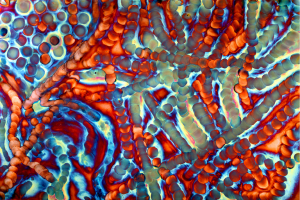
Chemistry on copper
Cheryl Safren manipulates chemicals in order to create images on copper panels (Figures 1-9). Some of the chemical reactions create textures coarse and grainy while others produce shiny and gossamer textures. It is a low-tech process employing thin kebab sticks, wooden styluses, Q-tips, cotton balls and rags when applying chemicals in liquid form, torches when using gases and a mortar and pestle for grinding chemicals in solid form.



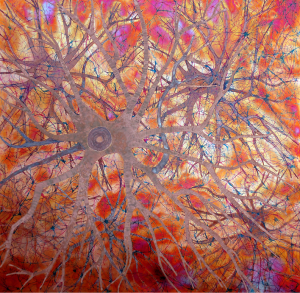
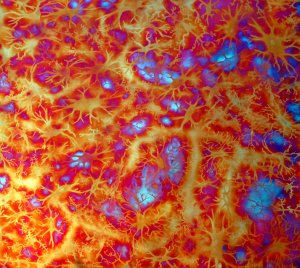
Some of the colors generated on these copper panels are known as interference colors and are produced by a transparent oxide film deposited on the metal surface. The colors develop when part of the light striking the oxide surface reflects and part passes through the film before reflecting off the metal below. When the delayed light reappears and combines with the surface light waves, they may either reinforce or cancel each other, generating a specific hue. The thickness of the oxide film dictates the color.
When the light hits the copper surface at any oblique angle the colors become vibrant and saturated. Shifting light on the metal surface and viewer movement are kinesthetic forces that alter our perception allowing us to discover something new each time we view the work.
About the artist
Cheryl’s artwork has been commissioned for many public art spaces around the country. Her work has also appeared in many magazines including Hyle, Chemical Engineering News and National Geographic Science.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.