Prevalence and factors associated with false positive suspicion of acute aortic syndrome: experience in a patient population transferred to a specialized aortic treatment center
Introduction
Patients with time sensitive acute medical emergencies often present to community hospitals and subsequently require transfer to tertiary centers for definitive treatment. Modeled on the success of statewide trauma networks, a number of regional systems for the management of acute medical emergencies including ST-elevation myocardial infarction (STEMI), cardiac arrest, stroke, and acute aortic syndrome (AAS), defined as acute aortic dissection, intramural hematoma, or penetrating atherosclerotic ulcer, have been developed (1-8). The creation of STEMI networks across the United States and Europe has led to a dramatic increase in the availability of timely primary percutaneous coronary intervention (PCI) and reduced door to balloon times, both of which have translated into a decreased mortality rate with this condition (4,8-10).
Created in 1996, the International Registry of Acute Aortic Dissection (IRAD) is the largest dataset of AAS patients to date and has significantly advanced the recognition, diagnosis and treatment of patients presenting with this medical emergency. Eligible patients in IRAD have a confirmed AAS diagnosis based on medical history, imaging study, direct visualization at surgery, or post-mortem examination (11-13). However, the initial diagnosis of acute aortic dissection and emergent triage is challenging due to the lack of biomarkers, complex differential diagnosis, and need for confirmatory imaging. Regional AAS networks have been created in order to accelerate diagnosis, transport, and treatment of these patients.
The upstream triage of care prior to arrival at a tertiary center, and the rate of diagnostic confirmation in patients emergently transferred for suspected AAS, is incompletely described. Understanding the frequency and causes of false positive activation in patients with suspected AAS provides opportunities to improve clinical care in AAS networks (14-16). The aim of this study is to report the prevalence and etiology of false positive activation for AAS in a consecutive series of patients transferred to a tertiary referral center.
Methods
AAS network and emergency transport system
The AAS network in the Cleveland Clinic Health System (CCHS) comprises of a tertiary academic center in downtown Cleveland and a number of referring hospitals in Ohio and neighboring states. At the main campus hospital, care for patients with AAS is organized within a specialized ‘Aorta Center’, staffed by a group of critical care cardiologists, cardiothoracic and endovascular surgeons, and cardiovascular imaging specialists/radiologists. Patients with confirmed or suspected AAS are emergently transferred by the Cleveland Clinic Critical Care Transport system (based on location, availability, weather and distance via ground, helicopter, or fixed-wing jet) directly to the CICU as described previously (17).
The Cleveland Clinic Critical Care Transport system can be activated for time sensitive medical emergencies via a single phone contact. While predominantly utilized for STEMI, this system is also designed and utilized for acute stroke as well as our AAS network. The central hospital and some hospitals within the CCHS share a ‘Picture Archiving and Communication’ PACS system, allowing shared access to radiologic studies. However, such sharing is not currently possible with the majority of other referring hospitals.
Selection of participants
The study cohort consisted of 150 consecutive patients, with a community hospital/emergency department diagnosis of suspected AAS, transferred to our institution by the critical care transport team, via ground ambulance (n=27), helicopter (n=110), or fixed wing jet (n=13) between March, 2010 to August, 2011. The initial data collection date of March, 2010 was chosen because our critical care transport team created a database of AAS transfers and began prospectively entering data on this date. Transport data was prospectively collected and added (CR and BA) to our Heart and Vascular Institute’s RedCaps© AAS database, modeled similarly to IRAD. Final diagnosis in this cohort of subjects was made by consensus agreement of the cardiac intensive care unit, vascular surgery, cardiothoracic surgery and cardiovascular imaging teams utilizing all available clinical, imaging and surgical data, also modeled after IRAD (11-13). If there were no available diagnostic images from the initial hospital, computed tomography (CT) imaging was performed utilizing our institution’s acute aortic dissection CT imaging protocols (Table 1). Patients were grouped into either confirmed dissection (Type A or Type B) or false positive suspicion (no pathology or no acute pathology). Patients with an initial ED diagnosis of penetrating aortic ulcer (3 patients) or intramural hematoma (4 patients)were reclassified as either Type A or Type B dissection based on the location of the lesion. The Cleveland Clinic Institutional Review Board approved this study, with a waiver of individual consent.

Full table
Analysis
Baseline characteristics were compared between the groups with confirmed AAS and those with false positive activation. Continuous variables were compared between groups using Student’s t-test with mean ± standard deviation (SD) reported. Categorical variables were compared between groups using Pearson’s chi-squared test with frequency and percent reported.
Results
Overall, 150 patients were transferred with a suspected diagnosis of AAS from 60 different hospitals with a median distance of 40 [interquartile range (IQR), 14-75] miles. A total of 133 patients (63 Type A and 70 Type B) had the diagnosis of AAS confirmed at the tertiary center. In 17 patients (11.3%) the diagnosis was not confirmed (‘false positive suspicion’), with 10 (58.8%) of these being suspected Type A and 7 (41.2%) suspected Type B. Baseline demographics for the confirmed AAS group and the false positive group are illustrated in Table 2. Demographics were similar in both groups.
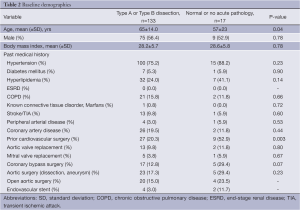
Full table
The overall in-hospital mortality for the 150 patients cohort was 9.3%. This included 13 patients in the confirmed dissection group (‘true positive group’ mortality =9.7%) and one patient in the false positive group (5.8%). One patient died during index hospital stay in the false positive cohort. A CT scan ruled out a Type B dissection in this patient who subsequently manifested mesenteric ischemia secondary to a superior mesenteric artery embolus and expired intra-operatively.
False positive suspicion by the referring institution was based on CT imaging in 15/17 cases. The remaining patients were transferred based on high clinical suspicion without pre-transfer imaging. Imaging utilized for the false positive group at both the initial and tertiary hospitals, is illustrated in Table 3.
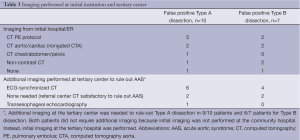
Full table
The pre-transfer suspicion for Type A dissections was not confirmed in ten patients (‘false positive Type A’). In one patient, the clinical suspicion (no pre-transfer imaging) was ruled out with a post-transfer ECG-synchronized CT imaging performed at the tertiary center. Review of the pre-transfer CT images from the initial hospital was satisfactory to rule out Type A dissection in two cases. In six patients, AAS was ruled out based on repeat imaging with an ECG-synchronized CT performed at the tertiary center. One patient had a TEE to rule out AAS due to renal insufficiency.
There were seven false positive suspicions of Type B dissections. In one patient the clinical suspicion (no pre-transfer imaging) was ruled out with a post-transfer ECG-synchronized CT imaging performed at the tertiary center. Review of pre-transfer imaging from the referral hospital was considered satisfactory to rule-out Type B dissection in two patients. Four patients underwent repeat (ECG-synchronized) CT to rule out AAS (Table 3).
A history of prior surgical or endovascular repair was seen in 5/17 (29.4%) cases with one patient having both history of open and endovascular repair (Table 2). History of prior ascending aortic surgery was seen in three (30%) patients in the false positive Type A group.
Discussion
This is the first study describing the prevalence and causes of false positive activation of emergency medical personnel for suspected AAS in an AAS network. In 11.3% of patients transferred to a tertiary center with an initial suspicion of an acute aortic dissection, the suspicion was not confirmed (false positive transfer). The unconfirmed suspicion was mainly a result of motion artifacts arising from non-ECG synchronized CT imaging that mimicked or could not clearly rule out an ascending aortic dissection. Just under one-third of the false positive transfers were associated with prior aortic surgery and 43% of false activation for Type B dissections had prior aortic intervention. These observations provide important insight for the future improvement of the organization and efficiency of acute aortic networks.
This reported rate of false positive activation lies between the reported false positive rate for other time sensitive emergencies including stroke treated with TPA (1.4-7%) (18,19) and STEMI (14-36%) (20,21), and should be considered in the clinical context of emergent presentation. Specifically, the referring emergency physician must weigh the risk of missing an uncertain but potentially lethal diagnosis of acute aortic dissection versus the negligible risk of transfer for further evaluation by experts.
AAS is complex, relatively rare when compared to PE and acute coronary syndrome (ACS), and can be difficult to diagnose (11,22). The emergency physician triages patients based on clinical presentation and initial test results, weighing probabilities for various serious diseases including aortic dissection, pulmonary embolism, and ACS (23). For that reason and taking into account availability of CT protocols in most emergency departments, a chest CT primarily assessing and protocoled for PE (but also with attention for aortic dissection) is the most commonly performed study. These protocols have advantages in the evaluation of PE with regards to bolus timing and enhancement of the smaller, distal pulmonary branches; however motion of the aortic root during the cardiac cycle can create motion artifacts in a significant percentage of non-synchronized aorta studies (24-27). In contrast to prior reports of nearly 100% diagnostic accuracy of CT aorta (CTA), this study also shows the potential for false diagnosis in non-ECG CTA studies (28).
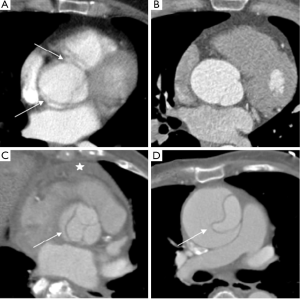
Alternatively, ECG-synchronized protocols (retrospective gating or prospective triggering) reduce or eliminate motion artifact cause by the cyclic motion of the aortic root and ascending aorta (Table 1 and Figures 1-3). These ECG-synchronized ‘gated’ protocols have a high sensitivity and specificity for evaluating AAS and are typically used if aortic disease of the aortic root and ascending aorta is the primary concern. However, their use is not universal for several reasons, including the cost of software, technical expertise, and increased radiation exposure (24,28). In fact, the vast majority of hospitals in our referral network do not have around-the-clock access to ECG-synchronized CT capabilities in the ED. Furthermore, for the reasons discussed above, these protocols would likely not be the first choice in patients presenting with poorly defined chest pain syndromes.
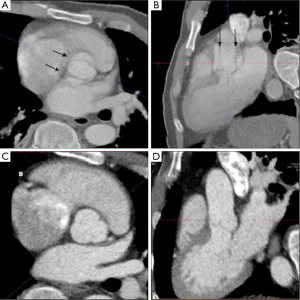
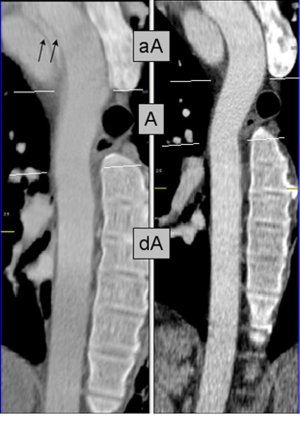
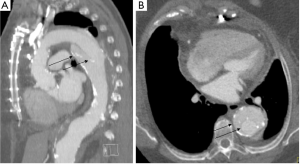
The emergent evaluation of AAS in patient populations with prior aortic surgery adds further complexity. Physicians and radiologists in community hospitals may be unfamiliar with the post-surgical anatomy of various surgical, endovascular, and hybrid repair techniques (29,30). Slightly <30% of the false positive cases occurred in patients with a prior history of aortic surgery or endovascular repair, with 60% of these cases requiring repeat imaging following transfer (Figure 4). In each instance the local hospital did not have access to prior imaging for comparison. Perhaps such patients should be encouraged to carry a copy of their most recent CT scan (on disc or USB drive) like we encourage many patients with chronic ST elevations to have a copy of their ECG. However, it is likely that both the referring hospital and tertiary center may still prefer patient transfer for definite diagnosis, image interpretation and close monitoring. From a tertiary center perspective, it is difficult to triage this complex disease over the phone. For example, in this analysis two separate patients presented one month post ascending aorta repair with chest pain. On both occasions, a non contrast CT performed at a local ED resulted in suspicion for new dissection. There was not access to prior CT imaging and both the initial and receiving hospitals favored transfer for definitive care. A subsequent ECG-synchronized CTA at our institution showed no change from prior CT.
Emerging concepts of telemedicine, based on shared picture archiving and communicating system (PACS) servers and cloud technology may have potential to increase communication and image sharing between referring EDs and tertiary centers (31). A regional network protocol in Minnesota has reported success with using a standard protocol with transmission of the CT images through a systems network (3). It is unclear whether the widespread use of ECG-synchronized CT and telemedicine would be safer and cost effective. Further study of this could prove useful.
It could be argued that lack of reader experience may also contribute to the incidence of false positive suspicion of dissection. However, the number of false positive transfers in which subsequent review of the initial imaging study reversed the initial suspicion (without need for repeat imaging) was relatively limited.
Limitations
This is a single center study with inherent bias and as such is not generalizable to all institutions or similar networks. Patients in this analysis were transferred by our institution’s critical care transport team, therefore patients transferred by other transport teams or patients presenting directly our tertiary ER were not captured, however we believe this number to be small.
Conclusions
The rate of false positive activation lies between the reported false positive rate for other time sensitive emergencies like stroke treated with TPA and STEMI, and has to be considered in the clinical context of emergent presentation. It is primarily driven by uncertainty secondary to motion-artifact of the ascending aorta and difficult to interpret complex anatomy following prior aortic surgery. Network-wide standardization of imaging strategies and improved consultation between referring and receiving centers may reduce the incidence of false positive activation.
Acknowledgements
Thanks to Matthew Bunte, MD and Imran Ahmad, MD for contributions in organizing and generating the prospective database. (Meetings: Abstract poster presented at European Society of Cardiology 2012 in Munich Germany on August 27th, 2012).
Disclosure: The authors declare no conflict of interest.
References
- Boyd DR, Dunea MM, Flashner BA. The Illinois plan for a statewide system of trauma centers. J Trauma 1973;13:24-31. [PubMed]
- Cowley RA, Hudson F, Scanlan E, et al. An economical and proved helicopter program for transporting the emergency critically ill and injured patient in Maryland. J Trauma 1973;13:1029-38. [PubMed]
- Harris KM, Strauss CE, Duval S, et al. Multidisciplinary standardized care for acute aortic dissection: design and initial outcomes of a regional care model. Circ Cardiovasc Qual Outcomes 2010;3:424-30. [PubMed]
- Henry TD, Sharkey SW, Burke MN, et al. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007;116:721-8. [PubMed]
- Jacobs AK. Regional systems of care for patients with ST-elevation myocardial infarction: being at the right place at the right time. Circulation 2007;116:689-92. [PubMed]
- LaMonte MP, Bahouth MN, Magder LS, et al. A regional system of stroke care provides thrombolytic outcomes comparable with the NINDS stroke trial. Ann Emerg Med 2009;54:319-27. [PubMed]
- Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010;121:709-29. [PubMed]
- Ting HH, Rihal CS, Gersh BJ, et al. Regional systems of care to optimize timeliness of reperfusion therapy for ST-elevation myocardial infarction: the Mayo Clinic STEMI Protocol. Circulation 2007;116:729-36. [PubMed]
- Aguirre FV, Varghese JJ, Kelley MP, et al. Rural interhospital transfer of ST-elevation myocardial infarction patients for percutaneous coronary revascularization: the Stat Heart Program. Circulation 2008;117:1145-52. [PubMed]
- Jollis JG, Roettig ML, Aluko AO, et al. Implementation of a statewide system for coronary reperfusion for ST-segment elevation myocardial infarction. JAMA 2007;298:2371-80. [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [PubMed]
- Moore AG, Eagle KA, Bruckman D, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol 2002;89:1235-8. [PubMed]
- Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg 2009;37:149-59. [PubMed]
- Firstenberg MS, Crestanello JA, Sai-Sudhakar CB, et al. Ascending aortic dissection: look again before you leap. Ann Thorac Surg 2008;85:1782-4. [PubMed]
- Gologorsky E, Karras R, Gologorsky A, et al. Transesophageal echocardiography after contrast-enhanced CT angiography in the diagnosis of type A aortic dissection. J Card Surg 2011;26:495-500. [PubMed]
- Shanmugam G, McKeown J, Bayfield M, et al. False positive computed tomography findings in aortic dissection. Heart Lung Circ 2004;13:184-7. [PubMed]
- Aggarwal B, Raymond C, Jacob J, et al. Transfer of patients with suspected acute aortic syndrome. Am J Cardiol 2013;112:430-5. [PubMed]
- Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med 2003;42:611-8. [PubMed]
- Artto V, Putaala J, Strbian D, et al. Stroke mimics and intravenous thrombolysis. Ann Emerg Med 2012;59:27-32. [PubMed]
- McCabe JM, Armstrong EJ, Kulkarni A, et al. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate-SF registry. Arch Intern Med 2012;172:864-71. [PubMed]
- Larson DM, Menssen KM, Sharkey SW, et al. “False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA 2007;298:2754-60. [PubMed]
- Harris KM, Strauss CE, Eagle KA, et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation 2011;124:1911-8. [PubMed]
- Madder RD, Raff GL, Hickman L, et al. Comparative diagnostic yield and 3-month outcomes of “triple rule-out” and standard protocol coronary CT angiography in the evaluation of acute chest pain. J Cardiovasc Comput Tomogr 2011;5:165-71. [PubMed]
- Manghat NE, Morgan-Hughes GJ, Roobottom CA. Multi-detector row computed tomography: imaging in acute aortic syndrome. Clin Radiol 2005;60:1256-67. [PubMed]
- Cademartiri F, Pavone P. Advantages of retrospective ECG-gating in cardio-thoracic imaging with 16-row multislice computed tomography. Acta Biomed 2003;74:126-30. [PubMed]
- Roos JE, Willmann JK, Weishaupt D, et al. Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology 2002;222:271-7. [PubMed]
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008;75:7-9, 12, 15-7 passim. [PubMed]
- Yoo SM, Lee HY, White CS. MDCT evaluation of acute aortic syndrome. Radiol Clin North Am 2010;48:67-83. [PubMed]
- Conradi L, Baldus S, Treede H, et al. The best of both worlds: staged hybrid approach to complex cardiac disease in a patient at high surgical risk. Thorac Cardiovasc Surg 2013;61:421-4. [PubMed]
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005;112:2619-26. [PubMed]
- Schoenhagen P, Falkner J, Piraino D. Transcatheter aortic valve repair, imaging, and electronic imaging health record. Curr Cardiol Rep 2013;15:319. [PubMed]


