Treatment of hypertension in patients with renal artery stenosis due to fibromuscular dysplasia of the renal arteries
Introduction
Fibromuscular dysplasia (FMD) is a rare noninflammatory and nonatheroslerotic vascular disease that mainly affects the renal arteries, but less commonly the carotids and vertebral arteries (1-3). FMD was first described in 1938 by Leadbetter and Burkland (4), and it was later classified pathologically, by Harrison and MacKormack in 1971 (5). The most common type of FMD is the “medial fibroplasias” (80-90%) with its characteristic picture of the “string of beads” pattern. Less common types are “intimal fibroplasia” (<10%) and “adventitial fibroplasia” (<5%) (3). The disease affects mostly young females between the ages of 30 and 50 years (3,6). The exact prevalence of FMD in the general population is not currently known. In subjects evaluated for kidney donation the prevalence of FMD was 2.6% (68/2,640) of subjects evaluated (7). Of the 68 subjects, 59 (86.8%) were females, mean age 52±10 years. Similar prevalence was noted in a US Registry of 447 patients with FMD. In this series, the prevalence of females was 91% with a mean age 51.9±13.4 years (8). FMD should be considered in a young person, usually female, who presents with severe hypertension and headaches in the absence of obesity, use of contraceptives, and history of parenchymal renal disease. Early diagnosis and treatment is very important for good long-term results. In these patients, the treatment of choice is percutaneous renal angioplasty (PTRA) with stent placement in selected cases ± medical therapy, which frequently leads to very good control of hypertension. For this review we performed a Medline search of the English language literature using the terms RAS and FMD of the renal arteries from January 2008 to December 31 2013. From the 58 papers reviewed, 19 pertinent papers were selected. The information from these papers together with collateral literature will be discussed in this concise review.
Pathophysiology of FMD
FMD of the renal arteries is a noninflammatory vascular disease, which commonly affects the renal, carotid, and vertebral arteries. However, FMD can also affect arteries in other vascular territories. As described above, renal artery FMD (RAFMD) typically presents anatomically in three different types, including “medial”, “intimal”, and “adventitial” fibroplasia (3).
Medial FMD
This type is the most common accounting for 80-90% of the cases. It presents with its classic appearance the “string of beads” due to the alternating areas of constrictions and post-stenotic dilatations of the renal artery (Figure 1). The lesions affect mainly the medium or distal third of the main renal artery, but they may extend into the proximal portion of the renal artery branches. This lesion can be bilateral in 60% of the cases.
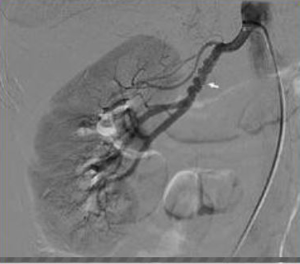
Intimal FMD
This is the second most common (10%) presentation of FMD. It is due to collagen deposition within the intimal complicated often by a fragmented or duplicated internal elastic lamina. Angiographically, it is distinct from the medial fibroplasia because the intimal fibroplasias cause a focal fibrotic constriction that results in a concentric stenosis or long tubular lesion.
Adventitial FMD
This is the least common (<5%) presentation of FMD. It is usually due to the hypertrophy of connective tissue at the junction of the medial and adventitial layers of the renal artery (3). These lesions cause unifocal FMD, which can be found at the ostium, the trunk, or the bifurcation of the renal arteries.
RAFMD affects mostly young women in the prime of their life. The most common clinical symptoms are severe hypertension, headaches, pulsatile tinnitus, and dizziness. The pathophysiologic cause of hypertension in unilateral RAFMD is activation of the renin-angiotensin-aldosterone system (RAAS) secondary to postenotic drop of renal artery pressure and renal ischemia. The increased systemic BP leads to pressure diuresis from the contralateral unobstructed kidney causing plasma volume contraction and further stimulation of RAAS. In these cases, relieve of the obstruction leads to prompt reduction of the activity of RAAS and decrease in BP. In bilateral RAFMD, there is activation of RAAS but the kidneys are not subjected to increased systemic BP and therefore, do not respond with pressure diuresis. In these patients, the hypertension is due to a combination of volume expansion and RAAS activation, and bilateral angioplasty with relief of obstruction in both kidneys will lead to prompt reduction of the activity of RAAS and BP. In contrast to atherosclerotic renal artery stenosis (ARAS), RAFMD almost never, leads to complete obstruction of the renal artery.
Extrarenal manifestations of FMD
Although FMD mainly affects the renal arteries, it can less frequently affect arteries in other vascular territories, as described in the following case studies.
Carotid arteries
Medial FMD (string of beads) of the right internal carotid (ICA) was found in a 52-year-old man who was diagnosed with an acute right posterior parietal brain infarct. This infarct was due to complete occlusion of right ICA from a dissection. The patient was treated successfully with aspirin and clopidogrel without further sequelae (9).
In another case, medial FMD of both carotid arteries was diagnosed in a 52-year-old female, who presented with severe hypertension, severe headaches, right ocular pain, diplopia and amaurosis fugax (10). Both renal arteries were normal. She was treated conservatively with antihypertensive and antiplatelet (aspirin) drugs and had no symptoms one year later.
Brachial arteries
Medial FMD of the left brachial artery was diagnosed in an 83-year-old man with end stage renal disease causing access problems for hemodialysis. The brachial stenosis was treated successfully with balloon angioplasty (11).
Ulnar and radial arteries
Medial FMD was diagnosed in a 20-year-old male who presented with ischemia and cyanosis of the right hand of six weeks duration. Examination revealed significant stenosis of the right ulnar artery, but the radial artery was not significantly narrowed. He was successfully treated with excision of the diseased segment of the ulnar artery with end to end anastomosis (12).
Mesenteric arteries
A rare case of periadventitial FMD of the right mesenteric artery was diagnosed in an elderly patient complaining of abdominal pain, constipation, and bowel distention. He was treated successfully with a right hemicolectomy. The rest of the colon and cecum were normal (13).
External iliac and femoropopliteal arteries
Two cases of medial FMD with obstruction of the iliac and femoropopliteal arteries were diagnosed. In one case a 63-year-old woman presented with disabling left leg claudication (ABI 0.37) and hypertension. She was diagnosed with medial FMD with bilateral common iliac artery aneurysms, and alternating aneurysms and stenoses of both popliteal and superficial femoral arteries. She was treated successfully with resection of the aneurysms and reconstruction of the superficial femoral arteries (14). The second case concerns a 37-year-old man presented with sudden onset of back pain. He was diagnosed with acute pancreatitis and thrombus with dissection of the celiac trunk and right external iliac artery. He was treated successfully for his pancreatitis and with a transluminal coil embolization of the iliac trunk.
Diagnostic evaluation of RAFMD
Catheter-based angiography remains the gold standard for the accurate diagnosis of RAFMD, because it can visualize the main renal arteries as well as the smaller branch vessels. In addition, catheter-based angiography has the advantage that a pressure wire and intravascular ultrasound (IVUS) imaging can be used to visualize the fibrous rings and webs that cause the intravascular obstruction and increase the pressure gradient (Figure 2), as well as evaluate the success of PTRA in cracking the rings (15-17). Other diagnostic modalities like CT angiography (CTA) and contrast-enhanced magnetic resonance angiography (MRA) display good specificity in the detection of RAFMD, and are thus recommended as imaging modalities for its diagnosis (18). In a study by Willoteaux et al. (18), in patients with RAFMD the sensitivity and specificity of contrast-enhanced MRA were 97% and 93%, respectively for the accurate diagnosis of main renal artery disease and it was highly correlated with digital subtraction angiography. In addition to these diagnostic methods, the measurement of translesional systolic blood pressure (SBP) gradient during catheter angiography should be measured as well. A systolic SBP gradient ≥20 mmHg is considered diagnostic for significant RAS (19). Another noninvasive method to evaluate the severity of RAS is the use of duplex scan (20). With this method the peak systolic velocity (PSV) in the stenosed renal artery can be measured. From this measurement, the ratio of PSV of the renal artery to PSV of the aorta (RAR) can be determined. The diagnostic values for significant RAS from this test are listed in Table 1.
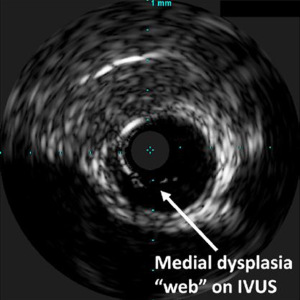
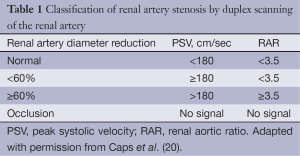
Full table
Treatment of patients with RAFMD
The treatment of choice in patients with RAFMD with hypertension is PTRA, with or without stent placement. This leads to successful control of BP either alone or less frequently in combination with pharmacological antihypertensive treatment. In contrast, in patients with ARAS, PTRA is less frequently successful in lowering the BP and will often require the addition of medical therapy. Another difference between RAFMD and ARAS is that RAFMD almost never causes renal functional impairment while ARAS is very frequently associated with hypertension and renal functional impairment. The American Heart Association has issued guidelines for the treatment of ARAS, which may help guide therapeutic approaches for patients with RAFMD (21). These guidelines regarding initial medical or interventional therapy are summarized in Table 2.
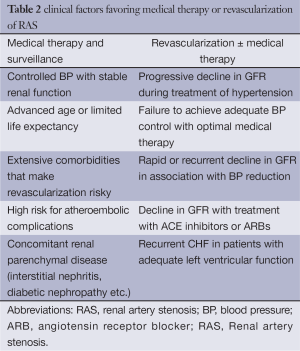
Full table
The following paragraphs summarize findings from studies evaluating the effectiveness and safety of PTRA in patients with RAFMD and hypertension either alone or in combination with antihypertensive drugs.
Øvrehus et al. (22), retrospectively analyzed the results of 12 patients with FMD and hypertension who were treated with PTRA and followed for an average of 13 years. In five of 12 (41.7%) the BP was normalized without medical therapy. Their BP dropped from a baseline of 170/100 to 130/78 mmHg after the intervention and the number of antihypertensive drugs was decreased from 2.7 to 0. In the other seven (58.3%) the BP dropped from a baseline 177/99 to 153/90 mmHg and the number of drugs was decreased from 3.6 to 1.0. Their BP and treatment remained the same for the whole follow-up period of 1,000 days (33.3 months). There was no change in the serum creatinine values in both groups. This study does not provide any information regarding the sex and age of patients.
Davies et al. (23), also analyzed retrospectively the results of 29 female patients with RAFMD, mean age 45 years (range, 18-80 years), who were treated with PTRA ± stent between January 1999 and December 2007. The baseline BP in the 21 (72.4%) of responders to PTRA was 169±20/92±13 mmHg, and in the eight (27.6%) nonresponders to PTRA the baseline BP was 180±36/ 91±14 mmHg. History of hypertension ≤8 years was present in 86% of the patients. PTRA without stent was initially used in all patients and stent was used only in cases of non successful PTRA or complications from the procedure. The patency rates with primary and assisted PTRA were 66% and 87%, respectively at five years. The hypertension was either improved or cured in 72% and 71% of patients, respectively at three months and five years after the procedure. The predictors of long-term success were the duration of hypertension ≤8 years, eGFR ≥60 mL/min/1.73 m2, ipsilateral kidney size ≥9 cm, fasting blood glucose <110 mg/dL and triglycerides <150 mg/dL.
Barrier et al. (24), also analyzed retrospectively, the findings of 30 patients with the less common types of FMD of unifocal and nonmedial dysplastic renal arteries, who were treated between 1994 and 2006. Of the 30 patients, 24 (80%) were females and six (20%) were males mean age 29.6 years (range, 13-51 years). Hypertension was the main presentation for treatment in 28/30 patients. Of the other two, one was treated for worsening of preexisting renal failure and the other for renal protection of a solitary kidney. The renal function of the 28 patients was within normal limits. All patients were treated initially with PTRA and stent was used only, when PTRA was not successful (seven patients). The immediate procedural success rate was 65% and increased to 82% with the additional procedure. Procedural complications occurred in 30%, but they were not serious and all were managed successfully. The initial success rate with improved or cured hypertension was 92%, but hypertension recurred later in 42% of patients. After the second procedure the BP benefit increased to 78% and was maintained for 84 months.
Thatipelli et al. (25), also made a retrospective analysis of the data from 16 older patients treated with PTRA for RAFMD and hypertension between 1999 and 2005.The mean age of the patients was 66±11 years and 14 (88%) of the patients were women. The median follow-up period was 21 months (range, 1.03-85.77 months). The duration of hypertension ranged from two to 24 years and 13 patients had developed severe, resistant to treatment hypertension. Two patients had chronic renal failure with serum creatinine levels 1.5 and 2.4 mg/dL, respectively. A total 21 procedures were performed, 18 on the right renal artery, one on the left renal artery and two were bilateral. Of the 21 procedures, 18 (95%) failed 12.8 months later. The main reason for the failure was prolonged duration of hypertension in 14 (93%) of patients. The mean number of antihypertensive medications before and after PTRA was 2.75±1.25 and 3.0±1.24, respectively (ns). The percentage of failures for BP control at 1, 6, and 12 months was 42%, 58%, and 79%, respectively. This study highlights the poor-, short- and long-term hypertension success rates of PTRA in older patients with RAFMD and hypertension.
Mousa et al. (15), also analyzed the data from 35 patients with RAFMD, of whom 32 (91%) were women. The mean age of the patients was 61.9 years and the main reason for treatment was sustained hypertension. In these patients 43 procedures were performed on 43 renal lesions and the initial success rate was 100%. Of the 35 patients, 27 had isolated RAFMD, 4 had RAFMD with atherosclerosis in the same artery, three with atherosclerosis in the opposite artery and 17 (48.6%) had bilateral RAFMD. The short-term reduction of SBP with PTRA was from (mean ± SEM) 162.2±3.8 to 132.6±2.4 mmHg (P<0.001), and for the diastolic BP (DBP) from 83.4±2.3 to 65.5±3.1 mmHg (P<0.001). The long-term (4.8±0.5 years) reduction in SBP was 142.6±3.6 mmHg from baseline (P<0.001), and in DBP 76.5±2.4 mmHg from baseline (P=0.02). Also, the number of antihypertensive medications was decreased from a baseline of 3.1±0.24 to 2.7±0.27 (P=0.03). Freedom from recurrent hypertension at one, two, and three years was 93%, 75%, and 41%, respectively. The eGFR increased with treatment in 26 patients, from a baseline of 71.1 to 75.6 mL/min (P=0.354). In ten patients with baseline eGFR of <60 mL/min, it increased to >60 mL/min in only two patients. The long-term patency rate was 95%, 71%, and 50% at one, five, and nine years, respectively, whereas the assisted primary patency rate remained at 100% for the nine years of follow-up. This study also, demonstrates the poor long-term response of BP to treatment in older patients.
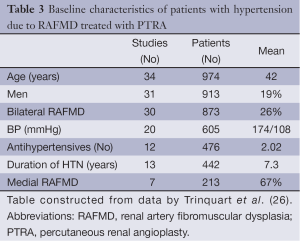
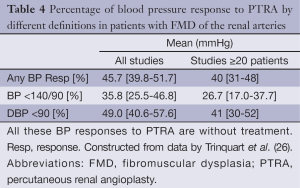
Trinquart et al. (26) examined BP control and complication rate from PTRA in patients with RAFMD in a review and meta-analysis of 47 studies comprising 1,616 patients. Their baseline clinical characteristics are listed in Table 3. There was a great heterogeneity among the studies reporting complete data. In the 31 studies reporting complete data, the mean age of the patients was 42 years with women accounting for 81% of patients with RAFMD. The most common lesion was medial RAFMD, which was present in 67% of patients, and 26% of patients had bilateral disease. Technical success rate was reported in 27 studies (838 patients, 997 procedures) with a success rate of 88.2%. The percentage rate of BP response to PTRA without concomitant medical therapy for all 47 studies (1,426 patients) and for the 22 studies (1,142 patients) with ≥20 patients using different criteria for BP control is listed in Table 4. The follow-up of the patients varied from one to 99 months. Additionally, in this large meta-analysis the percentage of hypertension control rate was inversely related to the age of the patients confirming previous studies. The combined procedural complication rate reported in 20 studies (663 patients) was 11.8% (95% CI, 8.2% to 15%) (26).
Full table
Full table
Discussion
RAFMD is a fairly uncommon disease and a rare cause of hypertension. Its prevalence in the general population is not currently known, but it is estimated from populations undergoing screening for potential kidney donation. In a recent study that evaluated 2,640 living kidney donors free of hypertension at the time of screening, 68 (2.6%) had RAFMD (7), whereas in a previous study of 1,862 subjects evaluated as potential kidney donors, 3.8% (71/1,862) had RAFMD (27). In this study as well the mean age of the subjects was 50.8 years and 75% were females. However, among patients with renovascular hypertension (RVH), the incidence of RAFMD is about 10%, since the greatest majority (80-90%) of patients have ARAS (28). RAFMD is a disease that affects mostly females in the prime of their lives, although it can occur at any age. Of 68 subjects identified with RAFMD among the 2,640 subjects screened for kidney donors, 59 (86.8%) were women mean age 52±10 years (7). Also, in the US Registry of 447 patients with RAFMD, 91% were women mean age 51.9±13.4 years (8). The disease has also been described in children and adolescents 2-18 years old (29). FMD is a noninflammatory fibrodysplastic condition affecting primarily the renal arteries, but also the carotid and vertebral arteries as well as other arteries of the body (9-14). Its etiology is not known at present, and its hereditary nature is disputable, although it has been found in close relatives of patients with RAFMD. In a retrospective analysis of 104 patients with RAFMD, 11% had a history of familial disease (30). Cigarette smoking has also, been implicated as a possible cause of RAFMD (31). In a study of 337 French patients with RAFMD, the proportion of smokers was 30% compared to 18% for non smokers (P<0.001). The most common clinical presentations of patients with RAFMD are severe, uncontrolled hypertension, pulsatile tinnitus and dizziness. Renal failure is a rare symptom of RAFMD and only occurs with complications of the disease like renal artery dissections or thrombosis due to aneurysms in bilateral RAFMD (32). In contrast in patients with ARAS, renal failure is a common presentation (28). Another difference between RAFMD and ARAS is that PTRA ± stent is the treatment of choice for RAFMD, whereas this modality is not always successful in patients with ARAS and most studies have shown no difference between interventional and medical therapy in lowering the BP (19,28). In addition, PTRA in patients with RAFMD is not associated with atheroembolic complications of the kidneys in contrast to ARAS where these complications are frequent due to atherosclerotic disease and the overall prognosis is more favorable in patients with RAFMD. With respect to BP response to treatment, the age and duration of hypertension are very important. In a recent review and meta-analysis, the age and the duration of hypertension were inversely associated with the BP response to treatment (26). In contrast, the response or cure of hypertension after PTRA in children is very high because the age and duration of hypertension are much smaller (29). It is quite possible that long-standing hypertension in patients with RAFMD could damage the opposite unprotected kidney leading to sustained hypertension, which could become unresponsive to PTRA. Therefore, the early diagnosis and treatment of patients with RAFMD is very critical. In addition to age and long-standing hypertension, the presence of risk factors such as diabetes mellitus, dyslipidemia, and renal insufficiency are contributing factors to the poor response of BP to treatment and these risk factors should be treated together with the treatment of hypertension. The treatment of hypertension due to RAFMD can be either medical, interventional, or the combination of both. The drugs of choice are those that block the RAAS, such as angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and direct renin inhibitors (DRI) since this type of hypertension is renin dependent. In addition, RAAS blockers can be used in combination with diuretics, calcium channel blockers (CCB) and beta-blockers (b-blockers). Occasionally, in severe bilateral RAFMD the use of RAAS blockers could lead to significant increase in serum creatinine and BUN due to drop in glomerular filtration pressure from the decrease in the efferent arterial pressure produced by these drugs (33). This complication is completely reversible with the discontinuation of the medications. Another potential modality of treatment in patients with RAFMD is renal denervation. Recently, a case of renal denervation was reported in a 62-year-old female with severe, resistant to medical therapy hypertension. Renal denervation resulted in additional BP reduction to the one achieved by the baseline medical therapy (34). However, large experience with the use of denervation for the treatment of patients with hypertension due to RAS is not available because these patients were excluded from participation in the large denervation studies. Unfortunately, the largest denervation study SIMPLICITY 3 (35), was recently stopped prematurely, because the BP reduction was not better than placebo (sham denervation). Therefore, the results of this study will, most likely, put in jeopardy the future use of renal denervation for the treatment of resistant to treatment hypertension.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Touzé E, Oppenheim C, Trystram D, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke 2010;5:296-305. [PubMed]
- Olin JW, Sealove BA. Diagnosis, management, and future developments of fibromuscular dysplasia. J Vasc Surg 2011;53:826-36.e1.
- Persu A, Touzé E, Mousseaux E, et al. Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest 2012;42:338-47. [PubMed]
- Leadbetter WF, Burkland CE. Hypertension in unilateral renal disease. J Urol 1938;39:611-26.
- Harrison EG Jr, MacKormack LJ. Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin Proc 1971;46:161-7. [PubMed]
- Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med 2004;350:1862-71. [PubMed]
- Mckenzie GA, Oderich GS, Kawashima A, et al. Renal artery fibromuscular dysplasia in 2640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol 2013;24:1477-80. [PubMed]
- Olin JW, Froelich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia. Results in the first 447 patients. Circulation 2012;125:3182-90. [PubMed]
- Poppe AY, Minuk J, Glikstein R, et al. Fibromuscular dysplasia with carotid artery dissection presenting as an isolated hemianopsia. J Stroke Cerebrovasc Dis 2007;16:130-4. [PubMed]
- Mazza A, Zamboni S, Cuppini S, et al. Internal carotid artery fibromuscular dysplasia in arterial hypertension: management in clinical practice. Blood Press 2008;17:274-7. [PubMed]
- Margoles HR, Trerotola SO. Fibromuscular dysplasia of the brachial artery causing hemodialysis access dysfunction. J Vasc Interv Radiol 2009;20:1087-9. [PubMed]
- Khatri VP, Gaulin JC, Amin AK. Fibromuscular dysplasia of distal radial and ulnar arteries: Uncommon cause of digital ischemia. Ann Plast Surg 1994;33:652-5. [PubMed]
- Chaturvedi R, Vaideeswar P, Joshi A, et al. Unusual mesenteric fibromuscular dysplasia- a rare cause for chronic intestinal ischaemia. J Clin Pathol 2008;61:237. [PubMed]
- Okazaki J, Guntani A, Homma K, et al. Fibromuscular dysplasia of the lower extremities. Ann Vasc Dis 2011;4:143-9. [PubMed]
- Mousa AY, Campbell JE, Stone PA, et al. Short- and long-term outcomes of percutaneous transluminal angioplasty/stenting of renal fibromuscular dysplasia over ten-year period. J Vasc Surg 2012;55:421-7. [PubMed]
- Prasad A, Zafar N, Mahmud E. Assessment of renal artery fibromuscular dysplasia: angiography, intravascular ultrasound (with virtual histology), and pressure measurements. Catheter Cardiovasc Interv 2009;74:260-4. [PubMed]
- Gowda MS, Loeb AL, Crouse LJ, et al. Complementary roles of color-flow duplex imaging and intravascular ultrasound in the diagnosis of renal artery fibromuscular dysplasia: should renal arteriography serve as the “gold standard”? J Am Coll Cardiol 2003;41:1305-11. [PubMed]
- Willoteaux S, Faivre-Pierret M, Moranne O, et al. Fibromuscular dysplasia of the main renal arteries: comparison of contrast-enhanced MR angiography with digital ultrasound angiography. Radiology 2006;241:922-9. [PubMed]
- Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal artery stenosis. N Engl J Med 2014;370:13-22. [PubMed]
- Caps MT, Perissinotto C, Zierler RE, et al. Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998;98:2866-72. [PubMed]
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al. Atherosclerotic peripheral vascular disease symposium II. Intervention for renal artery disease. Circulation 2008;118:2873-8. [PubMed]
- Øvrehus KA, Andersen PE, Jacobsen IA. Treatment of renovascular hypertension by transluminal angioplasty- 13 years experience in a single centre. Blood Press 2007;16:335-40. [PubMed]
- Davies MG, Saad WE, Peden EK, et al. The long-term outcomes of percutaneous therapy for renal artery fibromuscular dysplasia. J Vasc Surg 2008;48:865-71. [PubMed]
- Barrier P, Julien A, Guillaume C, et al. Technical and clinical results after percutaneous angioplasty in nonmedial fibromuascular dysplasia: outcomes after endovascular management of unifocal renal artery stenosis in 30 patients. Cardiovasc Intervent Radiol 2010;33:270-7. [PubMed]
- Thatipelli MR, Huettl EA, McKusick MA, et al. Angioplasty for renal artery fibromuscular dysplasia in older hypertensive patients. Angiology 2009;60:714-8. [PubMed]
- Trinquart L, Mounier-Vehier C, Sapoval M, et al. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension 2010;56:525-32. [PubMed]
- Cragg AH, Smith TP, Thompson BH, et al. Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology 1989;172:145-7. [PubMed]
- Chrysant SG. Current status of angioplasty in atherosclerotic renal artery stenosis for the treatment of hypertension. J Clin Hypertens (Greenwich) 2013;15:694-8. [PubMed]
- Srinivasan A, Kishnamurthy G, Fontalvo-Herazo L, et al. Angioplasty for renal artery stenosis in pediatric patients. An 11-year retrospective experience. J Vasc Interv Radiol 2010;21:1672-80. [PubMed]
- Pannier-Moreau I, Grimbert P, Fiquet-Kempf B, et al. Possible familial origin of multifocal renal artery fibromuscular dysplasia. J Hypertens 1997;15:1797-801. [PubMed]
- Savard S, Azarine A, Jeunemaire X, et al. Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension 2013;61:1227-32. [PubMed]
- Dursun B, Yagci B, Batmazoglu M, et al. Bilateral renal infarctions complicating fibromuscular dysplasia of renal arteries in a young male. Scand J Urol Nephrol 2012;46:73-7. [PubMed]
- Chrysant SG, Dunn M, Marples D, et al. Severe reversible azotemia from captopril therapy. Report of three cases and review of the literature. Arch Intern Med 1983;143:437-41. [PubMed]
- Kelle S, Teller DC, Fleck E, et al. Renal denervation in fibromuscular dysplasia. BMJ Case Rep 2013;2013. pii: bcr2013010204.
- Press Release: Medronic announces U.S. Renal Denervation Pivotal Trial fails to meet primary efficacy endpoint while meeting primary safety endpoint. Available online: http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=1889335&highlight


