
Discordant clinical presentation and pathophysiology: insights with cardiac magnetic resonance
Case presentation
A 23-year-old man was admitted to our hospital with hemodynamically stable wide-complex ventricular tachycardia (VT) for electrophysiological examination and potential ablation therapy. Three years prior to this admission, the patient had presented with palpitation and loss of consciousness while playing basketball. An electrocardiogram showed wide-complex VT and Holter monitoring identified 4,814 premature ventricular beat/24 h with 2 different morphologies. However, electrophysiological examination failed to induce the ventricular arrhythmias. The patient refused implantation of an implantable cardiac defibrillator and was discharged on metoprolol. Two years ago, the patient presented with chest distress and fatigue. He was diagnosed with “myocarditis” and received supportive treatment in other hospital. He continued to have episodes of chest pain and 1 week prior to admission he presented with chest pain, palpitation and syncope. He was then referred to our tertiary center.
Detailed review of available but limited medical records did not provide evidence to support or rule-out inflammatory injury of the myocardium prior to or at the time of the initial presentation with VT. Further documentation about the prior diagnosis and treatment of myocarditis was insufficient; neither imaging studies nor endomyocardial biopsy (EMB) were performed.
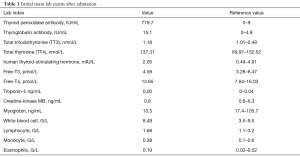
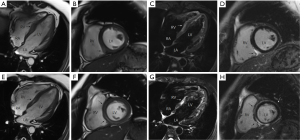
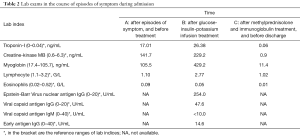
During the current admission, a chest X-ray was unremarkable. Initial labs were significant for elevated thyroid peroxidase antibody (TPOAb) of 779.7 IU/mL (reference range, 0–9 IU/mL), and thyroglobulin antibody (TgAb) of 15.1 IU/mL (reference range, 0–4.9 IU/mL), but total triiodothyronine (TT3), total thyroxine (TT4), human thyroid-stimulating hormone (hTSH), free-T3 (FT3), free-T4 (FT4) were normal, troponin-I (TNI), creatine-kinase MB (CKMB), myoglobin (MYO) and whole blood count (WBC) were in the normal range (Table 1). Ultrasound exam showed heterogeneity of thyroid parenchyma. EMB was recommended, but the patient refused. Cardiac magnetic resonance (CMR) imaging was performed and revealed mildly thickened mid-level left ventricular septum, which showed mild hyperintensity lesion on T2-weighted image (T2WI). There was enhancement in subepicardial mid-level inferoseptal wall and right ventricular insertion on late gadolinium enhancement (LGE) image. Left ventricular ejection fraction (LVEF) was 48% (Figure 1A,B,C,D). Later that evening, the patient complained about acute chest distress and nausea. He had hypotension and his blood pressure (BP) was 89/50 mmHg. His other physical examination was unremarkable. Electrocardiographic monitoring showed frequent premature ventricular beat, but no widened QRS VT. Lab exams showed that TNI, CKMB, MYO were now significantly elevated, eosinophils and lymphocyte count were normal (Table 2, Figure 1A). Glucose-insulin-potassium infusion was given, but he continued to complain about intermittent chest pain. Repeat labs showed elevated levels of TNI of 26.38 ng/mL, CKMB of 229.2 ng/mL, MYO of 429.2 ng/mL (Table 2, Figure 1B). Epstein-Barr virus (EBV) serology showed elevated viral capsid antigen (VCA) IgG (47.6 U/mL) and EBV nuclear antigen (EBNA) IgG (254.0 U/mL), but VCA IgM and early antigen (EA) IgG were in the normal range (Table 2, Figure 1B), which indicated previous virus infection. Cytomegalovirus (CMV) antibody detection showed that anti-CMV IgG was positive and anti-CMV IgM was negative. Patient continued to complain about chest distress and nausea, BP was 90/40 mmHg, heart rate of 46 bpm. Considering clinical presentation, CMR findings, and lab results, a diagnosis of myocarditis was made and intravenous methylprednisolone (200 mg/Qd) and immunoglobulin (10 mg/Qd) was given for five days. Three days after methylprednisolone and immunoglobulin stopped, TNI of 0.06 ng/mL (reference range, 0–0.04 ng/mL) were nearly normal (Table 2, Figure 1C). The patient’s symptoms resolved.
Full table
Full table
Repeated CMR after the prior CMR for 8 days, demonstrated diffuse mid and subepicardial edema of left ventricle on T2WI. Diffuse mid and subepicardial enhancement of left ventricle on LGE. Left ventricular systolic function was stable (LVEF 48%; Figure 1E,F,G,H). Discharge medications are metoprolol, coenzyme Q10 and trimetazidine. At a 12 months follow-up visit, the patient reported intermitted mild chest discomfort and labs in other hospital showed mild elevation of TNI (0.14 ng/mL, reference range, 0–0.04 ng/mL). At the latest follow-up, the patient reported no discomfort. Echocardiography was performed, no segmental wall motion abnormalities was reported, and LVEF was 51%.
Discussion
The etiology of wide-complex tachycardia includes a wide spectrum of conditions. In our patient, the normal chest X-ray made active sarcoidosis unlikely. Because of the elevated TPOAb and TgAb and heterogeneity of the thyroid parenchyma, Hashimoto’s thyroiditis was considered. We hypothesized that previous virus infection (molecular mimicry mechanism), caused both myocardial and thyroid injury. The findings of the initial CMR exam with mild edema of the ventricular septum, may reflect subacute/chronic persistent/recurrent myocardial inflammation.
We considered immune-mediated myocarditis as the most likely cause of our patient presentation. Positive viral serology just indicates the interaction of the peripheral immune system with an infectious agent, polyclonal stimulation of antibodies (IgM and IgG) may lead to incorrect diagnosis (1). Serum cardiac autoantibodies are helpful for diagnosis of immune-mediated myocarditis, which should be tested (1). Definitive diagnosis relies on EMB, which supported by the World Health Organization and scientific statements by the European Society of Cardiology (ESC) (1,2). We recommended EMB, which the patient refused.
Empiric treatment with methylprednisolone and immunoglobulin was initiated with subsequent clinical improvement and decreased serum markers. According to clinical practice and experience, immunosuppression therapy was stopped after 5 days.
Interestingly, a subsequent repeat CMR exam showed that evidence of myocardial injury was more pronounced than on the initial study. As described in the ESC position statement on diagnosis of myocarditis, CMR has an established role for non-invasive assessment of myocardial edema, inflammation and necrosis in myocarditis (1,3). The permeability of cellular membranes is increased due to the inflammatory cell injury. Initially Na+ influx causes intracellular edema, then more severe injury allows for a net efflux of water and larger molecules such as troponin into the extracellular space, eventually leading to loss of cellular functions and necrosis (4). In acute stages of myocarditis, gadolinium contrast is distributed in the widened extracellular space and necrotic myocytes. Therefore LGE imaging reflects both edema and necrosis.
In our case, after a short course of aggressive treatment, the symptom disappeared and lab results normalized, but imaging evidence of myocardial injury persisted. The discordance between clinical observations, lab results and imaging findings raises questions about how best to guide therapy. On the one hand, persistent imaging findings may reflect incomplete resolution and would support prolonged pharmacological intervention. This is relevant, because development of dilated cardiomyopathy or other long-term complications are common in myocarditis, and suboptimal therapy strategies may partially explain the poor prognosis. On the other hand, resolution/improvement of imaging findings of edema and scar may lag-behind clinical/histologic resolution, similar to persistent finding on a chest X-ray after clinical resolution of pneumonia.
The optimal therapeutic strategy in these scenarios remains unclear. The balance of immune response by the host after viral entry is a major determinant of outcome (5). Modulating the immune response to control the infection meanwhile to avoid excessive tissue damage from the inflammatory response is difficult but important. Timing of CMR exams should be coordinated with the comprehensive workup and treatment approach. For the initial diagnosis, CMR should be performed prior to EMB in patient with suspected myocarditis (1). When CMR should be repeated during or after treatment is not well defined. In fact, currently, comprehensive clinical practice guidelines specific to the treatment of myocarditis do not exist (3). Treatment varies according to clinical scenario and physician’s experience, which may partially accounted for the diversity of prognosis. Our patient reported mild recurrent symptoms at a 12 months follow-up with mildly abnormal lab results.
Myocarditis is an inflammatory disease with numerous causes and complicated pathophysiological mechanism, CMR can provide valuable information for treatment of myocarditis both for clinical care and future research, similar to the situation with pericarditis (6-8). Future clinical trials, comparing treatment guided with and without imaging will be necessary.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (NSFC-81671647, NSFC-81771787).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-48, 2648a-d.
- Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996;93:841-2. [Crossref] [PubMed]
- Pollack A, Kontorovich AR, Fuster V, et al. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat Rev Cardiol 2015;12:670-80. [Crossref] [PubMed]
- Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475-87. [Crossref] [PubMed]
- Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet 2012;379:738-47. [Crossref] [PubMed]
- Alajaji W, Xu B, Sripariwuth A, et al. Noninvasive Multimodality Imaging for the Diagnosis of Constrictive Pericarditis. Circ Cardiovasc Imaging 2018;11:e007878. [Crossref] [PubMed]
- Kumar A, Sato K, Yzeiraj E, et al. Quantitative Pericardial Delayed Hyperenhancement Informs Clinical Course in Recurrent Pericarditis. JACC Cardiovasc Imaging 2017;10:1337-46. [Crossref] [PubMed]
- Cremer PC, Tariq MU, Karwa A, et al. Quantitative assessment of pericardial delayed hyperenhancement predicts clinical improvement in patients with constrictive pericarditis treated with anti-inflammatory therapy. Circ Cardiovasc Imaging 2015. [Crossref] [PubMed]


