
Effect of epicardial application of amiodarone-releasing hydrogel on heart rate in an animal model
Introduction
Postoperative atrial fibrillation (POAF) is a frequent complication after open heart surgery. According to the literature, POAF occurs in 30–65% of cardiosurgical patients. The occurrence of rhythm disturbances after open heart surgeries depends on the initial severity of the patient's condition, surgical intervention type, carefulness of monitoring and determination of what to consider as postoperative arrhythmia. POAF can be also caused by a number of reasons, among which: heart trauma, hypoxia, acidosis, autonomic regulation disorder, increased production of catecholamines, disturbance of water-electrolyte balance, pericarditis. Recently, the role of the inflammatory process in the pathogenesis of POAF has become more evident. The development of a systemic inflammatory response syndrome after cardiac surgery has a high frequency (1-3).
Despite the fact that episodes of POAF are usually short, their presence increases the risk of development of thromboembolic complications in a patient or acute heart failure. It also increases the length of hospital stay and the cost of treatment (4). The majority of POAF patients steadily progress into a persistent or long-term persistent form of AF. In this regard, timely and effective prevention of this arrhythmia in the early stages after the intervention becomes of particular interest (5).
Antiarrhythmic drugs (AADs) of I, III classes, β-adrenoblockers, calcium antagonists, digoxin, colchicine, and statins are usually used for POAF prevention.
Nevertheless, some authors report that digoxin, calcium antagonists and class I antiarrhythmic drugs (AADs) do not show any conclusive evidence on the efficacy of POAF prevention (6). Colchicine and statins, in turn, can be used to prevent POAF, as they have demonstrated good efficacy and an acceptable safety profile (7-11).
Amiodarone is one of the most effective AAD. However, its use in clinical practice is limited due to the high incidence of extracardiac side effects (dysfunction of the thyroid gland, pulmonary and hepatotoxicity). These effects are partly due to the need for systemic administration of the drug. In this connection, currently, the methods of topical application of medications, the local delivery of amiodarone particularly, are very prevalent (12,13).
Currently, the following alternative routes of amiodarone delivery in the heart are used: (I) intrapericardial infusion of amiodarone solution (12); (II) epicardial sputtering of adhesive hydrogels with amiodarone in situ (14); (III) two-layer strips or disks with amiodarone, sewn to the epicardium (15).
Intrapericardial infusion of amiodarone is ideal for patients with an intact pericardial sac. This way, a natural reservoir is created and space is provided for a drug delivery. Nevertheless, recent studies have shown that intrapericardial delivery of ibutilide, sotalol, or amiodarone does not lead to a higher conversion of AF and increases the risk of infection, local fibrosis of the myocardium and ventricular arrhythmias. This is partly due to the influence of amiodarone on the electrophysiological features of the ventricles of the heart (16).
Takeda et al. have shown that bilayer epicardial discs or strips with amiodarone provide a more local delivery of the drug and minimize the drug outflow to the pericardial fluid, which in turn contributes to the preservation of electrophysiological features of ventricles functions and causes a prolonged delivery of the drug via a biodegradable material (15). Nonetheless, epicardial strips are regarded as a complex method that requires a lot of time and has a limited use on the left atrial wall.
A group of scientists led by Wang et al. applied a new method for local application of amiodarone in the form of a hydrogel (14). The epicardial application of an adhesive hydrogel with amiodarone is a less invasive, well tolerated, fast-performed and an effective method for POAF prevention. The results show that this is a promising method with a lower risk of ventricular and systemic side effects development when compared to intravenous and oral amiodarone administration (17).
In the A.N. Bakulev National Medical Research Center of Cardiovascular Surgery, Russia, an experimental study with the method of local application of drugs on the heart was conducted: modeling of a postoperative pericarditis with an assessment of the effectiveness of biodegradable films that are based on gelatin with colchicine for the prevention of adhesions. This study demonstrated a satisfactory anti-inflammatory effect of gelatin films with colchicine (18). In Russia however, there is currently no experience on a local delivery of amiodarone.
The optimal method of local delivery of the drug on the heart has not been yet developed. The existing methods require careful and large-scale research work in order to adequately assess their effectiveness and safety.
The aim of this research work was to study the safety and efficiency of local epicardial use of the hydrogel with amiodarone, as well as the influence of its different dosage on the heart rate of mongrel rabbits.
Methods
The experimental study was conducted on 46 animals (26 males and 20 females) in the laboratory for modeling and studying the pathology of the heart and blood vessels of the A.N. Bakulev National Medical Research Center of Cardiovascular Surgery.
Animals keeping and all surgical manipulations were made in accordance with the sanitary norms and requirements for work with laboratory animals of the Russian Academy of Sciences and the Ministry of Health of Russia. The animals were kept on a diet (GOST P5025892) in compliance with the International Recommendations of the European Convention for the Protection of Vertebrates used for experimental purposes. The animals were taken out of the experiment in compliance with the “rules for carrying out work using experimental animals” and “rules for conducting studies using experimental animals”. The study was approved by Ethical Institutional Animal Use and Care Committee.
As a medicinal carrier, the material “Kolegel” was used. “Kolegel” is a hydrogel material based on the biopolymer sodium alginate with a drug (the viscosity of the polymer composition is 4,500 cps at a speed of 20 rpm, the consistency is 27,310 cp, the yield point is 110.5 dyne/cm2).
Sodium alginate is a natural polymer-polysaccharide. It serves as a thickener. The main technological parameter underlies this substance is the viscosity of the composition. The composition with an impregnated drug in this case is used to provide controlled delivery of the drug to the body cavity. The introduction of the liquid composition into the cavity will lead to its leakage, which will lead to insufficient concentration of the drug in the field of application. Difficulties with the distribution of material are possible when using an excessively thick material.
Epicardial application of the hydrogel material is performed in 36 (1–4 groups) of rabbits of different sexes weighing from 3 to 4 kg.
For the experiment, the animals were divided into 5 groups:
- Group No. 1 (n=8)—dose of amiodarone in hydrogel 1 mg;
- Group No. 2 (n=11)—dose of amiodarone in hydrogel 3 mg;
- Group No. 3 (n=7)—dose of amiodarone in hydrogel 6 mg;
- Group No. 4 (n=10)—the control group with the use of hydrogel material without amiodarone;
- Group No. 5 (n=10)—control with the use of the intravenous form of amiodarone in a daily dose of 60 mg. The animals of this group performed pericardiotomy without insertion of anything into the cavity. Amiodarone was administered intravenously, simultaneously with the surgery.
Three rabbits from each group were taken out of the experiment for macro- and microscopic examination of the heart in the following time gaps: 3, 7 and 14 days. The animals were withdrawn from the experiment by intravenous injection of 10 mL of 2% solution of potassium chloride in a state of anesthesia at the indicated observation times. The heart complex with the pericardium was taken for histological examination.
For the prevention of infectious complications, within 3 days after the intervention, cefazolin at a dose of 100 mg/kg was intramuscularly injected.
Rabbits with complicated postoperative wounds and rabbits who died due to acute cardiovascular insufficiency, inadequate anesthesia, and infection were excluded from the evaluation for the sake of the experiment purity and proper statistical processing of data.
The endpoints of the study were the efficacy in the heart rate reduction, as well as the lethality associated with the drug effects.
The following laboratory and instrumental methods were used:
- Invasive measurement of blood pressure in the ear artery [pressure sensors (Spektromed P-23-HL, USA)];
- Saturation measurement (also in the ear artery); measurement of the frequency of respiratory movements;
- Long-term heart rate monitoring on a standard “OmniCare 24C” monitor (Hewlett Packard, USA);
- ECG (standard leads by the “NIHON KONDEN” device); PQ, QRS, QTc were evaluated;
- General blood analysis. The toxic and infectious effects of the hydrogel were assessed by monitoring the level of white blood cells (WBC) and the WBC formula;
- Biochemical blood analysis. Aspartate aminotransferase (AST) and alanine transaminase (ALT) were monitored;
- Atropine test. All rabbits prior to the operation were examined with intravenous atropine at a dose of 50 µg with repeated ECG recording. Atropine was administered at different stages of the study: “Before the procedure”, “immediately after surgery”, “2 hours after the procedure”, “1 day after the procedure” and “3 day after the procedure”. The introduction of Atropine was provided in the design of the study for the purpose of drug-induced heart rate increase in order to assess the dynamics of heart rate in different groups, taking into account the different saturation of amiodarone in atrial myocardial tissues.
Histological examination. Assessment of the degree of inflammatory and fibrous changes in the myocardium of the atria, as well as changes in the thyroid gland and lungs, were performed.
Anesthesia
For the purpose of premedication, an intramuscular injection of atropine at a dose of 0.05 mg/kg was administered to the animals one hour prior to the operation. Next, a solution of xylanite 3 mg/kg and zoletil 100 5 mg/kg was intramuscularly administered directly before the operating field shaving, installation of an intravenous catheter and imposing a tracheostomy. Against the sedative effect for the infusion therapy, a venous catheter was placed into the outermost ear vein. Injection anesthesia was deepened by administering a propofol solution of 1.5 mg/kg. All operations were performed under conditions of artificial lung ventilation with the help of ID-RO-9 device in the normal ventilation mode with the dynamic (according to exhalation pressure) change in the respiratory volume of 18–20 mL/kg/min and the respiration rate of 16–22 min, with oxygen supply into the inhaled mixture up to 50%. For the intubation of the trachea, the tube No. 2.5/3 was used. Miorelaxation and deepening of anesthesia for the main stage of the operation was carried out by intravenous administration of a propofol solution of 10 mg/kg, zoletil 100 7.5 mg/kg and xylanite 5 mg/kg. The operations were performed under conditions of normothermia.
Surgical technique
The animal was placed on the right side. Then, the operating field was treated 2 times with iodine solution. Access to the heart was obtained through a lateral thoracotomy on the left side of IV–V intercostal space, the left lung was protected by gauze tampons. Further, a pericardiotomy of 1–2 mm in the atrial projection was performed. Using a syringe, the hydrogel with amiodarone was applied in the volume of 2 mL (in 4-fold concentrations) on the surface of both atria. The pericardial cavity was not sutured. In turn, the postoperative wound was sutured layer by layer. In order to prevent pneumothorax, active drainage was established.
Statistical analysis
The normality of the distribution of both quantitative and qualitative data was determined initially (the Shapiro-Wilk test was used). It was found that the structure of the obtained data is partially not described by the law of normal distribution. Therefore, further investigations of the dependencies were carried out using both parametric and nonparametric statistics.
In order to compare several dependent samples, the non-parametric Friedman ANOVA criterion with Bonferroni correction for the number of groups was used. For comparison of two independent samples, a nonparametric Mann-Whitney criterion and a parametric two-sided Student t-test (under normal distribution) were used. In order to compare several independent samples, the non-parametric criterion of Kruskal-Wallis with Bonferroni correction for the number of groups was used. The data is represented as M ± SD (for normal distribution of values) or Me (Q1; Q3) for the distribution of values other than normal. The reliability of the used statistical estimates was at least 95%. The used for calculations software was Microsoft Office Excel 2007 and STATISTICA 10.0 (Statsoft, USA).
Results
It is important to emphasize that the groups at their comparison were initially comparable in all studied instrumental and laboratory parameters—there was no statistically significant difference (Table 1).
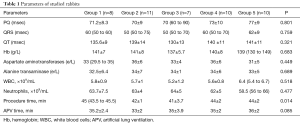
Full table
The dynamics of the average heart rate by groups is presented in Table 2. As can be seen from the table, the initial heart rate by the groups was comparable (P=0.443). After the atropine test, the mean heart rate between the groups also did not differ (P=0.285). After the intervention, there were statistically significant differences between the groups (Table 2).
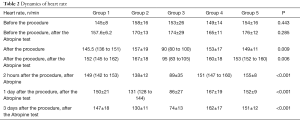
Full table
After the hydrogel with amiodarone application, the following changes in heart rate in the Groups were observed (Figure 1):
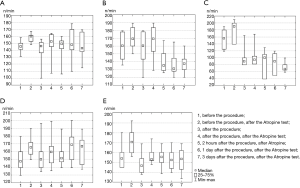
In Group No. 1 (Figure 1A) immediately after the intervention the heart rate was 167±18 beats/min, which is comparable to the initial heart rate in this group (P=0.866). After the administration of atropine, the heart rate significantly increased (P=0.012) up to 152 (145 to 162) beats/min. Two hours after the intervention and administration of atropine, the heart rate decreased to 149 (142 to 153), although the statistical significance was the threshold (P=0.049). After 1 and 3 days, there were no noticeable differences. The analysis of the heart rate dynamics at control points after hydrogel application (comparison of several dependent groups—the Friedman ANOVA criterion) shows that there is yet no statistically significant differences (P=0.244).
Group No. 2 (Figure 1B) had a similar dynamic of the heart rate that was observed immediately after the intervention and after the administration of atropine. However, 2 hours after the surgery and atropine administration, the heart rate dropped to 138±12 beats/min (P=0.003) and stayed within the indicated limits for at least 3 days.
Group No. 3 (Figure 1C) had a decrease in the heart rate immediately after hydrogel application and maintained at that level for at least 3 days. Moreover, with the gradual release of amiodarone from hydrogel, and its accumulation in the atrial tissues, this effect is more obvious (on day 3, the lowest heart rate in the group is noted).
In Group No. 4 (Figure 1D), the heart rate was the same before and after the surgery (P=0.061). The introduction of atropine, as expected, increased the heart rate. The analysis of the heart rate dynamics after the application of the hydrogel without amiodarone at control points (comparison of several dependent groups—the Friedman ANOVA criterion) showed no difference (P=0.076).
In Group No. 5 (Figure 1E), there was a significant reduction in the heart rate after administration of 60 mg of amiodarone (P=0.005), which increased after atropine and further remained without any significant dynamics (P=0.711).
Concerning the dynamics of PQ, QRS, QT intervals duration in groups, it is important to note the following: in groups No. 1 and No. 4—there was no statistically significant dynamics; in the 2nd group—the PQ interval increased (P=0.002); in the 3rd group—the PQ interval (P=0.001) and the QT interval (P<0.001) increased; in group 5—the QT interval also increased (P= 0.045) (Table 3).
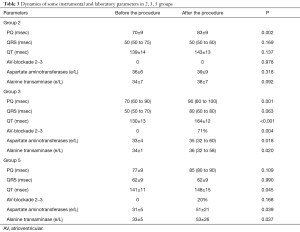
Full table
The violation of conductivity after the intervention was revealed in groups No. 3 and No. 5—in 71% and 20% of cases, respectively.
These groups also had a noticeable statistically significant increase in the level of liver enzymes (AST, ALT) (Table 3).
In all groups after the surgery, hemoglobin (Hb) levels decreased, and WBC and neutrophil levels, on the other hand, increased.
Histological examination
Generally, groups, where hydrogel with amiodarone was used, had a similar morphological pattern. Figure 2 shows the histological picture of the myocardium of the right atrium on the 14th day after the application of the hydrogel with amiodarone. Myocardium of the right atrium has a normal structure without visible changes in the light level and without signs of inflammation or damage. There are no ischemic and contractural injuries. Cardiomyocytes have a moderate uneven hypertrophy. Interstitium of the myocardium has a weakly expressed focal fibrosis (Figure 2).

Discussion
There are still unresolved issues of the use of drugs with local delivery—the duration of their action, toxicity, the preservation of the therapeutic effect of the drug, the path of elimination. These questions are especially important in cardiosurgical practice in connection with the fact that any toxic effects on the heart can lead to some constrictive changes in the myocardium, infectious and inflammatory complications, exudative pericarditis, etc. (17,18).
It is worth noting that there were no technical difficulties on the use of the hydrogel with amiodarone during conduction of this manipulation in the course of our study. The entire procedure for the hydrogel administration took only a few minutes, which makes it possible to significantly prolong the duration of the main stage of surgical intervention.
In the group with intravenous administration of amiodarone, there was a statistically significant increase in the level of liver enzymes. Obviously, these changes are due to the systemic effect of the drug, which is significantly reduced with a local epicardial application. With intravenous administration of the drug, a significant reduction in the heart rate was recorded, however, the development of cardiac conduction abnormalities, as well as prolongation of the QT interval, were failed to be avoided.
During the study of group No. 4, it was noted that the effect of the hydrogel on the heart was accompanied by an increase in the level of WBC, however, when compared with a group with intravenous administration, this increase is statistically insignificant. In this group, there was a significant increase in the heart rate that was apparently due to surgical manipulation. Taking these changes, as well as pathomorphological studies into account, it can be concluded that the hydrogel is not an aggressive infectious agent, despite direct application to the epicardium.
In group No. 3, the concentration of amiodarone before the start of the experiment was considered as toxic (6 mg), and after application there was an expected increase in the number of incidences of cardiac conduction disorders (up to 70%). There also was a significant increase in AST and ALT. The data of this group had an important role in the determination of the toxic concentration of amiodarone in the gel with its epicardial application.
The study results show the efficiency of local epicardial hydrogel with amiodarone application in the heart rate reduction. However, the safety of the method application was concerning. In this regard, our work was focused on the evaluation of the safety of the hydrogel application: we assessed the drug safety using laboratory and histological methods, ECG and functional research methods. According to the results of the work, it is worth noting that the technique has a sufficient safety profile.
For assessment of changes in the myocardium of the atria and ventricles, as well as pericardium, all rabbits underwent pathomorphological examination.
The results showed no inflammatory or post-inflammatory changes in the myocardium after hydrogel application with amiodarone. The myocardium after the application is structured. In comparison with the initial data, there was no progression of fibrosis. This histological picture is important since the initially considered main problem was the development of infectious and inflammatory changes due to the application of the hydrogel.
A possible complication due to the local application of amiodarone in the form of a hydrogel is the development of sinoatrial and AV blockades. In order to solve this problem, the correct calculation of amiodarone dosage in the gel is necessary. In this study, the most adequate dose of amiodarone in hydrogel was 3 mg. This concentration of the drug has demonstrated a significant decrease in the average heart rate, without development of atrial and AV blockages. With an increase in the concentration of amiodarone to 6 mg, there was a noted significant increase in the number of incidences of cardiac conduction abnormalities (up to 70%), and with a decrease in the dosage of amiodarone to 1 mg, there was no noticeable influence of amiodarone on the reduction in heart rate. The hydrogel itself does not affect the conductive system.
To evaluate the effectiveness of the hydrogel with amiodarone, we considered different options of control: determination of amiodarone concentration in the atria, blood, determination of the refractory period effectiveness by sewing up temporary myocardial electrodes. To assess the refractory period, insertion of temporary atrial electrodes would be necessary for a certain time. Given the anatomical features of the rabbit heart (its small size), it would technically complicate the study. Given the increased risk of additional injury to atrial myocardium when inserting atrial electrodes, we ultimately abandoned the idea. However, we decided that ECG monitoring and long-term heart rate monitoring would provide us with enough information to confirm the effectiveness of a local application of amiodarone.
It should be also noted that the economic component of the local technique for the use of amiodarone is of particular importance. When compared to intravenous and oral forms of the drug application, the latter one demands significantly less economic costs.
The results of the study demonstrated a sufficient effectiveness of a local epicardial application of the hydrogel with amiodarone in the reduction of the heart rate with a sufficient safety profile in mongerel rabbits.
Conclusions
Based on the conducted study, the following conclusions were drawn.
In an animal experiment, it was demonstrated that the hydrogel materials with amiodarone have sufficient safety for local epicardial delivery of the drug. With this method of drug use, systemic side effects are minimized, having a proper profile of effectiveness along.
The hydrogel with amiodarone is effective in the heart rate reduction in mongrel rabbits, compared to the control group and the group with intravenous drug administration (P<0.001). In this study, the most adequate dose of amiodarone in hydrogel was 3 mg. With this dosage, episodes of atrial and AV conduction disorders can be avoided.
In our opinion, the limitation of the study is the lack of measurement of amiodarone concentration in the blood and tissues of the atrial myocardium in the tested animals. This would provide us with more information on this study, but would significantly increase the financial costs of it. That is why we limited ourselves with those methods which were used in this study.
Acknowledgments
Funding: The article is written in the framework of the project of the Russian Science Foundation (grant no. 18-74-10064).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethical Institutional Animal Use and Care Committee (Protocol No. 3 dated 08.02.2017).
References
- Davis EM, Packard KA, Hilleman DE. Pharmacologic prophylaxis of postoperative atrial fibrillation in patients undergoing cardiac surgery: beyond beta-blockers. Pharmacotherapy 2010;30:274e-318e. [PubMed]
- Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003;108:3006-10. [Crossref] [PubMed]
- Minin SM, Nikitin NA, Shabanov VV, et al. Evaluation of CZT SPECT imaging for cardiac sympathetic innervation in healthy individuals and patients with atrial fibrillation. RusOMJ 2018;7:e0308. [Crossref]
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;123:104-23. [Crossref] [PubMed]
- Mitchell LB. CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation following cardiac surgery. Can J Cardiol 2011;27:91-7. [Crossref] [PubMed]
- Reinhart K, Baker WL, Ley-Wah Siv M. Beyond the guidelines: new and novel agents for the prevention of atrial fibrillation after cardiothoracic surgery. J Cardiovasc Pharmacol Ther 2011;16:5-13. [Crossref] [PubMed]
- Van Wagoner DR. Colchicine for the prevention of postoperative atrial fibrillation: a new indication for a very old drug? Circulation 2011;124:2281-2. [Crossref] [PubMed]
- Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 2011;124:2290-5. [Crossref] [PubMed]
- Bokeriya OL, Akhobekov AA, Shvarts VA, et al. Meta-analysis of clinical studies on the use of statins for prevention of atrial fibrillation soon after coronary bypass surgery. Klin Med (Mosk) 2016;94:85-92. [PubMed]
- Bockeria OL, Shvartz VA, Akhobekov AA, et al. Statin therapy in the primary prevention of early atrial fibrillation after coronary artery bypass grafting. Indian Heart J 2016;68:792-7. [Crossref] [PubMed]
- Bockeria OL, Shvartz VA, Akhobekov AA, et al. Statin therapy in the prevention of atrial fibrillation in the early postoperative period after coronary artery bypass grafting: a metaanalysis. Cor et Vasa 2017;59:e266-71. [Crossref]
- Ayers GM, Rho TH, Ben-David J, et al. Amiodarone instilled into the canine pericardial sac migrates transmurally to produce electrophysiologic effects and suppress atrial fibrillation. J Cardiovasc Electrophysiol 1996;7:713-21. [Crossref] [PubMed]
- Canbaz S, Erbas H, Huseyin S, et al. The role of inflammation in atrial fibrillation following open heart surgery. J Int Med Res 2008;36:1070-6. [Crossref] [PubMed]
- Feng XD, Wang XN, Yuan XH, et al. Effectiveness of biatrial epicardial application of amiodarone-releasing adhesive hydrogel to prevent postoperative atrial fibrillation. J Thorac Cardiovasc Surg 2014;148:939-43. [Crossref] [PubMed]
- Takeda T, Shimamoto T, Marui A, et al. Topical application of a biodegradable disc with amiodarone for atrial fibrillation. Ann Thorac Surg 2011;91:734-9. [Crossref] [PubMed]
- Vassallo P, Trohman RG. Prescribing amiodarone: an evidence- based review of clinical indications. JAMA 2007;298:1312-22. [Crossref] [PubMed]
- Darsinos JT, Karli JN, Samouilidou EC, et al. Distribution of amiodarone in heart tissues following intrapericardial administration. Int J Clin Pharmacol Ther 1999;37:301-6. [PubMed]
- Bokeriya LA, Bokeriya OL, Sivtsev VS, et al. Experimental Evaluation of Biodegradable Film Compositions Based on Gelatin with Colchicine. Bull Exp Biol Med 2016;161:414-8. [Crossref] [PubMed]


