Athernova, Cerenis, EliLilly, InfraReDx, Novartis, Resverlogix, and Sanofi-Regeneron. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review boards of the Royal Adelaide Hospital (ACTRN12612000594820), and all patients provided written informed consent.
References
- Karathanasis SK, Freeman LA, Gordon SM, et al. The Changing Face of HDL and the Best Way to Measure It. Clin Chem 2017;63:196-210. [Crossref] [PubMed]
- Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478-86. [Crossref] [PubMed]
- Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012;380:572-80. [Crossref] [PubMed]
- Kini AS, Vengrenyuk Y, Shameer K, et al. Intracoronary Imaging, Cholesterol Efflux, and Transcriptomes After Intensive Statin Treatment: The YELLOW II Study. J Am Coll Cardiol 2017;69:628-40. [Crossref] [PubMed]
- Honda S, Sidharta SL, Shishikura D, et al. High-density lipoprotein cholesterol associated with change in coronary plaque lipid burden assessed by near infrared spectroscopy. Atherosclerosis 2017;265:110-16. [Crossref] [PubMed]
- Goldstein JA, Maini B, Dixon SR, et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv 2011;4:429-37. [Crossref] [PubMed]
- Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, et al. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res 2011;52:2332-40. [Crossref] [PubMed]
- Talbot CPJ, Plat J, Ritsch A, Mensink RP. Determinants of cholesterol efflux capacity in humans. Prog Lipid Res 2018;69:21-32. [Crossref] [PubMed]
- Li XM, Tang WH, Mosior MK, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol 2013;33:1696-705. [Crossref] [PubMed]
- Rothblat GH, de la Llera-Moya M, Favari E, et al. Cellular cholesterol flux studies: methodological considerations. Atherosclerosis 2002;163:1-8. [Crossref] [PubMed]
- Madder RD, Smith JL, Dixon SR, et al. Composition of target lesions by near-infrared spectroscopy in patients with acute coronary syndrome versus stable angina. Circ Cardiovasc Interv 2012;5:55-61. [Crossref] [PubMed]
- Madder RD, Goldstein JA, Madden SP, et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2013;6:838-46. [Crossref] [PubMed]
- Madder RD, Wohns DH, Muller JE. Detection by intracoronary near-infrared spectroscopy of lipid core plaque at culprit sites in survivors of cardiac arrest. J Invasive Cardiol 2014;26:78-9. [PubMed]
- Kini AS, Baber U, Kovacic JC, et al. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol 2013;62:21-9. [Crossref] [PubMed]
- Oemrawsingh RM, Garcia-Garcia HM, van Geuns RJ, et al. Integrated Biomarker and Imaging Study 3 (IBIS-3) to assess the ability of rosuvastatin to decrease necrotic core in coronary arteries. EuroIntervention 2016;12:734-9. [Crossref] [PubMed]
- Moreno PR, Lodder RA, Purushothaman KR, et al. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation 2002;105:923-7. [Crossref] [PubMed]
- Schuurman AS, Vroegindewey M, Kardys I, et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur Heart J 2018;39:295-302. [Crossref] [PubMed]
- Alwaili K, Bailey D, Awan Z, et al. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim Biophys Acta 2012;1821:405-15. [Crossref] [PubMed]
- Hafiane A, Jabor B, Ruel I, Ling J, Genest J. High-density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. The American journal of cardiology. 2014;113:249-55. [Crossref] [PubMed]
- Tardif JC, Gregoire J, L'Allier PL, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 2007;297:1675-82. [Crossref] [PubMed]
- Zhou X, He W, Huang Z, et al. Genetic deletion of low density lipoprotein receptor impairs sterol-induced mouse macrophage ABCA1 expression. A new SREBP1-dependent mechanism. J Biol Chem 2008;283:2129-38. [Crossref] [PubMed]
- Venkateswaran A, Repa JJ, Lobaccaro JM, et al. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J Biol Chem 2000;275:14700-7. [Crossref] [PubMed]
- Fu X, Menke JG, Chen Y, et al. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem 2001;276:38378-87. [Crossref] [PubMed]
- Han J, Nicholson AC, Zhou X, et al. Oxidized low density lipoprotein decreases macrophage expression of scavenger receptor B-I. J Biol Chem 2001;276:16567-72. [Crossref] [PubMed]
- Terasaka N, Yu S, Yvan-Charvet L, et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest 2008;118:3701-13. [Crossref] [PubMed]
- Choi BJ, Prasad A, Gulati R, et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur Heart J 2013;34:2047-54. [Crossref] [PubMed]
- Terasaka N, Wang N, Yvan-Charvet L, et al. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci U S A 2007;104:15093-8. [Crossref] [PubMed]
- Adorni MP, Zimetti F, Billheimer JT, et al. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 2007;48:2453-62. [Crossref] [PubMed]
- Dohi T, Maehara A, Moreno PR, et al. The relationship among extent of lipid-rich plaque, lesion characteristics, and plaque progression/regression in patients with coronary artery disease: a serial near-infrared spectroscopy and intravascular ultrasound study. Eur Heart J Cardiovasc Imaging 2015;16:81-7. [Crossref] [PubMed]


