
Lower extremity arteries
Introduction
Lower extremities are an integral part of human body with prime function to provide mobility. Arterial system supplies blood to muscles and bones of lower limbs. Knowledge about anatomy and various imaging modalities available is important in identifying various disease conditions and its management. Ultrasound and doppler is quick, noninvasive with no known adverse effects. Ultrasound is operator, equipment dependent and has a learning curve for its use and interpretation. CT angiogram is rapid, provide quick overview of the extremity arteries and is widely used especially in emergency situations like acute limb ischemia. Drawback of CT is use of iodinated contrast media and potential risk of Contrast induced nephropathy. MRI can provide vital information without use of contrast media. It can also evaluate vessel wall and dynamic disease processes. MRI study is limited by its availability and prolonged scanning time. Catheter angiography is gold standard for evaluation of peripheral arterial diseases and has therapeutic role; however, it is invasive and requires trained operator for its safe performance.
Anatomy of lower limb arteries
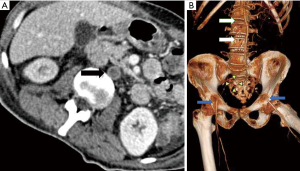
The lower extremity arteries start from common iliac artery origins from trifurcation of abdominal aorta into common iliacs and median sacral artery (Figure 1), towards anterior and left of the fourth lumbar vertebral body (1). The common iliac arteries bifurcates into Internal and external iliac arteries. The external iliac artery continues into the lower limbs as common femoral artery. The artery above inguinal ligament is known as external iliac artery and below is common femoral artery. The common femoral artery gives small branches namely superficial epigastric artery, external pudendal artery and the superficial circumflex artery prior to its bifurcation (2). The common femoral artery bifurcates into superficial and deep femoral artery also known as profunda femoris artery. The profunda femoris gives rise to medial and lateral circumflex femoral arteries and perforating branches to the thigh muscle (2). The superficial femoral artery continues along the medial side of the thigh through the adductor canal also known as hunter’s canal. The superficial femoral artery exits the adductor canal, courses posterior to the lower end of femur where it is known as popliteal artery after it exists the adductor hiatus in the region of popliteal fossa (2). The superficial femoral artery gives off the descending genicular branch prior to existing the adductor hiatus which gives supply to the knee and can also anastomose with other popliteal artery branches (1). Popliteal artery provides rich collaterals to the knee joint in the form of superior and inferior geniculate arteries on either side of the joint apart from middle genicular branch and muscular branches (Figure 2). For interventional purposes the popliteal artery is divided into three segments as follows: P1 segment, from intercondylar fossa to proximal edge of patella. P2 segment, from proximal part of patella to center of knee joint. P3 segment (below knee popliteal artery), from center of knee joint space to origin of anterior tibial artery (3).
Popliteal artery bifurcates into anterior tibial artery and tibioperoneal trunk approximately at the level of proximal tibiofibular joint (Figure 3). The anterior tibial artery courses along the lateral compartment of leg after piercing the interosseus membrane close to tibia and runs along anterior surface of interosseus membrane (2). The anterior tibial artery continues in foot as dorsalis pedis artery. At the level of malleoli the artery lies lateral to tendon of extensor hallucis longus which serves as anatomical landmark for clinical pulse palpation. Tibioperoneal trunk further divides into peroneal artery and posterior tibial artery which runs along posteromedial aspect of leg. The posterior tibial artery forms the medial and lateral plantar arches. The plantar arch gives rise to metatarsal and the plantar digital arteries. There are branches which communicate between the arches to the dorsalis pedis artery which hypertrophy in diseased states and help in maintaining blood supply to toes. Peroneal artery terminates into medial and lateral calcaneal branches above the ankle joint. These branches freely communicate with dorsalis pedis artery and posterior tibial artery and help in collateralizing the foot in case of diseased conditions (2).
Ultrasound
Ultrasound is readily available, inexpensive, rapid, noninvasive and accurate (4). Ultrasound is operator dependent and examination may be limited by arterial wall calcifications. For stenosis greater than 50% diameter reducing by angiography, duplex scanning had a sensitivity of 82%, a specificity of 92%, a positive predictive value of 80%, and a negative predictive value of 93% (5). For accurate assessment of velocity by duplex the sample volume cursor should be on the vessel studied and should include at least half to one third of the artery studied (6). The grade of stenosis is quantified based on direct B mode appearance of the lesion, indirect data such as peak systolic velocity, end diastolic velocity and the wave pattern of the spectral waveform. Monophasic waveform is a sign of significant lesion proximally which is characterized by the entire spectrum being on one side of the baseline, slow acceleration towards peak systolic velocity, low amplitude and continuous diastolic flow-parvus tardus wave pattern (7).
CT angiography (CTA)
CTA has high spatial resolution, rapid, noninvasive, depicts the exact anatomical location of lesions, length and extend of the lesions, probable diagnosis and etiology. Ionizing radiation is a concern in younger individuals and children and its use in pregnant females is restricted especially during first trimester. CTA is widely used for treatment planning, guides choice of hardware and access site. CTA demonstrates sensitivity of 87% and specificity of 93% in comparison to DSA in diagnosing occlusive peripheral arterial disease and a sensitivity of 92%, specificity of 91% and accuracy of 91% for detection of steno occlusive peripheral arterial disease (8).
CTA covers from level of diaphragm to toes in two phases. First phase helps in identification of proximal extent of occlusion or stenotic disease. Second phase helps in identification of reformation and distal runoff. Limitation from calcifications can be eliminated by using dual energy scans or plain mask images can be used to subtract from contrast images. The extent and severity of disease is characterized based on percentage reduction in the area of the lumen, number of lesions and also based on length of the diseased segment. Grade I is characterized as no or mild stenosis (less than or equal to 49% stenosis), grade 2-moderate stenosis (50% to 69% stenosis), grade 3-severe stenosis (70% to 99% stenosis) and grade 4-occlusion (100% luminal obliteration). Grade 3 and 4 are considered to be clinically significant (9). Based on length and degree of stenosis the lesions are classified into the various TASC II categories which are used as guide for revascularization (10).
MR angiography (MRA)
MRA is safe, noninvasive investigation with high tissue resolution. It is safe in patients with mild renal dysfunction, pregnant patients and children. MRA can also be performed without contrast using time of flight (TOF) angiography, Quiescent-Interval single-shot unenhanced MRA (QISS) (11) and T1 weighted dark blood MRA (12). Contrast MRA provides adequate information on distal reformation and runoff which is comparable to CTA. TOF has sensitivity of 43–67% and specificity of 74–89% for detection of significant (75%) stenosis (13). Contrast MRI has sensitivity of 96.6–97% and specificity of 95.2–96.1% (14).
Digital subtraction angiography
Digital subtraction angiography is invasive, highly accurate imaging technique with therapeutic implications. It involves use of iodinated contrast media however techniques like carbon dioxide angiography helps in limiting the use of iodinated contrast media. Carbon dioxide angiography is also preferred in patients with limited cardiac reserve as it does not increase intravascular volume since it gets rapidly dissolved in blood and excreted through lungs.
Specific diseases of extremity arteries
Atherosclerotic disease
Atherosclerotic diseases involve elderly individuals with risk factors like chronic Diabetes Mellitus and Hypertension. The disease is typically characterized by eccentric plaques (Figure 4) which may or may not have calcifications with reduction in compliance of vascular system resulting in biphasic wave pattern on doppler imaging. Clinically it manifests as gradually worsening claudication pain in the limbs, rest pain or as nonhealing ulcer in the limb with or without major tissue loss (10).
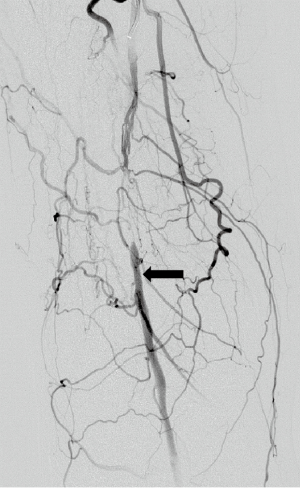
There are a number of collateral pathways in limb arteries. In iliac artery occlusions the lumbar arteries get recruited which further communicate with deep circumflex iliac artery and reform external iliac artery. In case of aortic and iliac involvement there is recruitment of intercostal collaterals through which the superior intercostal, internal mammary arteries and lumbar vessels anastomose via inferior epigastric and deep circumflex iliac arteries to reform the external iliac artery (15). There are rich collaterals within pelvis between branches of internal iliac arteries which gets recruited in case of common iliac artery or internal iliac artery proximal occlusions. These branches collateralize via obturator arteries, internal pudendal arteries, superior and middle rectal arteries (16). In case of common femoral artery occlusion there are collaterals from internal iliac branches namely obturator artery, internal pudendal artery, inferior and superior gluteal arteries which anastomose with medial and lateral collateral branches of profunda femoris which reforms profunda femoris and superficial femoral arteries (17). In cases of superficial femoral artery occlusions there are collateral pathways from profunda femoris artery which reforms distal part of superficial femoral artery or popliteal artery through descending geniculate artery or via genicular branches of popliteal artery.
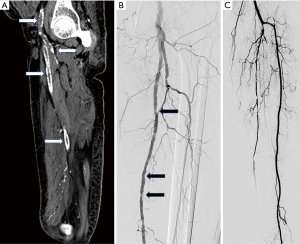
Popliteal artery aneurysms (Figure 5) are either true or false (pseudoaneurysm). True aneurysms have all 3 layers in their wall which are abnormally dilated and pseudoaneurysm have deficiency in at least one of the layers. Popliteal artery size of more than 7 mm is classified as aneurysm (18). The popliteal artery aneurysms are found to have high association with abdominal aortic aneurysms. Tuveson et al. reported popliteal artery aneurysm prevalence of 19.1% in patients of abdominal aortic aneurysms on radiological examination (19). They used size definition of more than 12 mm size to classify as popliteal aneurysm. Aneurysms clinically manifest with either rupture when they present with painful acute swelling in region of popliteal fossa or with distal thromboembolic phenomenon with acute limb ischemia or small gangrenous or pre-gangrenous changes in toes.
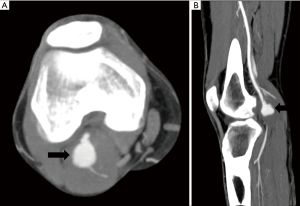
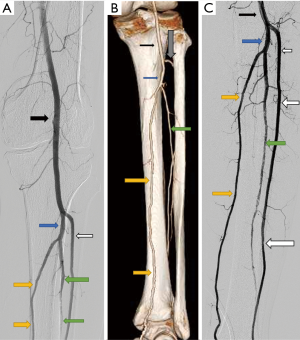
Popliteal artery entrapment syndrome (Figure 6), there is abnormal compression of popliteal artery due to abnormal course of popliteal artery, abnormal insertion of Medial head of gastrocnemius, or accessory slip or due to compression from popliteus muscle (18). It causes repeated trauma to artery and can result in thrombosis or distal thromboembolic phenomenon from microthrombi generated.
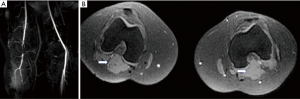
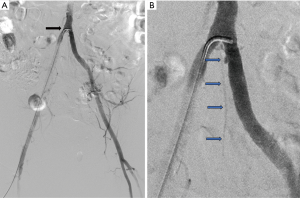
Cystic adventitial disease of popliteal artery (Figure 7) is described as formation of mucoid cysts in adventitia of artery causing luminal compression. Ultrasound typically shows anechoic or hypoechoic cystic structures in close relation to popliteal artery. MRI is considered to be extremely useful in suspected cases as the cysts appear hyperintense on T2 and variable intensity on T1 due to various degree of proteinaceous content within the cysts which are located in arterial wall and produce luminal narrowing on MR angiography (18).
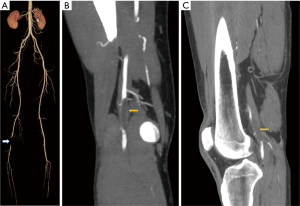
Arteriovenous malformations
Arteriovenous malformations are congenital disorder characterized by abnormal connection between the arteries and veins known as nidus with absence of intervening capillary bed. Arteriovenous fistula is characterized by direct communication between the artery and vein. Arteriovenous fistulas are either congenital or acquired due to trauma, penetrating injuries or iatrogenic. They are seen in young or middle age individuals and present with symptoms of pain, swelling or torrential bleeding. Long standing arteriovenous malformations or fistulas may present with hypertrophy of the limb (Figure 8) and may be associated with cardiac failure. Ultrasound shows dilated lower limb veins and bunch of serpiginous vascular structures with arterial wave pattern. The feeding arteries show low resistance monophasic wave pattern of high velocity which differentiates it from pattern of parvus tardus which is typically low velocity flow. CT or MR angiogram confirms the diagnosis and demonstrates feeding arteries and outflow veins. DSA delineates the site of fistula or nidus, along with information on the arterial feeders and venous outflow. DSA demonstrates early filling of the venous system which is diagnostic of an arterial venous malformation.
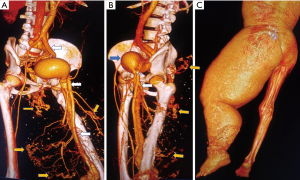
Vasculitis
A number of vasculitis conditions including substance abuse affect lower limb arteries (20). These conditions typically appear as arterial wall thickening in acute phase associated with thrombosis of vessels (Figure 9) which later on lead to stenotic lesions, dissections and aneurysm formation. Doppler is an early, rapid effective tool for diagnosis of vasculitis and CT, MRI (vessel wall imaging) or PET CT helps in identification of disease activity based on contrast enhancement or radiotracer uptake respectively (21).
Buerger disease
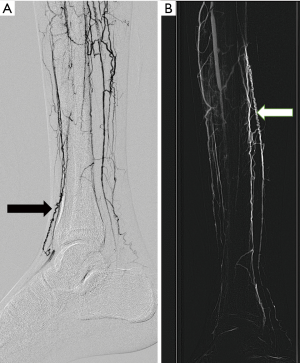
Thromboangitis obliterans is a segmental occlusive inflammatory disease of small and medium sized arteries and veins of the extremities affecting young adults below the age of 45, history of tobacco abuse is a consistent finding. Superficial thrombophlebitis and Reynaud’s phenomenon occur in approximately 40% of patients (22). Radiologically it involves below knee vessels, proximal vessels are essentially normal and there is no calcification of involved vessels. Angiographically there are corkscrew collaterals (Figure 10) along occluded vessels (23).
Conclusions
Knowledge of basic anatomy of lower extremity arteries and various imaging methods available for its evaluation helps one to select appropriate imaging modality to answer specific clinical questions and reach a confident diagnosis.
Acknowledgments
The authors would like to thank Dr. Sandeep S Hedgire, MD for his contribution towards this article in the form of Images for subsection on cystic adventitial disease of popliteal artery and popliteal entrapment syndrome.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Butler P, Mitchell AWM, Ellis H, Healy JC. Applied radiological anatomy. Cambridge University Press; 2012:400.
- Bajzer Christopher T. Arterial supply of the lower extremities - Guide to Peripheral and Cerebrovascular Intervention - NCBI Bookshelf. Remedica Pub, 2004:291.
- Scheinert D, Werner M, Scheinert S, et al. Treatment of Complex Atherosclerotic Popliteal Artery Disease With a New Self-Expanding Interwoven Nitinol Stent: 12-Month Results of the Leipzig SUPERA Popliteal Artery Stent Registry. JACC Cardiovasc Interv 2013;6:65-71. [Crossref] [PubMed]
- Koshy CG, Chacko BR, Nidugala Keshava S, et al. Diagnostic accuracy of color Doppler imaging in the evaluation of peripheral arterial disease as compared To digital subtraction angiography. Vasc Dis Manag 2009;6:2-9.
- Kohler TR, Nance DR, Cramer MM, et al. Duplex scanning for diagnosis of aortoiliac and femoropopliteal disease: a prospective study. Circulation 1987;76:1074-80. [Crossref] [PubMed]
- Knox RA, Phillips DJ, Breslau PJ, et al. Empirical findings relating sample volume size to diagnostic accuracy in pulsed doppler cerebrovascular studies. J Clin Ultrasound 1982;10:227-32. [Crossref] [PubMed]
- Zacharia BE, Hickman ZL, Grobelny BT, et al. Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 2010;21:221-33. [Crossref] [PubMed]
- Sun Z. Diagnostic accuracy of multislice CT angiography in peripheral arterial disease. J Vasc Interv Radiol 2006;17:1915-21. [Crossref] [PubMed]
- Napoli A, Anzidei M, Zaccagna F, et al. Peripheral Arterial Occlusive Disease: Diagnostic Performance and Effect on Therapeutic Management of 64-Section CT Angiography. Radiology 2011;261:976-86. [Crossref] [PubMed]
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45 Suppl S:S5-67.
- Edelman RR, Sheehan JJ, Dunkle E, et al. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: Technical considerations and clinical feasibility. Magn Reson Med 2010;63:951-8. [Crossref] [PubMed]
- Mihai G, Chung YC, Merchant A, et al. T1-weighted-SPACE dark blood whole body magnetic resonance angiography (DB-WBMRA): Initial experience. J Magn Reson Imaging 2010;31:502-9. [Crossref] [PubMed]
- McCauley TR, Monib A, Dickey KW, et al. Peripheral vascular occlusive disease: accuracy and reliability of time-of-flight MR angiography. Radiology 1994;192:351-7. [Crossref] [PubMed]
- Loewe C, Schoder M, Rand T, et al. Peripheral Vascular Occlusive Disease: Evaluation with Contrast-Enhanced Moving-Bed MR Angiography Versus Digital Subtraction Angiography in 106 Patients. AJR Am J Roentgenol 2002;179:1013-21. [Crossref] [PubMed]
- Kim J, Won JY, Park SI, et al. Internal thoracic artery collateral to the external iliac artery in chronic aortoiliac occlusive disease. Korean J Radiol 2003;4:179-83. [Crossref] [PubMed]
- Hardman RL, Lopera JE, Cardan RA, et al. Common and Rare Collateral Pathways in Aortoiliac Occlusive Disease: A Pictorial Essay. AJR Am J Roentgenol 2011;197:W519-24. [Crossref] [PubMed]
- Petrov VF. Collateral arterial circulation of the leg in postcatheterization iliofemoral occlusion. Indian J Vasc Endovasc Surg 2018;5:120.
- Wright LB, Matchett WJ, Cruz CP, et al. Popliteal Artery Disease: Diagnosis and Treatment. RadioGraphics 2004;24:467-79. [Crossref] [PubMed]
- Tuveson V, Löfdahl HE, Hultgren R. Patients with abdominal aortic aneurysm have a high prevalence of popliteal artery aneurysms. Vasc Med 2016;21:369-75. [Crossref] [PubMed]
- Parsa P, Rios A, Anderson LN, et al. Isolated lower extremity vasculitis leading to progressive critical limb ischemia. J Vasc Surg cases Innov Tech 2017;3:119-22.
- Kermani TA, Warrington KJ. Lower extremity vasculitis in polymyalgia rheumatica and giant cell arteritis. Curr Opin Rheumatol 2011;23:38-42. [Crossref] [PubMed]
- Arkkila PET. Thromboangiitis obliterans (Buerger’s disease). Orphanet J Rare Dis 2006;1:14. [Crossref] [PubMed]
- Busch K. Buerger’s disease (thromboangiitis obliterans): clinical features and assessment by colour duplex ultrasound. Australas J ultrasound Med 2011;14:18-22. [Crossref] [PubMed]


