
The value of hand grip strength (HGS) as a diagnostic and prognostic biomarker in congenital heart disease
Introduction
Although today most patients born with congenital heart disease (CHD) survive into adulthood, morbidity and mortality of CHD are still high (1). The natural and unnatural history is often complicated by residues and sequels of the particular CHD, which includes heart failure, pulmonary vascular disease, arrhythmias, multi-organ involvement, comorbidities and cardiac death (2-4).
Depending on the type of CHD and the treatment status, CHD patients may experience muscle mass loss due to low levels of physical activity. Functional exercise capacity and physical reserves are frequently reduced, and may denote patients at risk for hospitalization or death (2,5,6). In order to identify high-risk patients in time, not only an increased awareness of the treating physician is required, but also effective, easily available and cost-effective biomarkers. Research from cardiological fields which are different from CHD suggests that hand grip strength (HGS), measured by a dynamometer, may provide reliable information on the clinical status of a patient and possibly predict adverse outcomes, because muscle strength is associated with all-cause mortality regardless of cardiorespiratory fitness (7-11).
Since there is little data on the use of HGS for clinical assignment in patients with CHD, the objective of this study was to describe the presence and degree of skeletal muscle dysfunction with HGS as an expression of health, fitness and the functional status in a large cohort of patients with CHD. Moreover, the mid-term survival of ACHD should be assessed depending on the HGS-test results.
Methods
This case control study was conducted in the Department of Congenital Heart Disease and Pediatric Cardiology of the German Heart Centre Munich.
All clinical evaluations were part of the regular management of the patients that these required for management of their medical conditions. No additional examination was performed for the sole purpose of the study. All data were assessed and analyzed in an anonymous way. Ethical approval has been waived because it is routine clinical data, and all patients gave written consent to their anonymous publication.
Patients were consecutively included from January 2013 until September 2013 in the order that they presented at our institution and were not selected in prior.
Inclusion criteria for the present study were a confirmed diagnosis of CHD. Exclusion criteria were obvious signs of muscle weakness or musculoskeletal abnormalities, lack of cognitive competence to consent to research, and refusal to consent. Also excluded were severely disabled patients with decompensated heart failure and patients confined to bed by illness.
Medical records were reviewed for patient demographics, cardiac and non-cardiac diagnosis, surgical, interventional or electrophysiological procedures, clinical condition and medication.
The severity of congenital heart anomaly was categorized as mild (ACC-Class I), moderate (ACC-Class II), or severe (ACC-Class III) according to Warnes (12,13). Likewise, disorders not mentioned in this classification were similarly classified according to the experience of the German Heart Center Munich. In addition, patients were assigned to one of five major groups depending on the type of underlying CHD: (I) complex CHD; (II) left heart anomalies/aortopathies; (III) right heart anomalies/anomalies of pulmonary valve or pulmonary artery; (IV) primary left-to-right shunt—either at pre-tricuspid or post-tricuspid level, or (V) other congenital heart anomalies (12).
Patients with Fontan circulation or severe chronic cyanosis (defined as arterial oxygen saturation ≤90%), or with a morphologic right systemic ventricle (transposition of the great arteries after Mustard or Senning procedure; congenitally corrected transposition of the great arteries), or with transposition of the great arteries after arterial switch operation, or with Ebstein’s anomaly, were analyzed separately.
Patients with CHD were compared with a control group (CG) of 124 healthy individuals who showed no pathological clinical signs of the musculoskeletal system.
The control subjects were volunteers recruited from a range of high schools, colleges, universities, companies and administration organizations in our geographical region.
HGS was measured according to a standardized protocol using a Jamar Hydraulic Hand Dynamometer (Jamar Hydraulic Hand Dynamometer Model 5030J1; Sammons Preston, Bolingbrook, IL, USA) (14). As recommended by the American Society of Hand Therapists, patients were instructed to sit in an upright position with shoulders adducted, elbow-flexed at 90°and forearms in neutral position (8,15). All participants were encouraged to give their best effort in isometric grip strength, with one repetition for each hand. The test was repeated 3 times, alternating between each hand. The maximum values of each hand were determined. Only maximum values of the dominant hand were used for statistical analysis. Age and sex differences were considered in a linear regression. HGS was classified as normal if the results fell within the 95% confidence interval or higher and abnormal if their results fell below this level.
In addition, all included patients were reevaluated from January until August 2018 in our outpatient clinic, or were contacted by telephone for the assessment of mid-term survival.
Statistical analysis
The descriptive data were expressed in mean values and standard deviations (mean ± SD). Differences in HGS between CHD, the healthy CG and CHD subgroup analysis were evaluated using linear regression models with gender and age as covariates. P-values <0.05 in a two-sided analysis were considered significant. STATA 14.2 (STAT Corp.) was used for statistical analysis.
Results
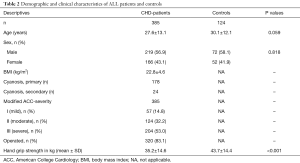
A total of 385 patients with CHD were suitable for the present analysis and were enrolled. The diagnosis of CHD was made in accordance to the specific expert guidelines (16,17). General characteristics and detailed underlying diagnosis are listed in Table 1. A total of 166 patients (43%) were female. Mean age at time of study inclusion was 27.6±13.1 years. The CG consisted of 124 healthy individuals. Mean age at the time of study inclusion was 30.1±12.1 years, 52 patients (42%) were female. Out of the 385 patients 320 (83%) had at least one previous heart operation for their CHD; 65 patients (16.9%) had not undergone any cardiac surgery (Tables 2,3).

Full table
Full table
Full table
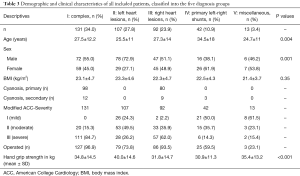
The classification according to the recommendations of the American College of Cardiology (ACC-Classification), modified by the Munich experience (12), showed simple defects in 57 (15%), moderate defects in 124 (32%), and severe defects in 204 (53%) cases.
Assigned to the type of underlying CHD 131 (34%) patients had complex CHD, 107 (30%) had left heart anomalies/aortopathies, 92 (24%) had right heart anomalies/anomalies of pulmonary valve or pulmonary artery, 42 (11%) had primary left-to-right shunt—either at pre-tricuspid or post-tricuspid level, and 13 (3.4%) had other congenital heart anomalies.
In the entire cohort of 385 patients 17 (4.4%) had a Fontan-circulation, and 24 (6.2%) had chronic cyanosis. Thirty-six patients, including 25 (6.5%) patients after Mustard or Senning procedure for complete transposition of the great arteries, as well as 11 (2.9%) patients with congenitally corrected transposition of the great arteries had a morphologic right systemic ventricle. An arterial switch operation for transposition of the great arteries was performed in 25 patients (6.5%), and 22 patients (5.7%) had Ebstein’s anomaly.
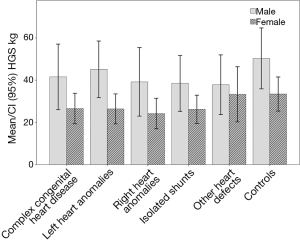
In the entire group of patients with CHD HGS was 35.1±14.6 kg and significantly reduced compared to the healthy CG (43.2±14.7 kg, P<0.001) (Figure 1) on average −5.71 kg (95% CI: −7.61 to −3.81 kg), controlled for sex and age.
Patients with left heart anomalies (Group II) showed highest values for HGS compared to the entire CHD group (mean 40.7±14.7 kg) and did not differ from the CG (P=0.102).
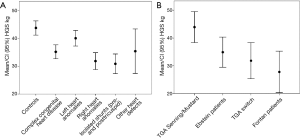
The lowest HGS (mean of 30.9±11.3 kg) was verified in patients with previous primary left-to-right shunts—either at pre-tricuspid or post-tricuspid level. In this group, HGS was −12.36 kg (95% CI: −16.67 to −8.04 kg) lower compared to healthy controls (Figure 2A).
Within the three ACC groups, there were no significant differences concerning HGS (P=0.207). CHD patients with cyanosis had −3.25 kg (95% CI: −6.18 to −0.31 kg) lower HGS values compared to acyanotic CHD patients (P=0.03). If controlled for sex and age, there were no significant differences detected (P=0.130).
Male subjects with CHD had significantly higher HGS values (mean 42.0±14.9 kg) compared to female subjects with CHD (mean 26.1±7.6 kg). Male subjects with CHD had −5.62 kg (95% CI: −8.37 to −2.87 kg) HGS compared to healthy male controls (P<0.001), female subjects with CHD had −6.00 kg (95% CI: −8.14 to −3.86 kg) HGS compared to healthy female controls, both controlled for age (P<0.001) (Figure 1).
Regarding specific groups of disorders, patients with a morphologic right system ventricle showed better results in HGS (5.56 kg, 95% CI: 1.75 to 9.37 kg) (P=0.004) whereas patients with Fontan circulation exhibit significantly lower HGS values (−5.43 kg, 95% CI: −10.44 to −0.43 kg) (P=0.033) (Figure 2B) compared to the entire CHD group, controlled for age and gender. In patients with a TGA after arterial switch operation or Ebstein’s anomaly those marked differences could not be detected.
During the observation period, only seven (1.8%) of the 385 patients enrolled in the study died. The mean time interval between the date of the test and the date of death was 422 days (range, 206–1,824 days). Detailed information is given in Table 4. The causes of death have not been established.
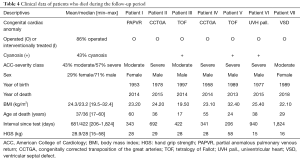
Full table
Discussion
In patients with CHD only scarce data exists about muscle strength and muscular endurance as indicators of physical and muscular fitness and as predictors of morbidity and mortality (18-21). In contrast, in the general population, several clinical and epidemiological studies have shown the predictive value of declining HGS concerning morbidity and all-cause mortality in the short and long-term (8,22-25).
In patients with CHD, this study provides the so far most comprehensive data on the use of HGS as a biomarker, including 385 children and adults (43% female, mean age 27.6±13 years; adults ≥18 years: n=277) with nearly all types and severity levels of CHD. The study demonstrates that HGS measured by a Hydraulic Hand Dynamometer can easily be used for the assessment of cardiac health, fitness and functional status.
Even if the mortality of patients with CHD has decreased, the morbidity is still high due to residua, sequelae or complications, and therefore most CHD defects require lifelong experienced cardiological follow up (26-30).
For a proper patient assessment, it is mandatory to understand the unique medical, social and psychological effects of living with a chronic heart disease from childhood, and for the clinical assignment it is worth knowing that only a loose correlation exists between the patient’s subjective perceived health status and the objective clinical status. Because the pathoanatomical and pathophysiological complexity of CHD causes diagnostic problems, there is a deep need for a reliable, easily applicable, repeatedly usable and cost-effective diagnostic and prognostic marker, which allows gaining a rapid, quantifiable assessment of the current condition of the patient. This is of outstanding importance, as the clinical impression of the treating physician can be misleading, especially if he is not experienced in the evaluation of the particular disorder. Furthermore, a decline in the functional status, defined as the ability to perform daily live activities, is challenging to assess in patients with CHD because the definition is vague and measurement is difficult (15). Moreover, several studies have suggested that the severity of the CHD and the illness course are only marginally associated with patient’s health perception, the supposed condition and the quality of life (31). Therefore, subjective statements of patients regarding their current being have to be put into a proper perspective.
By applying clinical examination, technical methods, stress tests and modern biomarkers, it is possible to better classify the current health state. However, the results of complex clinical, technical, or laboratory studies are often inconclusive, contradictory and certainly associated with considerable costs. Besides, several studies have contradicted the assumption, that a significant association exists between systemic ventricular function and health status (31).
Without any doubt, cardiopulmonary exercise testing is an objective diagnostic and prognostic instrument for the assessment of patients with CHD (5,32). However, this method is time-consuming, costly, and requires a lot of experience for a proper interpretation of the results. In comparison, the current study reveals that HGS-measurement is an easy applicable test that can be incorporated into the physical examination in order to help objectively assess the current state of muscular fitness (15).
Various studies in patients without CHD could reveal that reduced muscle strength, measured by HGS, was associated with an increased mortality risk (23,24,33,34).
The current data from patients with CHD proves that muscular function, measured by HGS, is decisively influenced by the type and status of the heart defect, the initial morphological diagnosis, the severity of the disease, to previous surgical or interventional procedures, criteria such as residuals, sequels and complications (e.g., cyanosis). Comparing the results from the study cohort with data from 124 healthy controls reveals that HGS in patients with CHD in general is significantly reduced.
The most pronounced impairments are present in female CHD-patients (P<0.001). This can be explained at least in part by the fact that hand strength depends on hand length, which varies according to gender, with men generally having larger hands (35). Age and gender stratified normative data for HGS are available from the literature (36).
A striking observation is that in our patients HGS was significantly lower in patients with cyanosis if compared to acyanotic patients (P=0.03). This might be consistent with previous studies detecting in children and adults with cyanotic CHD abnormalities in skeletal muscle metabolism presumably contributing to the reduced exercise tolerance in the affected patients.
The highest HSG values had patients with left heart lesions and did not differ from the CG (P=0.102). Surprising is the fact that HGS was lowest in patients with primary left-to-right shunt lesions (P<0.001), as from the clinical experience these patients usually mostly have mild CHD, a nearly well-preserved cardiac function and fewer medical problems relative to the patients in other CHD-groups. One might suspect that this is based on a bias, as patients with an uncomplicated course of a simple left-to-right shunt lesion are rarely seen in a tertiary care center, and the included patients had concomitant, perhaps non-cardiac problems.
Between specific groups of disorders (Fontan circulation, morphologic right systemic ventricle, TGA after arterial switch operation, Ebstein’s anomaly) also marked differences could be detected, where patients with Fontan circulation exhibited the lowest (P=0.033), and patients with a morphologic right system ventricle showed better HGS-values (P=0.004).
Our results are at least partly consistent with results from other studies in CHD, which contain conflicting data. While some studies verify a normal muscle development, others report on a reduced HGS (18,19,37,38). The informative value of these data is, however, limited because of low numbers of patients included, the predominance of children, the lack of complex anomalies and missing data about the clinical outcome.
The mechanism, however, by which low muscle strength develops and how it might predispose to an adverse outcome or death is widely unknown. HGS may be controlled by multiple physiological systems and there are certain considerations that particularly patients with heart failure or multiple chronic diseases suffer from skeletal muscle atrophy, altered muscle metabolism, and reduced mitochondrial-based enzyme levels that may lead to decreased muscle force. Moreover, reduced oxygen delivery to peripheral and respiratory muscles from reduced blood supply and/or hypoxemia may result in hypoxic muscle tissue, an increased CO2 retention and maladaptive changes in skeletal muscle (39-43). Low HGS could also be associated with subclinical inflammation, hyperthyroidism, increased interleukin-6, defects in insulin resistance and glucose metabolism and reduced insulin-like growth factor I (IGF-I) (43).
Certainly, HGS measurement, has some shortcomings as it is only an indicator of upper body strength and muscle function and cannot be accurately performed in patients who are unable or not willing to properly grip the dynamometer (15). However, from other studies data exist that handgrip strength is closely related to lower extremity force (39).
Further research is therefore needed to determine the potential benefits of using HGS as examination tool. It should be clarified more intensely, if a decrease in HGS over time is a predictor of a higher risk of dying from any cause, from heart disease, and of heart attacks or stroke. Therefore, in addition, the causative genetic, biological, and biochemical influences on muscle metabolism and function in CHD have to be elucidated (9). It has to be uncovered whether fitness interventions and inclusion in exercise-based rehabilitation programs, as recommended by the European Society of Cardiology for all adults with CHD, can help to improve muscle strength (44). Also the importance of supportive measures, including dietary counseling, nutritional supplementation, must be verified (6). Finally, it has to be elucidated in larger and in longer term studies whether changes of behavior and lifestyle can improve outcome and reduce the cardiovascular risk as well as morbidity and mortality of patients with CHD.
An important additional information concerns the prognostic significance of the HGS test in patients with CHD. Here, our results differ significantly from those of other patient collectives where the predictive value of the test for mortality assessment was high. For a prognosis of mortality, it is advisable to take an HGS measurement several times at defined points in time and thus to document the change in cardiac fitness in a way that can be easily implemented in everyday clinical routine. More valid information can be derived from the change of the HGS than from a punctual measurement at an unspecified point in time. Due to the low mortality rate in this sample, it is recommended to include larger samples in the future study with regard to a prediction of mortality.
Therefore, to get a reliable impression of the clinical status it may be more important to determine the individualized best result of each patient and to follow this, than to look for a group effect.
Study limitations
Certain limitations should be borne in mind when assessing our results. Strengths include the large sample size of enrolled patients with almost all types and severity grades of CHD.
This study was limited by the fact that only patients who agreed to participate voluntarily were included. It is not known to what extend the motivation of the patients may have biased the observations, as it is well known that individuals who volunteer to participate in research studies differ from those who do not choose to participate. Moreover, HGS cannot be accurately performed in patients who are unable or not willing to properly grip the dynamometer. One could also assume that handgrip strength is primarily an indicator of upper body strength and muscle function, but it is closely correlated with the lower extremity force (33).
In order to better correlate the entire clinical situation with the HGS data, comparative data on CPET, imaging modalities and biomarkers would be helpful. Since these tests are not routine, but only dependent on the clinical indication, we cannot provide data on these. Besides, in this study echocardiographic or invasive data about ventricular performance during exercise are lacking. However, until now it is unclear if even under resting conditions a significant correlation between systemic ventricular function and health status exists.
Another limitation is that the reasons for the reduction of HGS in CHD could not be explained, but the current study was not designed to prove cause and effect.
Finally, the sample of patients may not represent the pattern of CHD in the community and does not represent the typical population of CHD seen by a general practitioner, by a normal cardiologist or even in departments for cardiology.
Conclusions
In conclusion, functional status is difficult to assess in patients with CHD. The reasons and pathomechanisms for the alteration of physical performance in the included patients with CHD, expressed as reduction of HGS, could not be explained, but it may be speculated that they are depending of the severity of CHD, residual defects, myocardial and respiratory function and the level of individual physical activity and fitness. Although HGS may not be a suitable tool for assessing survival probability or identifying high-risk patients, the test is well suited to provide a reliable impression of functional muscle status as an indicator of the overall clinical situation of the patient.
Over and above that seems to be important to follow the included patients for some years to see whether HGS is of any significance beyond current assessments, particularly to identify patients at higher risk for cardiovascular complications or even death.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval has been waived because it is routine clinical data, and all patients gave written consent to their anonymous publication.
References
- Neidenbach R, Schelling J, Pieper L, et al. Sind Erwachsene mit angeborenen Herzfehlern ausreichend versorgt? Zeitschrift für Herz thorax und Gefasschirurgie 2017;31:S228-40.
- Hauser M, Lummert E, Braun SL, et al. Nichtkardiale Komorbiditäten bei erwachsenen Patienten mit angeborenen Herzfehlern: Häufigkeit und gesundheitspolitische Bedeutung. Zeitschrift für Herz thorax und Gefasschirurgie 2017;31:130-7.
- Lummert E, Hauser M, Vigl M, et al. Noncardiac comorbidities of congenital heart disease in adults. Am J Cardiol 2014;113:S109. [Crossref]
- Ministeri M, Alonso-Gonzalez R, Swan L, et al. Common long-term complications of adult congenital heart disease: avoid falling in a H.E.A.P. Expert Rev Cardiovasc Ther 2016;14:445-62. [Crossref] [PubMed]
- Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828-35. [Crossref] [PubMed]
- Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J 2014;35:1726-31. [Crossref] [PubMed]
- Ling CH, Taekema D, de Craen AJ, et al. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ 2010;182:429-35. [Crossref] [PubMed]
- Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266-73. [Crossref] [PubMed]
- Timpka S, Petersson IF, Zhou C, et al. Muscle strength in adolescent men and risk of cardiovascular disease events and mortality in middle age: a prospective cohort study. BMC Med 2014;12:62. [Crossref] [PubMed]
- Artero EG, Lee DC, Ruiz JR, et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. J Am Coll Cardiol 2011;57:1831-7. [Crossref] [PubMed]
- Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008;337:a439. [Crossref] [PubMed]
- Warnes CA. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol 2005;46:1-8. [Crossref] [PubMed]
- Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 2001;37:1170-5. [Crossref] [PubMed]
- Molenaar HM, Selles RW, Zuidam JM, et al. Growth diagrams for grip strength in children. Clin Orthop Relat Res 2010;468:217-23. [Crossref] [PubMed]
- Dowhan L, DeChicco R, Welsh R, et al. Comparison Between Handgrip Dynamometry and Manual Muscle Testing Performed by Registered Dietitians in Measuring Muscle Strength and Function of Hospitalized Patients. JPEN J Parenter Enteral Nutr 2016;40:951-8. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143-263. [Crossref] [PubMed]
- Greutmann M, Le TL, Tobler D, et al. Generalised muscle weakness in young adults with congenital heart disease. Heart 2011;97:1164-8. [Crossref] [PubMed]
- Kroonstrom LA, Johansson L, Zetterstrom AK, et al. Muscle function in adults with congenital heart disease. Int J Cardiol 2014;170:358-63. [Crossref] [PubMed]
- Muller J, Rottgers L, Neidenbach RC, et al. Reduced Handgrip Strength in Congenital Heart Disease With Regard to the Shunt Procedure in Infancy. Front Pediatr 2018;6:247. [Crossref] [PubMed]
- Smith MP, Muller J, Neidenbach R, et al. Better lung function with increased handgrip strength, as well as maximum oxygen uptake, in congenital heart disease across the lifespan. Eur J Prev Cardiol 2019;26:492-501. [Crossref] [PubMed]
- Beyer SE, Sanghvi MM, Aung N, et al. Prospective association between handgrip strength and cardiac structure and function in UK adults. PLoS One 2018;13:e0193124. [Crossref] [PubMed]
- Ortega FB, Silventoinen K, Tynelius P, et al. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ 2012;345:e7279. [Crossref] [PubMed]
- Sasaki H, Kasagi F, Yamada M, et al. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med 2007;120:337-42. [Crossref] [PubMed]
- Silventoinen K, Magnusson PK, Tynelius P, et al. Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: a population-based cohort study of one million Swedish men. Int J Epidemiol 2009;38:110-8. [Crossref] [PubMed]
- Perloff JK, Warnes CA. Challenges posed by adults with repaired congenital heart disease. Circulation 2001;103:2637-43. [Crossref] [PubMed]
- Bolger AP, Gatzoulis MA. Towards defining heart failure in adults with congenital heart disease. Int J Cardiol 2004;97 Suppl 1:15-23. [Crossref] [PubMed]
- Book WM. Heart failure in the adult patient with congenital heart disease. J Card Fail 2005;11:306-12. [Crossref] [PubMed]
- Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007;115:1039-50. [Crossref] [PubMed]
- Trojnarska O. Heart failure in the adult patient with congenital heart disease. Cardiol J 2007;14:127-36. [PubMed]
- Moons P, Van Deyk K, De Geest S, et al. Is the severity of congenital heart disease associated with the quality of life and perceived health of adult patients? Heart 2005;91:1193-8. [Crossref] [PubMed]
- Fredriksen PM, Veldtman G, Hechter S, et al. Aerobic capacity in adults with various congenital heart diseases. Am J Cardiol 2001;87:310-4. [Crossref] [PubMed]
- Gale CR, Martyn CN, Cooper C, et al. Grip strength, body composition, and mortality. Int J Epidemiol 2007;36:228-35. [Crossref] [PubMed]
- Lopez-Jaramillo P, Cohen DD, Gomez-Arbelaez D, et al. Association of handgrip strength to cardiovascular mortality in pre-diabetic and diabetic patients: a subanalysis of the ORIGIN trial. Int J Cardiol 2014;174:458-61. [Crossref] [PubMed]
- Wichelhaus A, Harms C, Neumann J, et al. Parameters influencing hand grip strength measured with the manugraphy system. BMC Musculoskelet Disord 2018;19:54. [Crossref] [PubMed]
- Massy-Westropp NM, Gill TK, Taylor AW, et al. Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC Res Notes 2011;4:127. [Crossref] [PubMed]
- Fricke O, Witzel C, Schickendantz S, et al. Mechanographic characteristics of adolescents and young adults with congenital heart disease. Eur J Pediatr 2008;167:331-6. [Crossref] [PubMed]
- Witzel C, Sreeram N, Coburger S, et al. Outcome of muscle and bone development in congenital heart disease. Eur J Pediatr 2006;165:168-74. [Crossref] [PubMed]
- Cortopassi F, Divo M, Pinto-Plata V, et al. Resting handgrip force and impaired cardiac function at rest and during exercise in COPD patients. Respir Med 2011;105:748-54. [Crossref] [PubMed]
- Berg HE, Tesch PA. Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand 1996;157:63-70. [Crossref] [PubMed]
- Terjung RL, Dudley GA, Meyer RA. Metabolic and circulatory limitations to muscular performance at the organ level. J Exp Biol 1985;115:307-18. [PubMed]
- Henriksson J, Reitman JS. Time course of changes in human skeletal muscle succinate dehydrogenase and cytochrome oxidase activities and maximal oxygen uptake with physical activity and inactivity. Acta Physiol Scand 1977;99:91-7. [Crossref] [PubMed]
- Cheung CL, Nguyen US, Au E, et al. Association of handgrip strength with chronic diseases and multimorbidity: a cross-sectional study. Age (Dordr) 2013;35:929-41. [Crossref] [PubMed]
- Hirth A, Reybrouck T, Bjarnason-Wehrens B, et al. Recommendations for participation in competitive and leisure sports in patients with congenital heart disease: a consensus document. Eur J Cardiovasc Prev Rehabil 2006;13:293-9. [PubMed]


