Current state-of-play in spontaneous coronary artery dissection
Introduction
Spontaneous coronary artery dissection (SCAD) is a non-iatrogenic, non-atherosclerotic coronary artery dissection, resulting in the formation of an intramural haematoma or false lumen in the coronary artery wall. This in turn can compress the true lumen, obstructing coronary blood flow and leading to an acute coronary syndrome (ACS). SCAD has recently become widely acknowledged as a significant cause of myocardial infarction (MI), particularly in premenopausal women. First described in 1931 (1), SCAD was first believed to be a rare cause of ACS; only being reported in small case series at autopsy. As a result, even only a few decades ago, it was believed to be a rare condition that affected peripartum women and was universally fatal (2). These conclusions have been challenged by recent landmark clinical studies and contemporary reviews (3-5). Increased awareness and diagnosis of SCAD have recently come about as a result of wider use of coronary angiography in patients presenting with ACS, a greater understanding of the angiographic variants of SCAD, the use of high resolution intravascular imaging techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), and heightened recognition of the disease through social media and scientific publications (6). Furthermore, these advances have uncovered the different characteristics of SCAD compared to atherosclerotic coronary artery disease, including risk factors, associated conditions, angiographic appearances, response to treatment and outcomes. Despite growing recognition and understanding, SCAD continues to be underdiagnosed, leading to potential mistreatment. Many important questions also remain unanswered relating to its underlying pathogenesis, including genetic, environmental, and hormonal predisposing factors, and the optimal treatment strategies to improve short and long-term outcomes and prevent recurrence. This review will provide a contemporary clinical perspective on SCAD and highlight current considerations, guidelines, and challenges in the management of this condition.
Epidemiology
Early studies speculated the incidence of SCAD to be between 0.2% and 1.1% in patients presenting for coronary angiography with ACS (7-9). This was corroborated by a large administrative US-based study that reported an incidence of 0.49% among 13,573,200 ACS presentations from 2004 to 2015 (10). These were largely based on the diagnosis of SCAD being made from the traditional angiographic appearance of a double lumen and therefore are likely to have missed more subtle angiographic presentations that mimic atherosclerotic disease and are now better recognised. New developments in angiographic classification of SCAD and the use of intravascular imaging techniques such as OCT and IVUS have enabled the incidence of SCAD to be more accurately estimated. As one example, a Japanese series used OCT to determine a diagnosis of SCAD in 4% of 326 patients presenting with ACS (11). Far more common is the incidence of SCAD in female patients below the age of 60 who present with ACS. An early retrospective study of 11,605 patients reported an incidence of 8.7% SCAD in this demographic (7). More recent reports indicate that SCAD is responsible for 22–31% of ACS in women under the age of 60, and up to 43% of pregnancy-related ACS (12-15). Despite these new insights, SCAD is still underdiagnosed and its true incidence remains unclear, highlighting the need for clinicians to be more informed about this condition (12,16,17).
SCAD is therefore a condition that primarily affects younger to middle aged women, although it occasionally occurs in males and older individuals. In 2012, Tweet et al. reported a mean age of 42.6 years with 82% females among a retrospective cohort of 87 patients presenting with SCAD at the Mayo Clinic, USA between 1979 to 2011 (18). In a second landmark study of 168 patients from Vancouver General Hospital published in 2014, 92% of patients were women, with mean age 52.1 years (19). Several smaller retrospective studies have subsequently found similar age and gender demographics, with data from six different series reporting that 92–95% SCAD patients were women with average ages ranging from 44 to 55 years-old (12,17,20-22). Recently, the largest yet, prospective, observational multi-centre study of 750 SCAD patients enrolled in Canada from 2014 to 2018 found that 88.5% were women, with mean age 51.8 years (23). Notably, 33.9% of these patients had no traditional cardiovascular risk factors, in keeping with numerous other contemporary studies (12,18-20,24,25).
Clinical presentation
Most commonly SCAD presents as ACS, often in younger females with a background of few, if any, traditional atherosclerotic risk factors. Of seven published SCAD cohorts shown in Table 1, between 82% and 98% of patients were female, 26–49% presented with STEMI, and mean age was between 43.6 and 52.0 years old. A history of migraine headaches has also been associated with SCAD, with one analysis of 40 patients finding a history of migraine in 17 (43%) (27). In the initial Vancouver series of 168 patients, Saw et al. reported emotional or physical stress as a precipitant in 56.5% of SCAD presentations (19), while more recent, prospective Canadian data identified precipitating stressors in 79.2% of 750 patients (23). Some series have observed elevated cardiac biomarkers consistent with myocardial infarction in all acute SCAD cases (18), while others have shown this to be the case in approximately three out of four (28). In an ACS cohort study from Japan, lower creatine kinase levels were observed in women <50 years old with a diagnosis of SCAD (n=45) compared to those without (n=55) (13). It follows that left ventricular function after SCAD-related ACS is often preserved, with Saw et al. previously noting that only 17.3% SCAD patients were left with an ejection fraction of less than 50% (19). Despite increasing knowledge about this condition, some patients with SCAD may not be referred for coronary investigations, as the focus of acute medical services is often on identifying high risk atherosclerotic ACS (4). For this reason, a high index of clinical suspicion for SCAD, as well as awareness and familiarity with angiographic variants are key to minimise delayed diagnosis or misdiagnosis.
Full table
Pathology
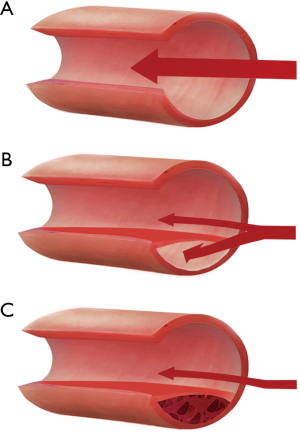
Traditional views on the pathological mechanisms and associated causes of SCAD were largely based on evidence from early case reports at autopsy (29,30). These highlighted two possible mechanisms: an intimal tear with subsequent false lumen formation, or an intramural haematoma, compressing the true lumen of the coronary artery and occluding blood flow (Figure 1). With the recent introduction of intracoronary imaging, intimal tears have become much easier to visualise; however, their prevalence remains uncertain. This is highlighted by variable findings from different OCT case series, which have been small with inherent selection bias. For example, while Paulo et al. observed intimal tears in 6 out of 8 SCAD patients (31), other studies have reported lower rates as low as 2 out of 17 patients (32). These findings indicate that a proportion of SCAD cases involve an isolated intramural haematoma. In the remaining cases, it is unclear whether the intimal tear is the cause of SCAD or if it is subsequent to an intramural haematoma. Interestingly, a recent analysis of 240 SCAD cases managed conservatively showed that those with an isolated intramural haematoma on presentation were more likely to experience progression or recurrent SCAD over a fourteen-day follow-up period (33). Furthermore, 20% of patients in this cohort had recurrent SCAD, in which an intimal tear was observed, suggesting that intimal disruption may occur secondary to initial intramural haematoma. Contrary to the more prevalent and traditional athero-occlusive mode of ACS, the role of thrombus in the pathophysiology of SCAD-related ACS is unclear. Many studies have shown an absence of thrombus in the coronary arteries during a SCAD event (17,19,32). However, one study that used OCT to image SCAD cases reported that all 11 patients studied had minor thrombi in either the true or false lumen (32). Further research into the role and clinical implications of thrombus in SCAD is needed to guide treatment.
Pathogenesis and associated conditions
Despite recent advances in our understanding from the widespread use of coronary angiography and the introduction of high-resolution intravascular imaging, there are still many uncertainties regarding the condition’s pathogenesis. Current consensus is that different genetic factors, hormonal influences, underlying acquired or genetic arteriopathies, and/or systemic inflammatory diseases increase susceptibility to SCAD, such that an increase in shear arterial wall stress can precipitate a spontaneous intramural haemorrhage. Furthermore, up to 80% of patients can recall a precipitating emotional or physical stressor (19), which is thought to speak to the integral role of shear wall stress in the pathogenesis of this condition. Various early case reports linked SCAD to pregnancy, arteriopathies, connective tissue disorders and systemic inflammatory diseases, and hypothesised that these conditions may predispose to SCAD (34-37). Larger cohort studies have confirmed that SCAD is frequently associated with underlying arteriopathies, negligible conventional cardiovascular risk factor profiles, and precipitating stressors (12,18-20). One study of 168 patients showed that the prevalence of diabetes, smoking, family history of coronary artery disease, and hyperlipidaemia were all less than 30% (19).
Female sex hormones and pregnancy
Historically, SCAD was often regarded as a disease of pregnancy that carried a high risk of fatal MI. More recent studies have suggested that pregnancy-associated SCAD comprises only a small percentage of total SCAD cases. Tweet et al. retrospectively analysed 189 cases of acute SCAD, only 7.9% of which were associated with pregnancy (17). This association and the predominance of females affected by SCAD suggest a pathophysiological involvement of female sex hormones in the pathogenesis of this condition. Long-term exposure to oestrogen and progesterone, both endogenous and exogenous, has been shown to impair arterial wall integrity and cause long-term changes in arterial architecture (38-40). Moreover, a substudy of the Vancouver cohort revealed that a significant proportion of SCAD cases were associated with active hormonal therapy (15%), hormone replacement therapy (27.3%), and more than three pregnancies (24.1%) or more than 2 births (27.8%), rationalising a cause-effect relationship between SCAD and female sex hormones (41). As a result, repeated pregnancies and use of hormonal therapies such as selective oestrogen receptor modulators or hormone replacement therapy are believed to increase the risk of SCAD in women.
Fibromuscular dysplasia (FMD)
FMD is an arteriopathy characterised by disorganisation, dysplasia, and/or destruction of connective tissue, smooth muscle cells, and fibroblasts, resulting in weakening of any of the three layers of an arterial wall (42). Although FMD most commonly affects the renal arteries, presenting with dissection, tortuosity, beading, or aneurysms, it has been shown to affect any arterial bed, with coronary artery FMD often manifesting as SCAD or angiographic tortuosity (43-45).
In 2005, Pate et al. described seven women presenting with ACS with concomitant renal FMD (46). Since then, larger case series have studied the relationship between SCAD and FMD. While an early small study reported the prevalence of FMD of renal and/or carotid arteries in 25% (3/12) of patients with SCAD (47), more recent studies have adopted more thorough angiographic screening for FMD and have found higher prevalence rates of 72% to 86% (19,20). Furthermore, in a recent report of 921 patients enrolled in the US FMD registry, 2.1% had a history of SCAD, supporting the link between the two (48).
Despite this well-established association, the role of screening for FMD in patients presenting with SCAD is not yet clear. Some tertiary care centres recommend screening for arteriopathies in all patients presenting with SCAD (3); however, no study has shown that this has an impact on outcomes. Screening can involve imaging of renal, iliac, femoral, and carotid arteries through ultrasound, CT angiography or invasive angiography, with increasing sensitivity respectively. As contemporary management of FMD is usually conservative (49), routine screening for it is currently unlikely to alter treatment. Additional research into how the concomitant presence of FMD should impact the management and influence the prognosis of SCAD will help clarify the value of screening.
Precipitants and triggering factors
Cases of SCAD frequently appear to be associated with an acute triggering event that presumably causes an increase in arterial wall shear stress, possibly mediated by acute changes in circulating catecholamine levels. Numerous retrospective case series have studied the incidence of stress triggers, and patient recall has varied between 22–57% (19,27); however, this may have been under-estimated as suggested by the latest, large-scale prospective data which have reported the presence of precipitating emotional stress in approximately 50% and physical stress in 30% (23). Valsalva-like manoeuvres such as coughing or retching, use of recreational drugs such as cocaine or amphetamines, or intense exercise have all been reported frequently prior to SCAD events (19). Although acute SCAD presentations have been associated with different triggers, key questions linger about the appropriate advice that patients should be given to prevent recurrences, especially in terms of their future lifestyle and need to avoid exercise, pregnancy or hormone therapy.
Molecular and genetic factors
Due to the typically sporadic and unpredictable nature of SCAD, its molecular and cellular basis remains largely unstudied. Similarly, genetic factors predisposing individuals to SCAD have not been well defined although new insights are beginning to emerge in the medical literature. Inherited connective tissue disorders such as Marfan’s Syndrome and Ehlers-Danlos Syndrome were initially linked to SCAD in small case reports, presumably predisposing to dissection due to compromised arterial wall integrity and increased fragility. Larger cohort studies have shown that these conditions are in fact only present in as few as 1–2% of SCAD cases (19,50). Therefore the yield of genetic screening for these conditions in SCAD patients has been very low and is not routinely recommended.
There are several published reports of pairs of relatives who have presented with SCAD. An analysis of 412 patients from the Mayo Clinic registry identified three first-degree and two second-degree related cases, giving a prevalence of 1.2% of familial cases (51). One study of a mother-daughter pair also identified possible genetic alleles that may predispose to the condition (52). Furthermore, a recent multinational study of 1,055 SCAD patients and 7,190 case controls has now reported on the first genetic link with SCAD, identifying an odds ratio of 1.67 (95% CI 1.50 to 1.86) per copy of the rs9349379-A allele of the PHACTR1/EDN1 gene locus (53), which was also previously found to be associated with FMD (54). Efforts to further characterise genetic correlations in SCAD are currently ongoing, including a large Mayo Clinic study that is aimed at identifying causative mutations (55).
Diagnosis
Accurate recognition and diagnosis of SCAD in patients with ACS is crucial, as the management and outcomes of SCAD vary substantially from atherosclerotic coronary disease. Although a high index of clinical suspicion should be assumed in patients presenting with ACS that have high-risk demographics—young, female and few conventional cardiovascular risk factors—patients outside this demographic may still present with SCAD, demonstrating the importance of diagnosing clinicians’ awareness and understanding of this condition. Most pivotal to diagnosis, is a proficiency in recognising angiographic variants of SCAD, and understanding the appropriate indications for proceeding to intravascular imaging.
Angiography
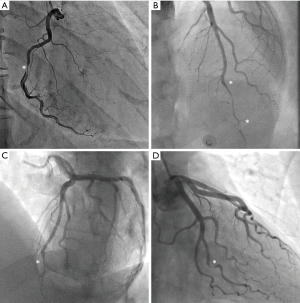
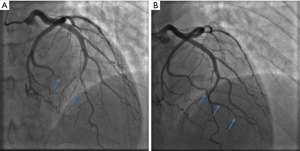
Traditionally, the pathognomonic appearance of SCAD on angiography described the presence of multiple lumens and extra-luminal contrast staining (56). With the advent of intravascular imaging, a greater understanding of angiographic variants has been obtained (57), resulting in a contemporary angiographic classification of SCAD proposed by Saw et al., which is now widely used (Figure 2) (56). This classification refers to Type 1 SCAD as the appearance of multiple lumens and extra-luminal contrast staining (Figure 2A). More recent studies have concluded that the Type 1 angiographic appearance is present in the minority of cases, with Rashid et al. observing a double lumen appearance in fewer than 20% (12). Type 2 SCAD is the most common angiographic manifestation, occurring in 52–67% in modern series (13,19,21). It is characterised by long diffuse and smooth stenosis, that can either be bordered by normal arterial segments, known as Type 2A (Figure 2B), or extend to the distal tip of the artery, known as Type 2B (Figure 2C). Type 3 SCAD mimics atherosclerosis as focal or tubular stenosis (Figure 2D). Current guidelines recommend that distinguishing this variant from atherosclerotic coronary artery stenosis may require further assessment with intravascular imaging (e.g., OCT, IVUS) (4,5). Additionally, Al-Hussaini et al. described Type 4 SCAD as a distal occlusion of the coronary artery (58), recognised by excluding embolic causes and subsequent evidence of vessel healing, in accordance with the natural history of SCAD.
Other important angiographic characteristics of SCAD include its predisposition to mid-to-distal coronary artery segments, predominant involvement of the left anterior descending artery, and association with coronary artery tortuosity (19). Notably, Eleid et al. studied 246 patients with SCAD against a control group of 313 patients using a semi-quantitative coronary artery tortuosity score, and found that tortuosity was far more common in patients with SCAD (78% vs. 17% in controls) and severe coronary artery tortuosity was predictive of recurrent SCAD (59). This suggests that further research into coronary artery tortuosity could be useful in risk stratification, diagnosis, management, and prognosis of SCAD.
IVUS/OCT
The use of IVUS to diagnose SCAD was first described in 2008 in a case series of four patients, where it was used when angiographic evaluation remained unclear (58). Over the past decade, the utilisation of intracoronary imaging has helped to advance our understanding, classification, and diagnosis of SCAD. Currently, intravascular imaging is used to clarify diagnosis in patients where angiographic uncertainty remains, particularly with the Type 3 angiographic appearance, and to guide coronary intervention and stent size and placement (Figure 3). Despite this, most SCAD can be diagnosed angiographically provided the clinician is vigilant and aware of the condition.
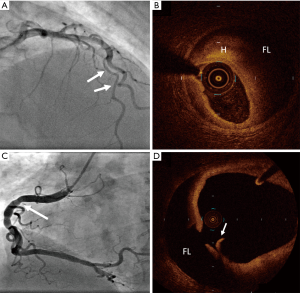
Both modes of intracoronary imaging are sufficient for diagnosis, and clinicians should become adept at interpreting features of SCAD with the intracoronary method available to them. OCT may be preferential for diagnosing SCAD due to its higher spatial resolution, resulting in a higher sensitivity for intimal tears, false lumens, intramural haemorrhages, and thrombi (4,5). Despite concern that intracoronary imaging may impose a greater than usual risk of iatrogenic dissection in SCAD (60), careful use of OCT and IVUS appears safe (32), and there have been few reports of complications. Regardless, any coronary catheter intervention in this cohort of patients should be performed with utmost care, and only when clinically indicated.
Computed tomography coronary angiography (CTCA)
In theory, CTCA has appeal for the diagnosis of suspected SCAD cases, as it is non-invasive and provides visualisation of the arterial wall as well as the lumen. However, it suffers from lower spatial and temporal resolution than conventional angiography, leading to lower sensitivity and risk of false negative results (61,62). One study reported three cases where SCAD was missed on CTCA on primary evaluation, and was only recognised retrospectively after the diagnosis had been made by invasive coronary angiography (61). Consequently, current guidelines do not recommend CTCA as a first-line investigation for acute SCAD (4,5), although there is emerging evidence of its utility in follow-up where it can help reassure clinicians and patients of spontaneous healing and recanalisation particularly in larger calibre arteries (22,63,64). Further evaluation is now needed to determine if CTCA should be used routinely for this purpose in clinical practice.
Management
Previously, the treatment of SCAD was largely extrapolated from management of atherosclerotic coronary disease, although this has now been brought into question (4,5). Although an early invasive strategy with revascularisation is widely advocated in ACS secondary to atherosclerotic disease, there are no randomised data to support coronary revascularisation with percutaneous coronary intervention (PCI) in ACS caused by SCAD. To the contrary, observational studies have consistently shown an increased risk of coronary complications from PCI performed in SCAD (17,19,21,25). Furthermore, conventional lipid lowering and antithrombotic therapies during and following ACS presentations are also primarily based on their anti-atherosclerotic and anti-thrombotic effects. However, SCAD has now been well characterised as a disease of younger women who typically have few recognisable cardiovascular risk factors and involves intramural haemorrhage of the coronary artery wall rather than thrombosis. Therefore, the role of these agents in SCAD is uncertain, particularly in the case of medications that prolong bleeding time; this is now reflected in the 2018 AHA Scientific Statement (5).
Conservative management
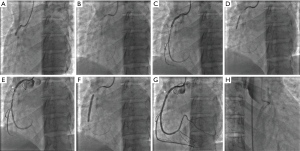
SCAD was initially considered to be predominantly fatal without intervention owing to the fact that early reports in the literature were primarily from autopsy studies (1). Contemporary observational data indicate that conservative management of haemodynamically stable patients without ongoing signs of ischaemia generally results in favourable outcomes (17,24). Spontaneous healing of dissections has been described in some follow-up studies. In the Mayo Clinic cohort, 73% of patients who had a prior diagnosis of SCAD showed complete angiographic healing over a median period of 876 days (17). Meanwhile, the published experience from Vancouver reported that all 79 patients with follow-up angiography had achieved spontaneous healing over a median interval of 161 days (19). Although resorption of intramural haematoma has been demonstrated within days by OCT (65), complete healing is thought to occur over months, and can be demonstrated angiographically (Figure 4). Despite this, early complications of recurrent ACS following SCAD most commonly occur over the first five days (18,19,25,66), and these patients may require urgent revascularisation. A recent report of 240 conservatively managed SCAD patients revealed that about one in six had recurrent SCAD within 14 days, all of whom presented with recurrent chest discomfort (33). Consequently, it has been recommended that patients with acute SCAD be observed in hospital for up to five days (4,5). In cases of haemodynamic instability, ongoing ischaemia or recurrent dissections, conservative therapy may not be appropriate. In this setting, pursuit of PCI or coronary artery bypass grafting (CABG) may be required; however, this decision needs to be carefully considered and individualised to the patient’s specific context.
Pharmacological therapy
Antithrombotic agents
With the recognition that the pathogenesis of SCAD often involves intramural bleeding into the vessel wall, the use of thrombolysis, antiplatelet therapy, and anticoagulants, inferred from treatment of atherosclerotic disease, has been brought under scrutiny. In the setting of an acute SCAD presentation, these interventions may increase the risk of further bleeding into the vessel wall, extending the dissection. Many case studies have illustrated that thrombolysis can result in extension of dissection, and even coronary artery rupture complicated by tamponade in SCAD patients (67-69), and thus, guidelines currently dictate that thrombolysis is contraindicated (4,5). There are limited data on anticoagulant therapy in SCAD patients; consequently, clinicians should be aware of the concerns about the potential adverse impact of their use, particularly the risk of progression of the dissection plane or early recurrence. Despite this, systemic clinical indications for anticoagulation (e.g., treatment of concurrent atrial fibrillation) should not be overruled without expert clinical opinion and decisions made on a case-by-case basis (5,58).
More controversial and unresolved is the use of antiplatelet therapy. Guidelines released by the AHA in 2014 recommended that patients diagnosed with SCAD undergoing PCI should receive standard antiplatelet therapy (70). However, no study has been performed to compare outcomes following use of dual antiplatelet therapy or aspirin monotherapy in the context of SCAD, particularly when managed conservatively. Furthermore, with recent developments in understanding that SCAD characteristically results from an intramural bleed of the coronary wall, use of antiplatelet agents could theoretically worsen its natural history and associated outcomes. Interestingly, a retrospective study of 139 patients demonstrated similar outcomes independent of antiplatelet therapy (25). Mostly, in the absence of complications, experts still recommend aspirin for at least a year following SCAD; however, given current uncertainty of the benefits versus risks, decisions for antiplatelet use in SCAD patients should be tailored to each individual case (4,5). Further research is required to determine efficacy of antiplatelet therapy in the setting of both acute SCAD and its long-term follow up.
β-blockers
Given the pathophysiological role of arterial wall shear stress in the pathogenesis of SCAD and the relatively high rates of recurrent dissection soon after an acute event, it has been speculated that β-blockers may help to reduce the risk of recurrence (3). Although this has not been studied systematically, extrapolation from the management of aortic dissection favours the use of β-blockers (71). Furthermore, data from Vancouver have illustrated a hazard ratio of 0.36 for recurrent SCAD in those managed with β-blockers (72). Further prospective research into the use of β-blockers in SCAD is needed to draw definitive conclusions regarding their use (including long-term continuation), however, in the absence of contraindications, they are recommended in the most recent expert SCAD guidelines (4,5).
Statins
Rationale for the routine use of statins has also been extrapolated from atherosclerotic ACS, with little biological plausibility or evidence of benefit. Although a retrospective study of 87 patients found a slightly higher recurrence of SCAD in patients on statins (18), this was not replicated in a larger prospective cohort of 327 patients (72), and may have been influenced by the date of index event or sample size. Notably, statin use after SCAD is not supported by the current guidelines unless indicated otherwise (e.g., for primary prevention of atherosclerotic events in individuals at high cardiovascular risk) (4,5). Currently, a clinical trial called SAFER-SCAD is investigating the efficacy of both Angiotensin Converting Enzyme inhibitors and statins in SCAD patients, and is estimated to be completed in June 2021 (73).
Percutaneous coronary intervention
In athero-thrombotic ACS, early coronary revascularisation and reperfusion of ischaemic myocardium through PCI has revolutionised treatment, short-term outcomes, and long-term prognosis (74). However, studies have consistently shown a low level of technical success in SCAD patients treated with PCI (3-5). Treating clinicians should also be aware that SCAD intervention heralds increased risk of iatrogenic dissection due to compromised vessel wall integrity associated with this condition, as well as the risk of guide wire passage into the false lumen and propagation of the false lumen. These technical complications have been associated with poorer clinical outcomes in SCAD cohorts. Tweet et al. reported technical failure of PCI in 53% of 189 patients (18). Furthermore, revascularisation did not reduce the risk of recurrent SCAD or repeated revascularisation. Similarly, in the Vancouver cohort PCI was successful in only 64% of cases, and long-term follow up was uncomplicated in only 30% of those patients treated with revascularisation (19). An Italian series of 139 patients also reported a relatively modest success rate of 72% from PCI, and showed a much higher incidence of in-hospital Major Adverse Cardiac Events (75) for those managed by PCI versus conservatively (16.1% vs. 3.8%, P=0.028) (25). In addition, across three cohorts totalling 491 patients, emergency CABG was required as bale out for complicated PCI in 9–13% cases (17,18,25).
Although selection bias is obviously an important consideration when interpreting the aforementioned observational registry data, conservative management is currently recommended for patients with acute SCAD who are clinically stable. Despite this, intervention may be required in select cases, such as those with ongoing significant ischaemia, haemodynamic instability, recurrent dissection, or large left main dissections. At present there are no guidelines regarding optimal PCI approach. Intervening clinicians should be meticulous, thorough, and vigilant in their approach to patients with SCAD. By avoiding deep catheter engagement, poor catheter tip positioning, catheter dampening, and strong contrast injection against the vessel wall, clinicians can decrease the likelihood of complications or poor technical success. Moreover, in patients with a tapering dissection, the artery often appears deceptively small and this can lead to undersizing of stents, difficulty in stent deployment and heightened risk of iatrogenic dissection, sometimes with fatal consequences (Figure 5). Decision to intervene with PCI in a SCAD patient should therefore be made by an experienced clinician, with careful evaluation and appraisal of each clinical case individually.
Alternative interventional approaches
Various case reports have illustrated technical success with various alternative interventional techniques and approaches; however, in the absence of large prospective clinical trials, no specific interventional strategy has been demonstrated as a superior alternative to PCI with conventional stenting. Further research into bioresorbable scaffolds (76), balloon angioplasty alone, use of cutting balloons (77), extended stent lengths, and intravascular imaging guidance for stent placement is required to optimise interventional approaches to patients presenting with SCAD requiring intervention.
Coronary artery bypass grafting
Published reports of CABG to treat acute SCAD have been around since the late 1980s (78). Generally, it is reserved for cases where revascularisation is required due to ongoing ischaemia or clinical instability when PCI is contraindicated, unsuccessful or complicated. In these situations, CABG has been demonstrated as an efficacious last resort treatment intervention. A retrospective study of the Mayo cohort indicated a 94% technical success rate and 100% in-hospital survival in SCAD patients selected for CABG (17). Other studies have demonstrated similar outcomes using arterial and venous conduits, both off-pump and robotic techniques (7,67,79). Not surprisingly, follow-up angiography of 16 patients in the Mayo cohort demonstrated a low rate of patent grafts probably due to spontaneous healing of the dissection, leading to re-establishment of native flow and subsequent conduit thrombosis (17). In these patients, the role of CABG may be limited to initially preserving coronary flow to the at-risk myocardium until the SCAD has healed and native coronary flow has been restored. Notwithstanding recent developments in our understanding of the natural history and management of SCAD, CABG can still be used as a life-saving intervention in SCAD patients where other treatment has failed.
Management of myocardial dysfunction
Following SCAD, patients with impaired left ventricular systolic function should be prescribed medication according to the current guidelines for post-MI management (4,5). This includes using maximally tolerated doses of an angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (35), a heart failure-selective β-blocker, and a mineralocorticoid receptor antagonist (MRA) (80). Care should be taken in younger patients where hypotension may limit increases in dosage. Furthermore, young females of reproductive age should be aware of the teratogenicity of ACEis, ARBs and MRAs.
Follow-up
Prognosis and recurrence
SCAD patients often experience uncertainty and confusion around the nature of their condition, their prognosis, and risk of recurrence; healthcare teams are often unfamiliar with SCAD and fail to recognise or inform patients of the significant difference between conventional atherosclerotic heart disease and SCAD (5). Contemporary, prospective North American data indicate a 30-day MACE rate of 8.8% after acute SCAD (23). Other evidence suggests that longer-term mortality is low. Tweet et al. reported Kaplan-Meier estimates of 10-year survival at 92% (18), while others have described similarly low mortality rates ranging from 0 to 6.6% over median follow-up periods of 2.8 to 6 years (13,21,25,72). However, morbidity and other components of MACE are more commonly experienced during follow-up due to considerable risk of recurrent dissection (Figure 6). Across three different cohorts which have so far reported on 2 to 3 years follow-up, rates of MACE were 10% to 30% (13,19,72), with almost 50% MACE observed over ten years in the Mayo registry (18). The incidence of recurrent SCAD has been variably reported in the literature. Some studies have observed as low as 5% recurrence at a median of two years (25), while estimates from the 2018 SCAD AHA statement have concluded rates of up to 30% at 4 to 10 years (5). Interestingly, analysis of recurrent cases has indicated that the vast majority (approximately 80%) occur at new sites in the coronary vasculature, suggesting that stenting will not reduce recurrence of SCAD significantly (18). Currently, no risk factors have been shown to affect recurrence of SCAD, although one study has inferred its association with coronary artery tortuosity (59). Further research into tortuosity and other prognostic factors, as well as the efficacy of treatment strategies, such as beta-blockers and other antihypertensive agents is much needed.
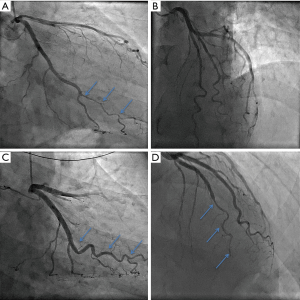
Left ventricular function assessment
Current guidelines recommend assessment of left ventricular dysfunction following MI regardless of the cause, to guide both medical and device therapy (80). This can be done by either echocardiography or cardiac magnetic resonance (CMR) imaging. Recently, a magnetic resonance imaging study of 18 SCAD patients demonstrated distribution patterns of delayed myocardial enhancement consistent with myocardial ischaemia, but did not reveal any prognostic features of recurrence, largely due to the small sample size (81). Further evaluation of the prognostic and clinical implications of CMR in the setting of SCAD is required.
Screening for FMD and other arteriopathies
As discussed earlier, SCAD is often the first presentation of an underlying arteriopathy, most commonly FMD (19). The presence of these arteriopathies may affect prognosis and management of SCAD patients. As a result, there has been recent discussion about the role of extra-coronary imaging to detect these arteriopathies in the follow-up of SCAD. The argument for this imaging is to screen for high-risk aneurysms or underlying arteriopathies. Current guidelines only agree that all patients with SCAD should undergo a thorough vascular physical examination of the abdominal aorta, carotid arteries, and peripheral arteries of the upper and lower limbs, and any examination findings should be followed up with a thorough vascular imaging work-up. More controversial, is the role of extra-coronary vascular imaging in follow-up of patients without abnormal clinical examination findings. Contemporary European guidelines advise imaging in all patients with SCAD (4). By comparison, the American guidelines recommend that such imaging should be considered in all SCAD patients, however, caution around the implications of the results of screening (5). These include false reassurance with a false negative result, anxiety with a true positive result, and risk of treatment-associated harm with a true positive result and subsequent intervention. Thus, it is recommended that extra-coronary imaging in SCAD follow-up should be approached with a shared-decision making model between patient and clinician. Long-term management of arteriopathies and unruptured intracranial aneurysms can be found in condition-specific guidelines (43,82).
Psychosocial impact and support
Often underestimated, under-recognised and poorly treated, SCAD has a substantial psychosocial impact on patients, amplified by the exposure to a substantial life-threatening emergency in a younger cohort, and often resulting in comorbid anxiety, depression, and post-traumatic stress disorder. Furthermore, treating teams are often unfamiliar with SCAD, confounding patients with atherosclerotic management principles. With this comes the uncertainty of management, prognosis, and recurrence. A cross-sectional study of 158 patients by Liang et al. showed that 33% had received treatment for depression, and 37% for anxiety (83). Another retrospective analysis by Pargaonkar et al. reported anxiety disorder (9.8%), major depression (8.4%), and post-traumatic stress disorder (5%) (84). Although current guidelines recommend screening for anxiety and depression after MI, this is often missed in both younger and female cohorts, leaving these patients susceptible to a lack of support and follow up (85). Given these substantial psychological comorbidities, appropriate and thorough follow-up with a well-informed multidisciplinary team is essential, and this can be assisted by online social support networks, which have emerged as an invaluable component to the management and follow-up of SCAD. Facebook group support networks, often patient initiated, have especially flourished and allowed SCAD patients to interact and support each other (86).
Cardiac rehabilitation
Despite clear evidence that cardiac rehabilitation improves symptom frequency, metabolic parameters, and well-being in patients with SCAD (87-90), current evidence indicates a low rate of referral and participation in cardiac rehabilitation programs (87,88,91). One contributor to this may be the physician or patient-held belief that precipitating stressors such as exercise may predispose patients to recurrent SCAD events. No study has confirmed a relationship between exercise and recurrent SCAD and many have demonstrated its safety (87-91), and thus, currently, a personalised cardiac rehabilitation program is an essential component of care in all patients with a history of SCAD (4,5).
Unanswered questions and future research direction
Although the risk factors, clinical features and angiographic appearances of SCAD are now far better characterised, questions still remain on the true incidence, natural history, ideal treatment, prevention of recurrence, the role of intravascular imaging in confirming diagnosis and genetic predisposition. Currently, there are five registered clinical studies that aim to answer some of these questions (Table 2). Despite this, prospective randomised controlled trials investigating medical treatment such as antiplatelet and beta-blocker therapy, as well as optimal coronary intervention strategies have not yet been undertaken. Collaborative research efforts establishing large multicentre SCAD databases and therapeutic intervention studies will help better understand the condition’s natural history and optimal treatment strategies.
Conclusions
SCAD is a challenging clinical entity that most commonly presents with ACS, frequently affecting younger women. Recent developments in angiographic characteristics and intravascular imaging have revolutionised our understanding of SCAD’s incidence and pathogenesis. Angiographically, SCAD often mimics atherosclerotic ACS, however, this is only a recent discovery. As a result, there remains uncertainty regarding its true incidence in the wider population. Research must still be done into SCAD’s ideal pharmacological and interventional management, and its underlying predisposing factors, including the importance of genetics. Finally, it is important that all clinicians remain vigilant and aware of this condition, as patient outcomes and treatment guidelines differ substantially from conventional atherosclerotic ACS.
Acknowledgments
The authors would like to acknowledge Lachlan Odell who prepared Figure 1. PJ Psaltis receives fellowship funding from the National Health and Medical Research Council (CDF1161506), National Heart Foundation of Australia (FLF102056) and Royal Australasian College of Physicians and Sylvia and Charles Viertel Charitable Foundation (VIERCI2017016). SJ Nicholls is a recipient of a Principal Research Fellowship from the National Health and Medical Research Council of Australia.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42: rupture. Br Med J 1931;1:667.
- Kamineni R, Sadhu A, Alpert JS. Spontaneous coronary artery dissection: report of two cases and a 50-year review of the literature. Cardiol Rev 2002;10:279-84. [Crossref] [PubMed]
- Saw J, Mancini GBJ, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2016;68:297-312. [Crossref] [PubMed]
- Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353-68. [Crossref] [PubMed]
- Hayes SN, Kim ESH, Saw J, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation 2018;137:e523-57. [Crossref] [PubMed]
- Tweet MS, Gulati R, Aase LA, et al. Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. Mayo Clin Proc 2011;86:845-50. [Crossref] [PubMed]
- Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250-4. [Crossref] [PubMed]
- Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv 2009;74:710-7. [Crossref] [PubMed]
- Hering D, Piper C, Hohmann C, et al. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol 1998;87:961-70. [Crossref] [PubMed]
- Krittanawong C, Kumar A, Virk HUH, et al. Trends in Incidence, Characteristics, and In-Hospital Outcomes of Patients Presenting With Spontaneous Coronary Artery Dissection (From a National Population-Based Cohort Study Between 2004 and 2015). Am J Cardiol 2018;122:1617-23. [Crossref] [PubMed]
- Nishiguchi T, Tanaka A, Ozaki Y, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2016;5:263-70. [Crossref] [PubMed]
- Rashid HN, Wong DT, Wijesekera H, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome--A single-centre Australian experience. Int J Cardiol 2016;202:336-8. [Crossref] [PubMed]
- Nakashima T, Noguchi T, Haruta S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol 2016;207:341-8. [Crossref] [PubMed]
- Saw J, Aymong E, Mancini GB, et al. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol 2014;30:814-9. [Crossref] [PubMed]
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014;129:1695-702. [Crossref] [PubMed]
- Tweet MS, Gulati R, Hayes SN. Spontaneous Coronary Artery Dissection. Curr Cardiol Rep 2016;18:60. [Crossref] [PubMed]
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777-86. [Crossref] [PubMed]
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579-88. [Crossref] [PubMed]
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645-55. [Crossref] [PubMed]
- Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44-52. [Crossref] [PubMed]
- Rogowski S, Maeder MT, Weilenmann D, et al. Spontaneous Coronary Artery Dissection: Angiographic Follow-Up and Long-Term Clinical Outcome in a Predominantly Medically Treated Population. Catheter Cardiovasc Interv 2017;89:59-68. [Crossref] [PubMed]
- Roura G, Ariza-Sole A, Rodriguez-Caballero IF, et al. Noninvasive Follow-Up of Patients With Spontaneous Coronary Artery Dissection With CT Angiography. JACC Cardiovasc Imaging 2016;9:896-7. [Crossref] [PubMed]
- Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a "conservative" therapeutic strategy. JACC Cardiovasc Interv 2012;5:1062-70. [Crossref] [PubMed]
- Lettieri C, Zavalloni D, Rossini R, et al. Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am J Cardiol 2015;116:66-73. [Crossref] [PubMed]
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015;115:1672-7. [Crossref] [PubMed]
- McGrath-Cadell L, McKenzie P, Emmanuel S, et al. Outcomes of patients with spontaneous coronary artery dissection. Open Heart 2016;3:e000491. [Crossref] [PubMed]
- Lindor RA, Tweet MS, Goyal KA, et al. Emergency Department Presentation of Patients with Spontaneous Coronary Artery Dissection. J Emerg Med 2017;52:286-91. [Crossref] [PubMed]
- Mathieu D, Larde D, Vasile N. Primary dissecting aneurysms of the coronary arteries: case report and literature review. Cardiovasc Intervent Radiol 1984;7:71-4. [Crossref] [PubMed]
- Claudon DG, Claudon DB, Edwards JE. Primary dissecting aneurysm of coronary artery. A cause of acute myocardial ischemia. Circulation 1972;45:259-66. [Crossref] [PubMed]
- Paulo M, Sandoval J, Lennie V, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging 2013;6:830-2. [Crossref] [PubMed]
- Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol 2012;59:1073-9. [Crossref] [PubMed]
- Waterbury TM, Tweet MS, Hayes SN, et al. Early Natural History of Spontaneous Coronary Artery Dissection. Circ Cardiovasc Interv 2018;11:e006772. [Crossref] [PubMed]
- Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Hum Pathol 1987;18:654-6. [Crossref] [PubMed]
- Sharma AK, Farb A, Maniar P, et al. Spontaneous coronary artery dissection in a patient with systemic lupus erythematosis. Hawaii Med J 2003;62:248-53. [PubMed]
- Hampole CV, Philip F, Shafii A, et al. Spontaneous coronary artery dissection in Ehlers-Danlos syndrome. Ann Thorac Surg 2011;92:1883-4. [Crossref] [PubMed]
- Aldoboni AH, Hamza EA, Majdi K, et al. Spontaneous dissection of coronary artery treated by primary stenting as the first presentation of systemic lupus erythematosus. J Invasive Cardiol 2002;14:694-6. [PubMed]
- Yip A, Saw J. Spontaneous coronary artery dissection-A review. Cardiovasc Diagn Ther 2015;5:37-48. [PubMed]
- Sheikh AS, O'Sullivan M. Pregnancy-related Spontaneous Coronary Artery Dissection: Two Case Reports and a Comprehensive Review of Literature. Heart Views 2012;13:53-65. [Crossref] [PubMed]
- Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol 1967;83:336-41. [PubMed]
- Eng L, Starovoytov A, Heydari M, et al. Spontaneous coronary artery dissection in women and association with hormonal stressors. Circulation 2015;132:A18913.
- Stanley JC, Gewertz BL, Bove EL, et al. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg 1975;110:561-6. [Crossref] [PubMed]
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014;129:1048-78. [Crossref] [PubMed]
- Olin JW. Expanding Clinical Phenotype of Fibromuscular Dysplasia. Hypertension 2017;70:488-9. [Crossref] [PubMed]
- Persu A, Van der Niepen P, Touze E, et al. Revisiting Fibromuscular Dysplasia: Rationale of the European Fibromuscular Dysplasia Initiative. Hypertension 2016;68:832-9. [Crossref] [PubMed]
- Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv 2005;64:138-45. [Crossref] [PubMed]
- Toggweiler S, Puck M, Thalhammer C, et al. Associated vascular lesions in patients with spontaneous coronary artery dissection. Swiss Med Wkly 2012;142:w13538. [PubMed]
- Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and Aneurysm in Patients With Fibromuscular Dysplasia: Findings From the U.S. Registry for FMD. J Am Coll Cardiol 2016;68:176-85. [Crossref] [PubMed]
- Michelis KC, Olin JW, Kadian-Dodov D, et al. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol 2014;64:1033-46. [Crossref] [PubMed]
- Henkin S, Negrotto SM, Tweet MS, et al. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart 2016;102:876-81. [Crossref] [PubMed]
- Goel K, Tweet M, Olson TM, et al. Familial spontaneous coronary artery dissection: evidence for genetic susceptibility. JAMA Intern Med 2015;175:821-6. [Crossref] [PubMed]
- Fahey JK, Williams SM, Tyagi S, et al. The Intercellular Tight Junction and Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2018;72:1752-3. [Crossref] [PubMed]
- Adlam D, Olson TM, Combaret N, et al. Association of the PHACTR1/EDN1 Genetic Locus With Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2019;73:58-66. [Crossref] [PubMed]
- Kiando SR, Tucker NR, Castro-Vega LJ, et al. PHACTR1 Is a Genetic Susceptibility Locus for Fibromuscular Dysplasia Supporting Its Complex Genetic Pattern of Inheritance. PLoS Genet 2016;12:e1006367. [Crossref] [PubMed]
- Genetic Investigations in Spontaneous Coronary Artery Dissection (SCAD). 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01427179?cond=SCAD&rank=1
- Saw J, Mancini GB, Humphries K, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv 2016;87:E54-61. [Crossref] [PubMed]
- Alfonso F, Paulo M, Dutary J. Endovascular imaging of angiographically invisible spontaneous coronary artery dissection. JACC Cardiovasc Interv 2012;5:452-3. [Crossref] [PubMed]
- Al-Hussaini A, Adlam D. Spontaneous coronary artery dissection. Heart 2017;103:1043-51. [Crossref] [PubMed]
- Eleid MF, Guddeti RR, Tweet MS, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656-62. [Crossref] [PubMed]
- Prakash R, Starovoytov A, Heydari M, et al. Catheter-Induced Iatrogenic Coronary Artery Dissection in Patients With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv 2016;9:1851-3. [Crossref] [PubMed]
- Eleid MF, Tweet MS, Young PM, et al. Spontaneous coronary artery dissection: challenges of coronary computed tomography angiography. Eur Heart J Acute Cardiovasc Care 2018;7:609-13. [Crossref] [PubMed]
- Alzand BSN, Vanneste L, Fonck D, et al. Spontaneous coronary artery dissection undissolved using cardiac computed tomography. Int J Cardiol 2016;229:1040-1. [Crossref] [PubMed]
- Guo LQ, Wasfy MM, Hedgire S, et al. Multimodality imaging of spontaneous coronary artery dissection: case studies of the Massachusetts General Hospital. Coron Artery Dis 2016;27:70-1. [Crossref] [PubMed]
- Tweet MS, Gulati R, Williamson EE, et al. Multimodality Imaging for Spontaneous Coronary Artery Dissection in Women. JACC Cardiovasc Imaging 2016;9:436-50. [Crossref] [PubMed]
- Lempereur M, Fung A, Saw J. Stent mal-apposition with resorption of intramural hematoma with spontaneous coronary artery dissection. Cardiovasc Diagn Ther 2015;5:323-9. [PubMed]
- Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol 2004;16:493-9. [PubMed]
- Andreou AY, Georgiou PA, Georgiou GM. Spontaneous coronary artery dissection: Report of two unsuspected cases initially treated with thrombolysis. Exp Clin Cardiol 2009;14:e89-92. [PubMed]
- Zupan I, Noc M, Trinkaus D, et al. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter Cardiovasc Interv 2001;52:226-30. [Crossref] [PubMed]
- Goh AC, Lundstrom RJ. Spontaneous Coronary Artery Dissection with Cardiac Tamponade. Tex Heart Inst J 2015;42:479-82. [Crossref] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-94. [Crossref] [PubMed]
- Clough RE, Nienaber CA. Management of acute aortic syndrome. Nat Rev Cardiol 2015;12:103-14. [Crossref] [PubMed]
- Saw J, Humphries K, Aymong E, et al. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol 2017;70:1148-58. [Crossref] [PubMed]
- Sedlak T. Statin and Angiotensin-converting Enzyme Inhibitor on Symptoms in Patients With SCAD (SAFER-SCAD). Available online: https://clinicaltrials.gov/ct2/show/NCT02008786?cond=scad&rank=6
- Ibánez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017;70:1082. [Crossref] [PubMed]
- Nogueira de Macedo R, de Paula Miranda S, Vieira da Costa RL. Spontaneous coronary artery dissection - a diagnosis to be considered in young patients presenting with acute myocardial infarction. The Journal of invasive cardiology 2009;21:E245-7. [PubMed]
- Camacho Freire SJ, Gomez Menchero AE, Roa Garrido J, et al. Bioresorbable Scaffolds in Spontaneous Coronary Artery Dissection: Long-Term Follow-Up in 4 Patients. Tex Heart Inst J 2017;44:405-10. [Crossref] [PubMed]
- Yumoto K, Sasaki H, Aoki H, et al. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc Interv 2014;7:817-9. [Crossref] [PubMed]
- Thayer JO, Healy RW, Maggs PR. Spontaneous coronary artery dissection. Ann Thorac Surg 1987;44:97-102. [Crossref] [PubMed]
- Faden MS, Bottega N, Benjamin A, et al. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart 2016;102:1974-9. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Tan NY, Hayes SN, Young PM, et al. Usefulness of Cardiac Magnetic Resonance Imaging in Patients With Acute Spontaneous Coronary Artery Dissection. . Am J Cardiol 2018;122:1624-9. [Crossref] [PubMed]
- Thompson BG, Brown RD Jr, Amin-Hanjani S, et al. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2368-400. [Crossref] [PubMed]
- Liang JJ, Tweet MS, Hayes SE, Gulati R, et al. Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev 2014;34:138-42. [Crossref] [PubMed]
- Pargaonkar V, Khandelwal A, Krishnan G, et al. Clinical presentation, management and prognosis of patients with spontaneous coronary artery dissection. J Am Coll Cardiol 2016;67:53. [Crossref]
- Dunlay SM, Witt BJ, Allison TG, et al. Barriers to participation in cardiac rehabilitation. Am Heart J 2009;158:852-9. [Crossref] [PubMed]
- Australian SCAD (Spontaneous Coronary Artery Dissection) survivors. Available online: https://www.facebook.com/groups/AustralianSCADsurvivors/
- Krittanawong C, Tweet MS, Hayes SE, et al. Usefulness of Cardiac Rehabilitation After Spontaneous Coronary Artery Dissection. Am J Cardiol 2016;117:1604-9. [Crossref] [PubMed]
- Chou AY, Prakash R, Rajala J, et al. The First Dedicated Cardiac Rehabilitation Program for Patients With Spontaneous Coronary Artery Dissection: Description and Initial Results. Can J Cardiol 2016;32:554-60. [Crossref] [PubMed]
- Pinto MC, Camargo RC, Filho JC, et al. Influence of cardiac rehabilitation in Primigravida with spontaneous coronary artery dissection during postpartum. Int Arch Med 2014;7:20. [Crossref] [PubMed]
- Silber TC, Tweet MS, Bowman MJ, et al. Cardiac rehabilitation after spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev 2015;35:328-33. [Crossref] [PubMed]
- Hayes SN. Spontaneous coronary artery dissection (SCAD): new insights into this not-so-rare condition. Tex Heart Inst J 2014;41:295-8. [Crossref] [PubMed]