Improved diagnosis and prognosis using Decisions Informed by Combining Entities (DICE): results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE)
Introduction
The role of late gadolinium enhancement (LGE) in magnetic resonance (MR) myocardial imaging is rapidly increasing due to the high-resolution and high-contrast nature of the cardiovascular magnetic resonance imaging (MRI) technique (1-4). The presence of LGE signal indicates an increase in extra cellular space, and in the context of ischemic heart disease, typically indicates a sub region of myocardium that may be permanently damaged, and consequently portends a poor prognosis (5-7). Further, myocardial perfusion imaging (MPI) could be performed along with an LGE examination to additionally identify regions of myocardium at risk of future infarct (6,8-11). However, the performance of MPI by MRI has dramatically lagged the performance and development of LGE, in large part due to the relatively low contrast between normal and at-risk regions of myocardium. Even when quantitative analysis of MRI MPI data is performed, it was recently noted by Bratis and Negal that “The development of an universal, reproducible, accurate and easily applicable tool in cardiovascular magnetic resonance (CMR) perfusion analysis remains a challenge and will substantially enforce the role of perfusion CMR in improving clinical care” (12). In a similar manner, interpretation of MPI data acquired using the more-established modality of gated single photon emission computed tomography (SPECT) examination also suffers from low and variable contrast, with contamination from well-documented sources of artifact (13-16). Further, even in cases where interpretation is restricted to evaluation of rest-stress myocardial perfusion reserve, the presence of noise and the variability of data places limits on reproducibility (17). Inclusion of demographic, risk factors, and symptom severity does not generally improve interpretation, since MPI is typically performed for patients at intermediate risk, largely identified using this additional data (18).
To improve the value of MPI data consider the following thought experiment (one that can only rarely be performed in practice): to compensate for the various sources of inaccuracy in MPI data, introduce a second modality to separately assess the myocardial perfusion status. The rationale for this approach being that the effects of random noise can be reduced by combining data and sources of bias that might adversely affect one modality may not be present in the second modality. We might expect that identification of low perfusion regions is more accurate when the two modalities are in agreement. However, in patients where the two modalities are divergent, a third test might be considered as a tiebreaker (at least in a thought experiment). It can be appreciated that the approach of pooling results between modalities rapidly leads to increased cost and is not generally a practical solution, but nevertheless it is useful to demonstrate the origin of the benefits of the approach introduced here: Decisions Informed by Combining Entities (DICE).
We hypothesize that removing systematic sources of bias and noise in MPI data leads to improved performance of the test. Here we introduce a new approach, DICE, whereby a regression model is generated that specifically incorporates image-measured variables, with an increasing number of variables systematically reducing noise. Initialization of the model requires acquisition and interpretation of data from two separate MPI tests performed on a common patient population. However, during implementation, data from only one MPI modality is required. Importantly, even during initialization, DICE does not require knowledge of additional test results such as coronary artery status or of outcome data.
Methods
Study population
Among the 935 Women’s Ischemia Syndrome Evaluation (WISE) study participants a sub-population consisting of 213 women with suspected myocardial ischemia were recruited and undergone a clinically indicated gated-SPECT evaluation, a study-directed MRI evaluation, and a clinically-indicated coronary artery angiographic examination. This prospective sub-study was performed at a single WISE site, the University of Alabama at Birmingham between the dates of November 1993 and October 1998, and included WISE participants with no contraindications for MR examination. All subjects provided written informed consent using forms and procedures approved by the university’s Institutional Review Board. The MR and gated-SPECT studies were performed on the same day and readers were blinded to all women’s data. Coronary artery cath data were available and read at a WISE core laboratory. Here, 70% was regarded as a high-grade stenosis.
Baseline MPI and left ventricular (LV) function evaluation
The WISE study design and methodology has been previously described (19-21). In brief, upon enrollment into WISE, demographic data, risk factors for coronary artery disease (CAD), medical and reproductive history, and functional capacity were collected as well as blood sampling for Lipid Core Laboratory evaluation. The study was structured with a pilot (n=64) and implementation (n=165) phase. During the pilot phase, the imaging protocol for the non-invasive approaches of MR and gated-SPECT underwent optimization, and each protocol was fixed for the implementation phase.
Gated-SPECT
The gated-SPECT examination was performed in parallel with the MR examination. A baseline gated-SPECT examination was obtained (ADAC, Milpitas, CA). Following this, women were sent to the MR suite where at three minutes following infusion of dipyridamole (0.56 mg/kg over four minutes) technetium-99m sestamibi (MIBI) was administered. Following the MR study, women returned to the nuclear cardiology laboratory for hyperemic gated-SPECT imaging. During the pilot phase, gated-SPECT studies were performed with either thallium-201 and MIBI used for post dipyridamole or with MIBI (low dose/high dose) used for both baseline and hyperemic gated-SPECT MPI (22). During the implementation phase, the MIBI (low dose/high dose) protocol was used exclusively for gated-SPECT MPI. The gated-SPECT MPI data were evaluated by a consensus of two or more readers experienced in the interpretation of gated-SPECT and without knowledge of the women’s prior clinical history or angiographic results (19). Gated-SPECT data were entered into an analysis program that fitted the data to a 3D model of the LV, and end-systolic and end-diastolic volumes were extracted with minimal user interaction. Data were of sufficient quality for the automatic volumetric extraction in 149 (66%) cases (from the implementation phase data). A consensus of experienced SPECT readers evaluated the SPECT data to adjudicate regions of myocardial perfusion deficit. Here a patient with at least one region of perfusion deficit is coded as ‘1’ for disease present and ‘0’ for disease absent.
MR
MR cine images were acquired using a Philips ACS 1.5T scanner (Philips Medical System, Best, The Netherlands). Non-invasive MPI was performed in the short axis orientation using a bolus injection of gadolinium (0.1 mmol/kg at a rate of 4-6 mL/sec) followed by a 10 mL saline flush. LV function was evaluated from serial cine slices acquired in the short-axis orientation. End-diastolic and end-systolic volumes were extracted from endocardial contours semi-automatically drawn using the MASS program (Medis, Leiden, The Netherlands). The myocardial wall thickness was measured at end-diastole in the horizontal long axis view at the mid-ventricular level in the septal wall and in the opposite free wall. The two measurements were averaged to form the mean myocardial wall thickness. A consensus of experienced MRI readers evaluated the MRI data to adjudicate regions of myocardial perfusion deficit, providing the qualitative reading, MRI qualitative perfusion assessment (MRIQL). Using previously described methods, a semi-quantitative reading (MRISQ) of the myocardial perfusion defect was performed (19,20). In brief, the product of the normalized uptake slope and signal gain from baseline to peak myocardial perfusion was evaluated (related to area under the uptake curve). If the product was less that 0.2 of that of the maximum for those with an adequate myocardial perfusion reserve, the region was designated as low perfusion. For patients with an inadequate myocardial flow reserve, a value of 0.3 of the maximum was used. In a similar manner to SPECT, a patient possessing at least one region with a perfusion deficit was coded as ‘1’ for disease present and ‘0’ for disease absent.
DICE modeling
Logistic regression analysis was performed to model the perfusion status of a target modality (e.g., gated-SPECT) by entering into the model the perfusion status determined by a second modality (e.g., MRIQL) along with variables such as ventricular volumetric data derived from the second modality. Only variables obtained from the second imaging modality were considered as candidates for the model. Variables were entered sequentially, and only those variables whose univariate test P-value <0.05 were included in the model, and only those terms that added to the model with a significance level of P<0.05 were retained. The logistic regression equation (LRE) thus generated was converted into probabilities by the standard formula
Values above a certain threshold indicate the presence of a perfusion deficit by the model (DICE model). The threshold was taken as the average perfusion score for the population based on the target modality (i.e., reflecting the percentage of patients that were positive using the target modality). Four DICE models were generated: SPECT modeling MRISQ, SPECT modeling MRIQL, MRIQL modeling SPECT, MRISQ modeling SPECT.
Follow-up procedures
Follow-up consisted of a scripted telephone interview performed by an experienced research coordinator at 6-week after enrollment and annually thereafter. The major adverse cardiovascular events (MACE) followed were cardiovascular-related mortality, first incidence of nonfatal myocardial infarction (MI) or hospitalization for congestive heart failure. Follow-up was 40±16 months. In the event of death, a death certificate and/or hospital record was obtained and a panel of experts adjudicated whether death was cardiovascular related using predetermined criteria.
Statistical analysis
Continuous values were presented as mean ± S.D. and categorical variables as percent frequency. Continuous clinical and demographic characteristics were compared between groups using the independent samples t-test; the chi-square test was used for categorical comparisons. Patients were grouped based on agreement between MPI tests between modalities. The performance of the DICE model was evaluated in receiver-operator characteristic (ROC) analysis, entering the DICE values both as a binarized value and separately as a continuum. The threshold for CAD was ≥70% stenosis. The area under the curve (AUC) for ROC analysis was compared between Original and DICE-assisted interpretation of MPI data for detection of CAD and for prediction of MACE. Kaplan-Meier log-rank statistics were compared between the Original and DICE-assisted MPI interpretation for time to MACE. The AUC and log-rank statistic readings were compared between Original and DICE-assisted readings using t-testing. To assess the performance of the DICE model on the number of parameters included, the model with the largest number of parameters was re-evaluated by manually removing one parameter at a time. All statistical tests were two-tailed and a P-value <0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, Illinois).
Results
Population characteristics and data
The mean age of women was 59±12 years (range 31-86 years); 34% were ethnic minorities, primarily African-Americans. Demographic data for all women and women categorized by MACE are summarized in Table 1. At the end of the 5-year follow-up period, MACE occurred in 25 women (12%) consisting of 12 deaths, 8 hospitalizations for congestive heart failure, and 5 MI’s. Of 230 women, 17 did not complete any imaging procedure, of the remaining 213 women, an additional 10 did not complete the MRI MPI examination, and 6 did not complete the SPECT MPI examination with complete MRI and gated-SPECT perfusion data available for approximately 95% of women (MRI, 95%, SPECT, 97%). Similarly, complete functional data were available in 188 (88%) for MRI, and 147 (69%) for gated-SPECT (LV function variables were available only for the implementation phase for SPECT).
Full table
DICE modeling
The LRE predicting SPECT using MRISQ data is
Where MRISQMSP is the DICE modeled SPECT result using the MRISQ perfusion assessment, ESVi (mL/m2) is end systolic volume index and Wall is the average myocardial wall thickness (mm), both measured by MRI. The threshold to signify a perfusion deficit was 0.25.
The LRE predicting SPECT using MRIQL data is
Where MRIQLMSP is the DICE modeled SPECT result using the MRIQL perfusion assessment, ESVi (mL/m2) is end systolic volume index and Wall is the average myocardial wall thickness (mm), both measured by MRI. The threshold to signify a perfusion deficit was 0.25.
The LRE predicting MRIQL using SPECT data is
Where SPECTMQL is the DICE modeled MRIQL result using the SPECT perfusion assessment and EDVi (mL/m2) is the SPECT-measured end diastolic volume index. The threshold to signify a perfusion deficit was 0.24.
The LRE predicting MRISQ using SPECT data is
Where SPECTMSQ is the DICE modeled MRISQ result using the SPECT perfusion assessment and EDVi (mL/m2) is the SPECT-measured end diastolic volume index. The threshold to signify a perfusion deficit was 0.21.
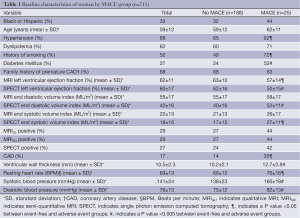
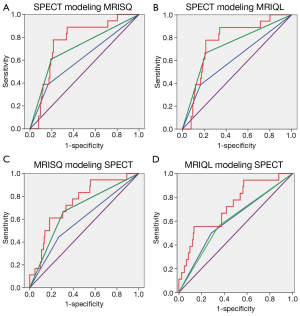
Variables that were entered and rejected in each model included: ejection fraction, end-systolic volume index, stroke volume, and linear cardiac dimensions. The MRISQ and MRIQL data shared common values for all cardiac variables derived from the functional and morphologic scans. Graphical plots of the probability functions generated for each model are shown in Figure 1, along with the threshold value applied to separate normal perfusion from low perfusion.
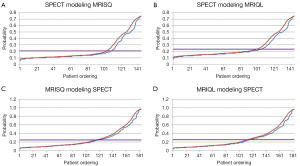
Congruent and incongruent studies
Figure 2 shows a Kaplan-Meier plot for the sub-set of patients where MRISQ and SPECT are in agreement (75% of patients) and a plot where the tests are in disagreement (25%). The log-rank statistic for the set where the two modalities agree is 5.5 (P<0.05), but collapses completely to 0.01 (P=0.9) for the set where the two modalities disagree. The average degree of agreement between modalities and between MRI readings is (74±3)%, and when analyzed using correlation analysis, MRISQ correlates with MRIQL with r=0.29, MRISQ correlates with SPECT with r=0.37 and MRIQL correlates with SPECT with r=0.38 (P<001 for each). Similarly, other variables measured separately by MRI and SPECT only agreed moderately: correlation r value for EF is 0.51 and for EDVi is 0.65 (P<0.001 for each). Conversely, the correlation agreement between the thresholded DICE models were as follows: MRISQMSP:MRIQLMSP =0.53, MRISQMSP:SPECTMQL =0.49, MRISQMSP:SPECTMSQ =0.49, MRIQLMSP:SPECTMQL =0.48, MRIQLMSP:SPECTMSQ =0.47, and SPECTMQL:SPECTMSQ =0.95. Excluding the outlier, SPECTMQL:SPECTMSQ, the average correlation for the DICE results is 0.49±0.02 which represents an increased from the Original correlation of 0.33±0.04 (P<001).
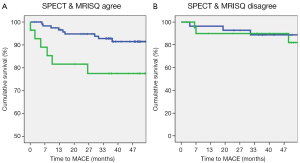
Original and DICE-assisted MPI predictors of CAD and MACE
Figure 3 shows the set of ROC curves for detection of CAD generated for the Original, threshold DICE and continuous DICE functions. The Original readings of the MPI data generated an average AUC of 0.7±0.01, which remained almost unchanged using the threshold DICE models (0.71±0.03, P=0.3) but increased significantly for the continuous DICE equation (0.77±0.03, P<0.01). Similarly, Figure 4 shows the set of ROC curves for prediction of MACE. The Original readings of MPI data generated an average AUC of 0.59±0.05, which trended to increase using the threshold DICE models (0.68±0.05, P=0.07) and increased using the continuous DICE equations (0.75±0.02, P<0.001).
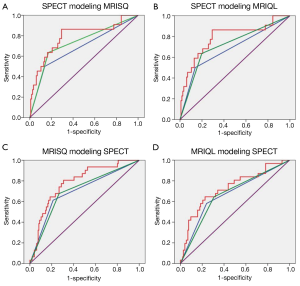
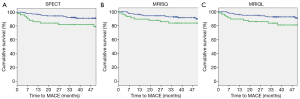
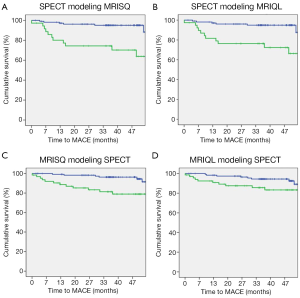
Kaplan-Meier survival curves were generated separately for the Original perfusion status readings and threshold DICE models. The three Kaplan-Meier plots generated for the Original readings had an average log rank statistic of 3.0±0.6, Figure 5. The four Kaplan-Meier plots for the threshold DICE models are shown in Figure 6, with an increased average log-rank statistic (10.6±5.0, P<0.05). With reference to the Kaplan-Meier plots, there was no difference in the annualized event rate of those identified with perfusion deficits between the Original vs. DICE [(3.2±.2)% vs. (4.3±1.4)%, P=0.2]. Conversely, the average event rate was higher for the Original vs. DICE for those identified as having normal perfusion [(1.6±0.17)% vs. (1.1±0.2)%, P<0.01].
Number of DICE model parameters
The DICE model given in Eq. [2] using MRISQ data to model SPECT was re-evaluated using 1, 2 and 3 parameters. Kaplan-Meier curves were generated for the Original, each reduced DICE model and the most complete DICE model. The log rank linearly increased from 2.2 to 10.03, given by the equation
Where NoP is the number of parameters (R2=0.98). The log-rank and percent survival by ischemic category are summarized in Table 2.

Full table
Discussion
DICE improved interpretation of MPI data by specifically incorporating terms related to the physical conditions of the patient. This was the case for both human and computer assisted interpretations. For the Original readings, the degree of discrepancy between the two MRI readings was comparable to the degree of discrepancy between MRI and SPECT with about 25% of patients having non-congruent findings. However, despite these widespread disagreements, the Original readings were broadly comparable to each other in diagnostic and prognostic value. With reference to Figure 4, the Original MPI readings indicated that about 60% of MACE occurred in patients without any evidence of a perfusion deficit. The DICE thresholded results captured approximately 60% of MACE. This increase in sensitivity was accomplished without altering the annualized event rates for positive patients between the Original and DICE-assisted readings [(3.2±.2)% vs. (4.3±1.4)%, P=0.2]. However, the annualized event rates in MPI negative patients were lower for DICE vs. Original readings [(1.1±0.2)% vs. (1.6±0.17)%, P<0.01]. Of significance, patients in the DICE-identified normal group are less likely to experiences an adverse event, potentially allowing further focus on patients within the high-risk group.
The DICE-assisted interpretation of MPI data improved detection of CAD as assessed by AUC in ROC analysis (while the thresholded DICE did not significantly increase detection, it can be appreciated that optimization of the threshold could be performed using knowledge of CAD of MACE). Further, myocardial perfusion status is not expected to agree completely with epicardial CAD status since physiologic compensation strategies (e.g., vasodilatation, collateral vessels) may have developed sufficiently to avoid perfusion deficits, while in other patients without significant epicardial coronary artery stenoses, perfusion deficits may be present due to other mechanisms such as microvascular disease (23). Further, the high degree of variability in the vascular response to the vasodilatation agent administered during MPI testing lowers sensitivity to CAD (24-26). While these phenomena may explain the relatively low correlation between MPI status for any one modality and CAD it likely does not adequately explain the differences noted between two modalities or between the two readings of one modality. Here, we identified physical conditions measured by each modality that influence interpretation of myocardial perfusion status. Incorporating these terms in a prediction model that seeks to homogenize the response between modalities/readings resulted in improved prediction of CAD and MACE.
While agreement between and within modalities increased vs. the Original interpretation, the agreement between the two SPECT models was an outlier with the correlation r=0.95. Given the relatively poor agreement between the two Original MRI perfusion readings (correlation r=0.29) it can be appreciated that the model is dominated by the physiologic measure of EDVi measured by SPECT, with a larger EDVi indicating the presence of low myocardial perfusion. Similarly, the DICE models for MRI data indicate that higher values of myocardial wall thickness and ESVi measured by MRI are also associated with an increased presence of low myocardial perfusion that is likely to be missed by the primary interpretation of MPI data. The physical interpretation is that hearts with higher physical dimensions are more likely to experience adverse events, but that the presence of adverse myocardial perfusion conditions is likely to be missed, both by MRI and by SPECT. Taken to an extreme this implies that evaluation of perfusion status in sufficiently large hearts (using modality-dependent criteria given by the DICE equations) should not be attempted due to the high likelihood of the presence of disease and the low likelihood of detection. Instead, patients thus identified may be considered candidates for evaluation at the cath lab without further evaluation of perfusion status. However, the criteria for identifying a heart as too large for MPI evaluation are modality dependent. Consider that the measure of EDVi by MRI and SPECT only moderately agree with each other, indicating a modality dependence to these measurements. Further, as noted in Eq. [6], as more terms are included in the model, the prediction of outcome increases. The physical conditions identified in the DICE models have the advantage, that, even if one or both modalities measure them inaccurately in an absolute sense, the relevant measure applicable for DICE modeling is that reported by the modality.
Limitations
The fidelity of each DICE model is reduced due to the lack of complete data, particularly for LV volumetric gated SPECT measurements. The relatively small data set necessitated use of pilot and implementation phase data, which may have added to variability of the results. We note that in each study phase the SPECT MPI interpretation was performed in a clinically standard manner, but that different imaging agents were used between the pilot and implementation phases. Data were only obtained from one site. Fewer data sets were available for gated-SPECT compared to MRI. Future research needs to focus on developing a prospective study to validate these findings in women and determine how they differ from men.
Conclusions
In women with suspected myocardial ischemia, agreement between modalities and between readings within one modality was improved using the DICE model. The DICE model incorporates physiologic variables that influence data interpretation; in this case modality-dependent measures of cardiac metrics. Modeling was accomplished without knowledge of diagnostic or prognostic outcomes, but nevertheless improved prediction of these (in ROC analysis).
Acknowledgements
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, R01-HL-073412-01, grants U0164829,U01 HL649141, U01 HL649241, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and QMED, Inc., Laurence Harbor, New Jersey, and the Edythe L. Broad Endowment, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
We are grateful to Bracco, Princeton, NJ, for providing the ProHance contrast agent.
Disclosure: The authors declare no conflict of interest.
References
- Kilner PJ, Geva T, Kaemmerer H, et al. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J 2010;31:794-805. [PubMed]
- Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation 2003;108:1945-53. [PubMed]
- Doyle M, Biederman RW. Future prospects in cardiac magnetic resonance imaging. Curr Cardiol Rep 2003;5:83-90. [PubMed]
- Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 2002;105:162-7. [PubMed]
- Kim RJ, Judd RM, Chen EL, et al. Relationship of elevated 23Na magnetic resonance image intensity to infarct size after acute reperfused myocardial infarction. Circulation 1999;100:185-92. [PubMed]
- Tsukiji M, Nguyen P, Narayan G, et al. Peri-infarct ischemia determined by cardiovascular magnetic resonance evaluation of myocardial viability and stress perfusion predicts future cardiovascular events in patients with severe ischemic cardiomyopathy. J Cardiovasc Magn Reson 2006;8:773-9. [PubMed]
- Chotenimitkhun R, Hundley WG. Identification of left ventricular myocardial ischemia and cardiac prognosis with cardiovascular magnetic resonance: updates from 2008 to 2010. Curr Cardiol Rep 2011;13:77-85. [PubMed]
- Bondarenko O, Beek AM, Hofman MB, et al. Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 2005;7:481-5. [PubMed]
- Bruder O, Schneider S, Nothnagel D, et al. EuroCMR (European cardiovascular magnetic resonance) registry: results of the German pilot phase. J Am Coll Cardiol 2009;54:1457-66. [PubMed]
- Greenwood JP, Younger JF, Ridgway JP, et al. Safety and diagnostic accuracy of stress cardiac magnetic resonance imaging vs exercise tolerance testing early after acute ST elevation myocardial infarction. Heart 2007;93:1363-8. [PubMed]
- Greenwood JP, Maredia N, Radjenovic A, et al. Clinical evaluation of magnetic resonance imaging in coronary heart disease: the CE-MARC study. Trials 2009;10:62. [PubMed]
- Bratis K, Nagel E. Variability in quantitative cardiac magnetic resonance perfusion analysis. J Thorac Dis 2013;5:357-9. [PubMed]
- Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374-9. [PubMed]
- Kühl HP, Lipke CS, Krombach GA, et al. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J 2006;27:846-53. [PubMed]
- Berman DS, Hachamovitch R, Shaw LJ, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: assessment of patients with suspected coronary artery disease. J Nucl Med 2006;47:74-82. [PubMed]
- Karamitsos TD, Leccisotti L, Arnold JR, et al. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging 2010;3:32-40. [PubMed]
- Goykhman P, Mehta PK, Agarwal M, et al. Reproducibility of myocardial perfusion reserve - variations in measurements from post processing using commercially available software. Cardiovasc Diagn Ther 2012;2:268-77. [PubMed]
- Ingkanisorn WP, Rhoads KL, Aletras AH, et al. Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J Am Coll Cardiol 2004;43:2253-9. [PubMed]
- Doyle M, Fuisz A, Kortright E, et al. The impact of myocardial flow reserve on the detection of coronary artery disease by perfusion imaging methods: an NHLBI WISE study. J Cardiovasc Magn Reson 2003;5:475-85. [PubMed]
- Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global Mr myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010;3:1030-6. [PubMed]
- Pohost GM, Hung L, Doyle M. Clinical use of cardiovascular magnetic resonance. Circulation 2003;108:647-53. [PubMed]
- DePuey EG, Parmett S, Ghesani M, et al. Comparison of Tc-99m sestamibi and Tl-201 gated perfusion SPECT. J Nucl Cardiol 1999;6:278-85. [PubMed]
- Shufelt CL, Thomson LE, Goykhman P, et al. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovasc Diagn Ther 2013;3:153-60. [PubMed]
- Taillefer R, Ahlberg AW, Masood Y, et al. Acute beta-blockade reduces the extent and severity of myocardial perfusion defects with dipyridamole Tc-99m sestamibi SPECT imaging. J Am Coll Cardiol 2003;42:1475-83. [PubMed]
- Fragasso G, Lu C, Dabrowski P, et al. Comparison of stress/rest myocardial perfusion tomography, dipyridamole and dobutamine stress echocardiography for the detection of coronary disease in hypertensive patients with chest pain and positive exercise test. J Am Coll Cardiol 1999;34:441-7. [PubMed]
- Vigna C, Stanislao M, De Rito V, et al. Inaccuracy of dipyridamole echocardiography or scintigraphy for the diagnosis of coronary artery disease in patients with both left bundle branch block and left ventricular dysfunction. Int J Cardiol 2006;110:116-8. [PubMed]


