
Carotid plaque imaging profiling in subjects with risk factors (diabetes and hypertension)
Introduction
Carotid artery stenosis (CAS), due to the presence of atherosclerotic plaque (AP) is a frequent medical condition and a known risk factor for stroke (1); in fact, it has been estimated that 15% of ischemic strokes are caused by large vessel atherosclerosis (1-3). It is also known from literature that several risk factors promote the AP development, in particular aging, smoke, male sex, hypertension, hyperlipidemia, smoke, diabetes type 1 and 2, and genetic factors (4-7).
Several guidelines have been introduced for the treatment of symptomatic CAS (SCAS) and asymptomatic CAS (ACAS) (8). According to the recent guidelines of the European Society of Vascular surgery (ESC) and European Society of for Cardiovascular Surgery (ESCV) the degree of CAS is still considered the main feature to take into consideration for the management of CAS (9).
However, this paradigm is changing, mainly thanks to the technological innovation of the last 10 years: the study of carotid artery atherosclerosis is moving from the mere evaluation of the degree of stenosis (DoS) to the plaque composition in order to identify the “vulnerable plaque” (3,10); for example, a patient with low-grade stenosis and ulcerated CAP would benefit more from a revascularization procedure than one with a stable CAP with a thick fibrous cap (FC) that determines a high-grade stenosis (11). The recognition of these features is often challenging, but it can be helpful for improving the management of these patients.
This aim of this paper is to give a general overview on the main imaging features of Carotid AP (CAP) in subjects with diabetes and hypertension, focusing in particular on the pathogenetic mechanisms, histological features of CAPs, and on the imaging features of the single plaque subcomponents.
Pathogenesis and histological features of carotid artery plaque
The pathogenesis underlying plaque development is still matter of study and debate. Recent published papers suggest that the key pathogenetic mechanism is represented by a self-perpetuating propagating complex inflammatory process involving the arterial wall, called as positive feedback hypothesis (12). According to this hypothesis, low density lipoproteins (LDL) play a central role in this process. LDLs can move from the blood circulation to the arterial wall through endothelial cells by using specific scavenger receptors (12,13) sensitive to estrogen levels (12,14). The remodeling process of the arterial wall starts when LDLs accumulate beneath the intimal layer forming a lipidic core. The remodeling process of the arterial wall can be positive or negative (10,15,16): positive remodeling is characterized by dilation of the vessel wall following the increase CAP volume, with little or absent compromise of the vessel caliber, while negative remodeling is characterized by the reduction of the vessel lumen. LDLs tend to spontaneously oxidize (ox-LDL) because of their molecular instability, and ox-LDLs act as pro-inflammatory molecules and stimulate the recruitment of circulating monocytes from circulating blood (12). Once inside the plaque, macrophages themselves contribute to the inflammatory and growth process of the plaque phagocytizing ox-LDLs and becoming foam cells (17); further, the production of ox-LDLs and other proinflammatory molecules stimulates neoangiogenesis, proliferation of the intimal smooth muscle cells, and endothelial dysfunction (12). This inflammatory environment inside of the plaque promote the production of other ox-LDLs in a self-feeding process that determines the necrosis of the lipidic core, forming the so-called lipid-rich necrotic core (LRNC) (12,18), and the remodeling of the extracellular matrix with the production of a FC on the luminal surface (19). Inflammatory cells tend to accumulate mainly in the shoulder regions and near to the FC of CAP (3,20). The neovessels generated by the inflammatory response inside the AP are usually immature and fragile (21) and tend to break and to determine to intraplaque hemorrhage (IPH) (22). Necrotic debris, apoptotic cells, and extracellular matrix can act as nidus for development of calcifications (23); further, osteoblastic-like cells and multinucleated giant cells, that result morphologically similar to osteoclasts, are frequently found in CAP, especially in regions of calcification and fibrosis (23). Some examples of the above-mentioned histological components of CAP are reported in Figure 1.
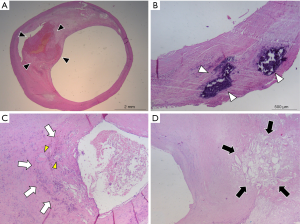
The erosion or rupture of the FC can determine the exposition of the necrotic core components to the blood flow, with release of embolic particles able to reach the distal brain vessels, and with activation of the coagulation cascade and formation of a superimposed thrombus that compromise the arterial lumen (18); the clinical manifestation of this process is the ischemic syndrome (18).
According to the pathological process above described, APs can be classified in six different types by using the well-validated criterion of the American Heart Association (AHA) (11,24) (Table 1): type I AP is characterized by isolated deposition of macrophages foam cells in the arterial wall, type II AP are fatty streak lesions characterized by intracellular lipidic deposits, whereas in type III AP the deposition of lipids is also extracellular (11,24). Type IV lesions are known also as “atheroma” and they are clinically relevant: this type of AP is characterized by the presence of a dense lipidic core that consists of macrophages and inflammatory cells and no FC or surface defects are present (11,24). On the other hand, type V AP is characterized by the presence of FC and of neoangiogenesis inside the lepidic core: this type of plaque can three different subtypes: Va characterized by the presence of a lipid core; Vb characterized by the presence of a partially calcified lipidic core; Vc with a lipid-poor core (11,24). Type VI AP are characterized by fissured FC with hemorrhage and thrombotic deposits (11,24). A similar system was introduced by Cai et al. for classifying APs on magnetic resonance (MR) imaging (32).
Full table
The composition of the plaque can differ also in relation to different factors, included the common risk factors for atherosclerosis such as diabetes and hypertension. A pathological study by Spagnoli et al. (33) in fact evidenced that CAP of patients with hypertension are characterized by the presence of numerous mononuclear cells, whereas the CAP of patients with hypercholesterolemia are rich in foam and mononuclear cells and are covered by a thinner FC; in contrast, the CAP of patients with smoking habit is associated with few mononuclear and giant cells, bigger quantities of connective tissue and greater incidence of thrombosis and calcifications. Lastly, CAP of patients with diabetes (both type 1 and 2) are characterized by the presence of large amount of connective tissue, presence of numerous giant cells and few foam cells (33), and a recent research by Yahagi et al. (34) evidenced that AP of patients with diabetes generally exhibit larger necrotic core and more inflammatory cells when compared to those of non-diabetic patients.
Statins demonstrated to be effective in stabilization of CAP (35); however, their effects on APs are still not well understood (36), even if it is known that higher levels of statins reduce plaque volume sustained not by reduction of necrotic core but mainly by increase of dense calcium volume (37).
Imaging features of carotid artery plaque subcomponents
The stenosis of the carotid artery, as well as the status of the carotid arterial wall, the volume of the CAP and its subcomponents can be analyzed in vivo by different noninvasive imaging modalities, included ultrasound (US), contrast enhanced US (CEUS), computed tomography (CT) and Dual Energy Computed Tomography (DECT), MR and nuclear medicine, in particular 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) combined with CT or MR (38). Their characterization is fundamental in order to assess the vulnerability of CAP (3), underlying that the main components that make a CAP “vulnerable” are: (I) presence of thin or fissured FC, (II) presence of LRNC and calcifications, and (III) presence of inflammation and IPH (3,10,22). In the following paragraph we will analyze the features of these subcomponents, in particular those to be taken into account for accurate evaluation of plaque’s risk of rupture in patients with risk factors for atherosclerosis. A list of the principle markers of plaque vulnerability is reported on Table 1.
We invite the readers to refer to the Expert Consensus Recommendations of the Vessell Wall Imaging Group of the American Society of Neuroradiology (ASNR) (10) for the imaging protocols of study of CAP.
Quantitative measurements of lumen and carotid artery plaque volume
One of the best known and widely studied parameters of risk of stroke is represented by CAS degree (8,9); for example, in order to reduce the risk of stroke, carotid revascularization through endarterectomy or stenting placement is indicated for patients with a recent (<6 months) history of stroke/transient ischemic attach (TIA) and CAS degree between 70–99% estimated using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method (9,39). Smoking habit (40), diabetes (6) and hyperlipidemia (41) are all independent risk factors associated to internal CAS due to the presence of CAP.
CAS degree can be widely studied by US, CT and MR (3) (Figure 2): the best method is represented by MR because of its contrast resolution and excellent reproducibility, that makes it optimal for cross-sectional and longitudinal studies. CT is faster than MR, but the presence of calcifications can lead to overestimation of wall areas (3). US is widely available, accurate and reproducible for plaque and stenosis measurements, but it is observer dependent and calcifications can lead to acoustic shadowing (3).
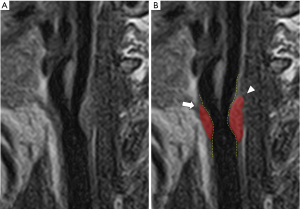
As well as the lumen stenosis, the increase of CAP volume predicts cardiovascular events (10,25,26), and some authors suggested that this parameter can be also a better parameter that could indicate the severity of atherosclerotic disease (42). Recently, a study by Lu et al. (43) evidenced that the progression of the CAP volume is associated with an increased risk of occurrence of cerebrovascular events). Beside CAP volume, CAP composition is another parameter to be taken into consideration: CAP composition has a crucial row in plaque’s stability (3,10). In the next paragraphs will be discussed the principle features of CAP, but it is interesting to note for example that the ratio IPH/ lipid volume is associated with cerebrovascular events as demonstrated by a recent study by Saba et al. (44). CT and MR are able to calculate the volume of the plaque: CT can identify and quantify calcified component, whereas accurate and reliable quantification of both IPH and LRNC is not possible; on the other hand, MR is the best technique for assessing IPH and LRNC (3,10). However, the use of dedicated algorithms can overcome the limitations of these techniques identifying and precisely quantifying plaque tissue characteristics on imaging: for example, a recent research by Sheahan et al. (45) evidenced that software algorithms are able to mitigate the beam hardening and blurring artifacts of routine CT angiography giving accurate quantification of CAP components with high correlation between imaging analysis and ex vivo histological data.
Fibrous cap thickness, surface morphology and lipid rich necrotic core
FC thickness, surface morphology and the lipidic content of CAP are strongly associated with systemic cardiovascular outcomes (3,46): unstable CAPs in fact are characterized by the presence of a thin FC and a large necrotic core (27), and the presence of ulcerations is considered a risk factor for stroke (3,47). Among the risk factors, it is remarkable to underline that diabetes.
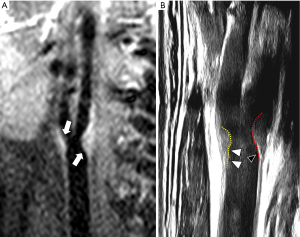
FC thickness has a pivotal role in CAP stability (3,10). It is remarkable to underline for example that type 2 diabetes is associated with thinning of the FC (48). Even if there is not unique consent about the distinction between “thin” from “thick” FC, a FC thickness <200 µm is considered as reference value for identifying “thin” FC (49-51). The other parameter to be taken into account in the evaluation of CAP is the FC status in terms of surface morphology; this can be smooth, irregular (presence of small irregularities ranging from 0.3 to 0.9 mm) or ulcerated (presence of cavities 1 mm depth) (Figure 3) (3). An intact FC is associated to low-risk plaque rupture, whereas the risk of rupture is mild for thin FC and high for fissured FC, and the best technique for assessing FC is MR (28). Intact FC is often not usually well detectable on proton density (PD), T1 and T2 sequences (52), but on time-of-flight (TOF) sequence it commonly appears as a hypointense juxtaluminal band: in case of thin FC this band could be absent, whereas in case of fissured FC the absence of juxta-luminal band is associated with the presence of plaque hemorrhage and/or mural thrombus that appears as a mild hyperintense area next to the lumen (28,49-53). The use of T1-weighted sequence after gadolinium-based contrast medium injection can be used to improve tissue characterization between the FC and the underlying lipidic core (54,55). FC thickness evaluation is feasible also with other technique, even if with suboptimal results; for example, on US it appears as a hyperechoic juxtaluminal structure on contact with the hypoechoic circulating blood (27). Even if US is often able to distinguish between thin and thick FC (56), it is important to remember that this methodology is operator dependent and that even modern US scanners have a spatial resolution between 200–600 µm (47); similarly, the low spatial resolution of modern CT scanners (0.5–0.625 mm) is not optimal for the study of FC (56), and FC cannot be differentiated from soft plaque component (3). On the other hand, CT angiography is considered excellent for the evaluation of the surface morphology and superior to MR because of its superior spatial resolution, whereas US is not considered the technique of choice even if the use of US contrast medium and the application of 3D methods can improve ulceration detection (3).
LRNC is a predictive parameter of increased risk of stroke (3,29). It is known in fact that LRNC size is predictor of FC disruption (10,57), in particular when LRNC area exceeds 40% of vessel wall area (26). As exposed above, LRNC is constituted by cholesterol crystals, debris and calcium deposits in variable percentages (3,11,33). Both CT and MR are able to identify the LRNC (3). LRNC can be easily detected on MR imaging as a focal hypointense area on T2-weighted sequences (10,58,59), and as a focal non-enhancing region within the carotid vessel wall on post-contrast T1-weighted images (10,55,60). CT is superior to MR in detection of calcium components (3), but it is not able to distinguish LRNC from IPH because these two entities show attenuation values <60 Hounsfield units (3,61). US is not useful for differentiating the main plaque components, and in particular it is not able to differentiate between IPH and LRNC (3).
Inflammation and neovascularization
As seen above, the presence of inflammation in the atherogenic process promotes the angiogenetic process (20), and it is considered a marker of plaque vulnerability (30). In the last years several researches were conducted to evaluate the utility of 18FDG-PET in combination with CT (62,63) or MR (64) for the study of CAP inflammation but, even if it is considered the best imaging method for accurate detection of CAP inflammation, there is still no consensus on cut-off of 18FDG uptake for identifying and quantifying it (3,65). MR studies on ex vivo carotid samples using iron nanoparticles (i.e., ultrasmall superparamagnetic irox oxide or P947) that can be incorporated by phagocytic cells within the CAP determining loss of signal on T2* sequences, have shown promising results for the evaluation of inflammation (10,66,67). However, inflammatory process and neoangiogenesis can be indirectly evidenced also by using dynamic contrast enhanced MR (DCE-MR): in fact the study by Kerwin et al. (68) found an association between the plaque enhancement measured by DCE-MR and inflammation, and in particular the transfer contrast (Ktrans) resulted to be correlated with the histologic markers of inflammation (presence of macrophages, neovasculature and loose matrix); further elevated Ktrans values were found in smokers patients when compared to non-smokers. Another study by Kerwin et al. (69) purposed average KTRANS within the adventitia as quantitative measurement related to the extent of vasa vasorum. According to these findings, to the study by Millon et al. (70) and the considerations by Wasserman (71), it is possible to affirm that gadolinium enhancement of CAP is related to inflammatory process and vulnerable plaque phenotype.
Contrast enhanced US (CEUS) allows researchers to detect intraplaque vascularization thanks to its high spatial and temporal resolution (72,73). The plaque enhancement on CEUS can be subjectively classified in three grades according to the regions of the CAP in which microbubbles spread: (I) mild, when microbubbles can be seen in outer parts of CAP; (II) moderate, when microbubbles are visible both inside the CAP and in its shoulder regions, and (III) severe, when microbubbles can be observed in all the plaque regions, including the apex (74). However, further studies are needed in order to normalize and standardize the CEUS technique for clinical practice (73), even if it is remarkable to underline that CEUS has been already used in experimental studies in order to monitor the response to statins treatment, as seen for example in the study by Tian et al. (75) that found that atorvastatin significantly inhibits the development of adventitial vasa vasorum.
It has been demonstrated also on CT that contrast enhancement of CAP is correlated with neoangiogenesis (Figure 4) (3,10): for example, a correlation between contrast enhancement of the CAP and microvessels density in CT was found by Saba et al. (76), and a more recent study by Romero et al. (77) evidenced that the enhancement of CAP reflecting neoangiogenesis is strongly associated with acute neurological symptoms in patients with internal CAS between 50–70% assessed by NASCET (39).
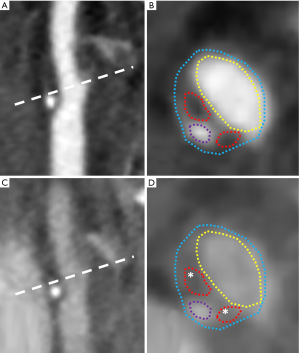
IPH
IPH is another mark of CAP vulnerability (22,31). IPH can occur because the neovascularization promoted by the inflammatory process is characterized by the presence of immature and not-well structured microvessels (22,78). Neovessels are more fragile and easier to break in response to the physiological blood pressure, blood flow and wall shear stress when compared to the normal microvessels (79-81).
IPH appears echolucent on US, and it is quite similar to the aspect of the LRNC (22,81,82). As we have seen in the previous paragraph, CEUS is able to detect intraplaque vascularization, even if the technique is still not standardized in clinical practice (73), but to the best of our knowledge up to date no studies have demonstrated the capability of this technique to identify IPH (22). The identification of IPH on CT angiography is still debated (83), but a recent research by Saba et al. (84) suggested that a HU threshold <25 after contrast medium injection is indicative of the presence of IPH. However, up to now, MR is the technique of choice for evaluating IPH (22): in fact, with this imaging technique IPH can be detected with a sensitivity between 82–97% and a specificity between 74–100% (28). However, the age of IPH influence its appearance on MR because of the different oxidative status of the iron inside the hemoglobin (85): IPH usually appears hyperintense on T1-weighted and TOF sequences and variable signal on T2-weighted and PD sequences (22). T1-weighted fast spin-echo (T1-FSE), T1-weighted sampling perfection with application-optimized contrasts using different flip angle evolution (T1-SPACE) and T1-weighted Magnetization Preapred Rpid AAcquisition Gradient Echo (T1-MPRAGE) are the sequences that show the higher sensitivity and specificity for the detection of IPH (86), whereas other sequences such as the multicontrast atherosclerosis characterization (MATCH) sequence (87) and the simultaneous non-contrast angiography and IPH (SNAP) sequence (88) have shown promising results for IPH evaluation.
Features of vulnerability in high risk subjects
Several risk factors often coexist at the same time in patients with CAT interacting and influencing one to each other, and for this reason it is difficult to study the specific effects of every single risk factor (89-92). However, in the last years some papers have been authored demonstrating that some categories of patients, accepted as high risk, have peculiar features in their carotid artery plaques, and it is possible that advanced algorithm would make easier to detect and characterize the single effect of these risk factors in the future (93). There are several categories that can be considered at high risks, but in the following section we will focus our attention to the hypertension and type 2 diabetes mellitus (T2DM).
Hypertension
Hypertension is considered one of the strongest biomarker associated with the occurrence of cerebrovascular events as demonstrated in a recently published paper by Flint et al. (94): researchers analysed data derived from a population of 1.3 million adults, and they found that both systolic and diastolic hypertension influence the risk of adverse cardiovascular events included myocardial infarction, ischemic stroke and hemorrhagic stroke, regardless the threshold used for the definition of hypertension (blood pressure values ≥140/90 mmHg or ≥130/80 mmHg).
How does this reflect in CAP composition? To the best of our knowledge, not many studies have investigated this aspect, taking into account the fact that more than a single risk factors are often present at the same time in a patient; however, we can make some considerations. As previously seen, Spagnoli et al. (33) evidenced that the presence of numerous mononuclear cells characterized the CAP of hypertensive patients. A recent study by Fassaert et al. (95) analyzed 1,684 underwent to carotid endarterectomy, and they found that patients with pre-operative hypertension (defined as systolic blood pressure ≥160 mmHg) a statistically significant association between systolic hypertension and presence of calcifications, macrophages, lipid core >10% of plaque area, microvessels and IPH, and increased diastolic blood pressure with macrophages, lipid core and IPH, and all these features are typical of the vulnerable CAP. It is also know that hypertension is associated with increased wall volume as reported by Chien et al. (96); in the same article, it is also interesting to underline that patients in therapy with angiotensin converting enzyme inhibitors (a category of drugs commonly used for the treatment of hypertension) is associated with increased thickness of the FC of CAP. Lastly, it is important to underline that hypertension influences also the progression of CAP: a recent study by Lu et al. (97) that analyzed the annual segment-specific progression of CAP by using serial contrast enhanced MR evidenced that hypertension and smoke are risk factors for the progression of CAP located above the bifurcation but not for those located below the bifurcation. The main features of. plaque vulnerability above mentioned are resumed in Table 2.

Full table
T2DM
Simirarly to hypertension, the diabetes status is considered a features of increased risk in terms of cardvioascular mortality as shown by Rawshani et al. (98), and it is also known that the prevalence of atherosclerosis and CAP is higher in patients with T2DM (99). A study by He et al. (100) for example evidenced a relatively high prevalence of non-calcified and non-obstructive CAP in a cohort of 195 patients with T2DM that suffered of TIA or stroke.
The specific effects of T2DM on carotid remodeling and CAP composition remains elusive even if some researches focused on this topic. T2DM acts independently on the atherosclerotic process and it has been demonstrated by Esposito et al. (101) that T2DM is associated to the development of vulnerable plaque irrespectively of the degree of carotid stenosis. T2DM in particular influences CAP composition, acting on inflammation and on the deposition of calcium (34). As previously seen, CAP of patients with diabetes are characterized by the presence of numerous inflammatory cells (in particular giant cells), fewer foam cells, larger amount of connective tissue and larger necrotic core when compared to those of non-diabetic patients (33,34); another recent study by Menegazzo et al. (102) confirmed these data analyzing carotid endarterectomy specimens from 59 patients and finding that although the plaque composition of and the degree of calcifications were similar between diabetic and non-diabetic patients, there were statistically significant differences in terms of plaque calcium component and expression of genes related to inflammation. It is reasonable considering that these features can be also observed on imaging, but to the best of our knowledge only one clinical research by Laugesen et al. (103) analysed the progression of CAP in diabetic patients on MR imaging: the authors compared 100 patients with T2DM (duration <5 years) and 100 healthy controls, finding that patients with T2DM showed increased plaque burden and negative remodeling compared with healthy controls. The main features of plaque vulnerability above mentioned are resumed in Table 3.

Full table
Lastly, it is important to remember that CAP is predictive of underlying silent coronary atherosclerosis prevalence and severity in patients with T2DM (104) and that for this reason some authors suggest that carotid US might be a valuable prognostic tool for this category of patients (105), even if the occurrence of CAP can be underestimated by using US when compared to CTA as demonstrated by Ramanathan et al. (106).
Conclusions
In the last years the evaluation of CAP by imaging has changed from the sole evaluation of the DoS to the evaluation of the plaque subcomponents in order to try to identify the “vulnerable” plaques. Some recent studies evidenced that risk factors for atherosclerosis such as diabetes and hypertension tend to modify CAP composition, that can be detected also by imaging, but further studies are needed to better understand their impact on the evolution of the atherosclerotic process and to optimize the treatment strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.01.13). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from July 2019 to June 2021. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Flaherty ML, Kissela B, Khoury JC, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013;40:36-41. [Crossref] [PubMed]
- Petty GW, Brown RD Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999;30:2513-6. [Crossref] [PubMed]
- Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. [Crossref] [PubMed]
- Woo SY, Joh JH, Han SA, et al. Prevalence and risk factors for atherosclerotic carotid stenosis and plaque: A population-based screening study. Medicine (Baltimore) 2017;96:e5999. [Crossref] [PubMed]
- Mathiesen EB, Joakimsen O, Bønaa KH. Prevalence of and risk factors associated with carotid artery stenosis: the Tromsø Study. Cerebrovasc Dis 2001;12:44-51. [Crossref] [PubMed]
- Noh M, Kwon H, Jung CH, et al. Impact of diabetes duration and degree of carotid artery stenosis on major adverse cardiovascular events: a single-center, retrospective, observational cohort study. Cardiovasc Diabetol 2017;16:74. [Crossref] [PubMed]
- Lee TH, Cheng ML, Shiao MS, et al. Metabolomics study in severe extracranial carotid artery stenosis. BMC Neurol 2019;19:138. [Crossref] [PubMed]
- Abbott AL, Paraskevas KI, Kakkos SK, et al. Systematic Review of Guidelines for the Management of Asymptomatic and Symptomatic Carotid Stenosis. Stroke 2015;46:3288-301. [Crossref] [PubMed]
- Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763-816. [Crossref] [PubMed]
- Saba L, Yuan C, Hatsukami TS, et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018;39:E9-31. [Crossref] [PubMed]
- Brinjikji W, Huston J 3rd, Rabinstein AA, et al. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016;124:27-42. [Crossref] [PubMed]
- Poston RN. Atherosclerosis: integration of its pathogenesis as a self-perpetuating propagating inflammation: a review. Cardiovasc Endocrinol Metab 2019;8:51-61. [Crossref] [PubMed]
- Armstrong SM, Sugiyama MG, Fung KY, et al. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc Res 2015;108:268-77. [Crossref] [PubMed]
- Ghaffari S, Naderi Nabi F, Sugiyama MG, et al. Estrogen Inhibits LDL (Low-Density Lipoprotein) Transcytosis by Human Coronary Artery Endothelial Cells via GPER (G-Protein-Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arterioscler Thromb Vasc Biol 2018;38:2283-94. [Crossref] [PubMed]
- Fukuda K, Iihara K, Maruyama D, et al. Relationship between carotid artery remodeling and plaque vulnerability with T1-weighted magnetic resonance imaging. J Stroke Cerebrovasc Dis 2014;23:1462-70. [Crossref] [PubMed]
- Virmani R, Burke AP, Kolodgie FD, et al. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J Interv Cardiol 2003;16:267-72. [Crossref] [PubMed]
- Yu XH, Fu YC, Zhang DW, et al. Foam cells in atherosclerosis. Clin Chim Acta 2013;424:245-52. [Crossref] [PubMed]
- Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 2014;276:618-32. [Crossref] [PubMed]
- Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852-66. [Crossref] [PubMed]
- van Dijk AC, Truijman MT, Hussain B, et al. Intraplaque Hemorrhage and the Plaque Surface in Carotid Atherosclerosis: The Plaque At RISK Study (PARISK). AJNR Am J Neuroradiol 2015;36:2127-33. [Crossref] [PubMed]
- Parma L, Baganha F, Quax PHA, et al. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol 2017;816:107-15. [Crossref] [PubMed]
- Porcu M, Anzidei M, Suri JS, et al. Carotid artery imaging: The study of intra-plaque vascularization and hemorrhage in the era of the "vulnerable" plaque. J Neuroradiol 2019. [Crossref] [PubMed]
- Qiao JH, Mishra V, Fishbein MC, et al. Multinucleated giant cells in atherosclerotic plaques of human carotid arteries: Identification of osteoclast-like cells and their specific proteins in artery wall. Exp Mol Pathol 2015;99:654-62. [Crossref] [PubMed]
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355-74. [Crossref] [PubMed]
- Rozie S, de Weert TT, de Monyé C, et al. Atherosclerotic plaque volume and composition in symptomatic carotid arteries assessed with multidetector CT angiography; relationship with severity of stenosis and cardiovascular risk factors. Eur Radiol 2009;19:2294-301. [Crossref] [PubMed]
- Wannarong T, Parraga G, Buchanan D, et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke 2013;44:1859-65. [Crossref] [PubMed]
- Devuyst G, Karapanayiotides T, Ruchat P, et al. Ultrasound measurement of the fibrous cap in symptomatic and asymptomatic atheromatous carotid plaques. Circulation 2005;111:2776-82. [Crossref] [PubMed]
- Saba L, Anzidei M, Marincola BC, et al. Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol 2014;37:572-85. [Crossref] [PubMed]
- Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071-7. [Crossref] [PubMed]
- Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med 2015;278:483-93. [Crossref] [PubMed]
- Michel JB, Virmani R, Arbustini E, et al. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J 2011;32:1977-85, 1985a, 1985b, 1985c.
- Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368-73. [Crossref] [PubMed]
- Spagnoli LG, Mauriello A, Palmieri G, et al. Relationships between risk factors and morphological patterns of human carotid atherosclerotic plaques. A multivariate discriminant analysis. Atherosclerosis 1994;108:39-60. [Crossref] [PubMed]
- Yahagi K, Kolodgie FD, Lutter C, et al. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler Thromb Vasc Biol 2017;37:191-204. [Crossref] [PubMed]
- Konishi T, Funayama N, Yamamoto T, et al. Stabilization of symptomatic carotid atherosclerotic plaques by statins: a clinico-pathological analysis. Heart Vessels 2018;33:1311-24. [Crossref] [PubMed]
- Bittencourt MS, Cerci RJ. Statin effects on atherosclerotic plaques: regression or healing? BMC Med 2015;13:260. [Crossref] [PubMed]
- Banach M, Serban C, Sahebkar A, et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med 2015;13:229. [Crossref] [PubMed]
- Goel S, Miller A, Agarwal C, et al. Imaging Modalities to Identity Inflammation in an Atherosclerotic Plaque. Radiol Res Pract 2015;2015:410967.
- Donnan GA, Davis SM, Chambers BR, Gates PC. Surgery for prevention of stroke. Lancet 1998;351:1372-3. [Crossref] [PubMed]
- Tell GS, Polak JF, Ward BJ, et al. Relation of smoking with carotid artery wall thickness and stenosis in older adults. The Cardiovascular Health Study. The Cardiovascular Health Study (CHS) Collaborative Research Group. Circulation 1994;90:2905-8. [Crossref] [PubMed]
- Kerenyi L, Mihalka L, Csiba L, et al. Role of hyperlipidemia in atherosclerotic plaque formation in the internal carotid artery. J Clin Ultrasound 2006;34:283-8. [Crossref] [PubMed]
- Xu D, Hippe DS, Underhill HR, et al. Prediction of high-risk plaque development and plaque progression with the carotid atherosclerosis score. JACC Cardiovasc Imaging 2014;7:366-73. [Crossref] [PubMed]
- Lu M, Peng P, Cui Y, et al. Association of Progression of Carotid Artery Wall Volume and Recurrent Transient Ischemic Attack or Stroke: A Magnetic Resonance Imaging Study. Stroke 2018;49:614-20. [Crossref] [PubMed]
- Saba L, Micheletti G, Brinjikji W, et al. Carotid Intraplaque-Hemorrhage Volume and Its Association with Cerebrovascular Events. AJNR Am J Neuroradiol 2019;40:1731-7. [PubMed]
- Sheahan M, Ma X, Paik D, et al. Atherosclerotic Plaque Tissue: Noninvasive Quantitative Assessment of Characteristics with Software-aided Measurements from Conventional CT Angiography. Radiology 2018;286:622-31. [Crossref] [PubMed]
- Sun J, Zhao XQ, Balu N, et al. Carotid Plaque Lipid Content and Fibrous Cap Status Predict Systemic CV Outcomes: The MRI Substudy in AIM-HIGH. JACC Cardiovasc Imaging 2017;10:241-9. [Crossref] [PubMed]
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415-25. [Crossref] [PubMed]
- Milzi A, Burgmaier M, Burgmaier K, et al. Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study. Cardiovasc Diabetol 2017;16:152. [Crossref] [PubMed]
- Saba L, Potters F, van der Lugt A, et al. Imaging of the fibrous cap in atherosclerotic carotid plaque. Cardiovasc Intervent Radiol 2010;33:681-9. [Crossref] [PubMed]
- Virmani R, Ladich ER, Burke AP, et al. Histopathology of carotid atherosclerotic disease. Neurosurgery 2006;59:S219-27; discussion S3-13.
- Redgrave JN, Gallagher P, Lovett JK, et al. Critical cap thickness and rupture in symptomatic carotid plaques: the oxford plaque study. Stroke 2008;39:1722-9. [Crossref] [PubMed]
- Mitsumori LM, Hatsukami TS, Ferguson MS, et al. In vivo accuracy of multisequence MR imaging for identifying unstable fibrous caps in advanced human carotid plaques. J Magn Reson Imaging 2003;17:410-20. [Crossref] [PubMed]
- Hatsukami TS, Ross R, Polissar NL, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959-64. [Crossref] [PubMed]
- Soloperto G, Casciaro S. Progress in atherosclerotic plaque imaging. World J Radiol 2012;4:353-71. [Crossref] [PubMed]
- Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005;112:3437-44. [Crossref] [PubMed]
- Picano E, Paterni M. Ultrasound tissue characterization of vulnerable atherosclerotic plaque. Int J Mol Sci 2015;16:10121-33. [Crossref] [PubMed]
- Ota H, Yu W, Underhill HR, et al. Hemorrhage and large lipid-rich necrotic cores are independently associated with thin or ruptured fibrous caps: an in vivo 3T MRI study. Arterioscler Thromb Vasc Biol 2009;29:1696-701. [Crossref] [PubMed]
- Toussaint JF, LaMuraglia GM, Southern JF, et al. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 1996;94:932-8. [Crossref] [PubMed]
- Trivedi RA. MRI-derived measurements of fibrous-cap and lipid-core thickness: the potential for identifying vulnerable carotid plaques in vivo. Neuroradiology 2004;46:738-43. [Crossref] [PubMed]
- Wasserman BA, Smith WI, Trout HH 3rd, et al. Carotid artery atherosclerosis: in vivo morphologic characterization with gadolinium-enhanced double-oblique MR imaging initial results. Radiology 2002;223:566-73. [Crossref] [PubMed]
- Wintermark M, Jawadi SS, Rapp JH, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008;29:875-82. [Crossref] [PubMed]
- Rudd JH, Myers KS, Bansilal S, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007;50:892-6. [Crossref] [PubMed]
- Liu J, Kerwin WS, Caldwell JH, et al. High resolution FDG-microPET of carotid atherosclerosis: plaque components underlying enhanced FDG uptake. Int J Cardiovasc Imaging 2016;32:145-52. [Crossref] [PubMed]
- Hyafil F, Schindler A, Sepp D, et al. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur J Nucl Med Mol Imaging 2016;43:270-9. [Crossref] [PubMed]
- Johnsrud K, Skagen K, Seierstad T, et al. (18)F-FDG PET/CT for the quantification of inflammation in large carotid artery plaques. J Nucl Cardiol 2019;26:883-93. [Crossref] [PubMed]
- Chan JM, Monaco C, Wylezinska-Arridge M, et al. Imaging of the vulnerable carotid plaque: biological targeting of inflammation in atherosclerosis using iron oxide particles and MRI. Eur J Vasc Endovasc Surg 2014;47:462-9. [Crossref] [PubMed]
- Ouimet T, Lancelot E, Hyafil F, et al. Molecular and cellular targets of the MRI contrast agent P947 for atherosclerosis imaging. Mol Pharm 2012;9:850-61. [Crossref] [PubMed]
- Kerwin WS, O'Brien KD, Ferguson MS, et al. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 2006;241:459-68. [Crossref] [PubMed]
- Kerwin WS, Oikawa M, Yuan C, et al. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med 2008;59:507-14. [Crossref] [PubMed]
- Millon A, Boussel L, Brevet M, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012;43:3023-8. [Crossref] [PubMed]
- Wasserman BA. Advanced contrast-enhanced MRI for looking beyond the lumen to predict stroke: building a risk profile for carotid plaque. Stroke 2010;41:S12-6. [Crossref] [PubMed]
- Huang R, Abdelmoneim SS, Ball CA, et al. Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J Am Soc Echocardiogr 2016;29:491-502. [Crossref] [PubMed]
- Rafailidis V, Charitanti A, Tegos T, et al. Contrast-enhanced ultrasound of the carotid system: a review of the current literature. J Ultrasound 2017;20:97-109. [Crossref] [PubMed]
- Saha SA, Gourineni V, Feinstein SB. The Use of Contrast-enhanced Ultrasonography for Imaging of Carotid Atherosclerotic Plaques: Current Evidence, Future Directions. Neuroimaging Clin N Am 2016;26:81-96. [Crossref] [PubMed]
- Tian J, Hu S, Sun Y, et al. Vasa vasorum and plaque progression, and responses to atorvastatin in a rabbit model of atherosclerosis: contrast-enhanced ultrasound imaging and intravascular ultrasound study. Heart 2013;99:48-54. [Crossref] [PubMed]
- Saba L, Lai ML, Montisci R, et al. Association between carotid plaque enhancement shown by multidetector CT angiography and histologically validated microvessel density. Eur Radiol 2012;22:2237-45. [Crossref] [PubMed]
- Romero JM, Pizzolato R, Atkinson W, et al. Vasa vasorum enhancement on computerized tomographic angiography correlates with symptomatic patients with 50% to 70% carotid artery stenosis. Stroke 2013;44:3344-9. [Crossref] [PubMed]
- Teng Z, Sadat U, Brown AJ, et al. Plaque hemorrhage in carotid artery disease: pathogenesis, clinical and biomechanical considerations. J Biomech 2014;47:847-58. [Crossref] [PubMed]
- Michel JB, Martin-Ventura JL, Nicoletti A, et al. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014;234:311-9. [Crossref] [PubMed]
- Teng Z, He J, Degnan AJ, et al. Critical mechanical conditions around neovessels in carotid atherosclerotic plaque may promote intraplaque hemorrhage. Atherosclerosis 2012;223:321-6. [Crossref] [PubMed]
- Tuenter A, Selwaness M, Arias Lorza A, et al. High shear stress relates to intraplaque haemorrhage in asymptomatic carotid plaques. Atherosclerosis 2016;251:348-54. [Crossref] [PubMed]
- Grønholdt ML, Wiebe BM, Laursen H, et al. Lipid-rich carotid artery plaques appear echolucent on ultrasound B-mode images and may be associated with intraplaque haemorrhage. Eur J Vasc Endovasc Surg 1997;14:439-45. [Crossref] [PubMed]
- Ajduk M, Pavić L, Bulimbasić S, et al. Multidetector-row computed tomography in evaluation of atherosclerotic carotid plaques complicated with intraplaque hemorrhage. Ann Vasc Surg 2009;23:186-93. [Crossref] [PubMed]
- Saba L, Francone M, Bassareo PP, et al. CT Attenuation Analysis of Carotid Intraplaque Hemorrhage. AJNR Am J Neuroradiol 2018;39:131-7. [Crossref] [PubMed]
- Cappendijk VC, Cleutjens KB, Kessels AG, et al. Assessment of human atherosclerotic carotid plaque components with multisequence MR imaging: initial experience. Radiology 2005;234:487-92. [Crossref] [PubMed]
- Ota H, Yarnykh VL, Ferguson MS, et al. Carotid intraplaque haemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology 2010;254:551-63. [Crossref] [PubMed]
- Dai Y, Lv P, Lin J, et al. Comparison study between multicontrast atherosclerosis characterization (MATCH) and conventional multicontrast MRI of carotid plaque with histology validation. J Magn Reson Imaging 2017;45:764-70. [Crossref] [PubMed]
- Shu H, Sun J, Hatsukami TS, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging: Comparison with contrast-enhanced MR angiography for measuring carotid stenosis. J Magn Reson Imaging 2017;46:1045-52. [Crossref] [PubMed]
- Colussi G, Da Porto A, Cavarape A. Hypertension and type 2 diabetes: lights and shadows about causality. J Hum Hypertens 2020;34:91-3. [Crossref] [PubMed]
- Ivanovic B, Tadic M. Hypercholesterolemia and Hypertension: Two Sides of the Same Coin. Am J Cardiovasc Drugs 2015;15:403-14. [Crossref] [PubMed]
- Virdis A, Giannarelli C, Neves MF, et al. Cigarette smoking and hypertension. Curr Pharm Des 2010;16:2518-25. [Crossref] [PubMed]
- van Rooy MJ, Pretorius E. Obesity, hypertension and hypercholesterolemia as risk factors for atherosclerosis leading to ischemic events. Curr Med Chem 2014;21:2121-9. [Crossref] [PubMed]
- Saba L, Biswas M, Kuppili V, et al. The present and future of deep learning in radiology. Eur J Radiol 2019;114:14-24. [Crossref] [PubMed]
- Flint AC, Conell C, Ren X, et al. Effect of Systolic and Diastolic Blood Pressure on Cardiovascular Outcomes. N Engl J Med 2019;381:243-251. [Crossref] [PubMed]
- Fassaert LMM, Timmerman N, van Koeverden ID, et al. Preoperative hypertension is associated with atherosclerotic intraplaque hemorrhage in patients undergoing carotid endarterectomy. Atherosclerosis 2019;290:214-21. [Crossref] [PubMed]
- Chien JD, Furtado A, Cheng SC, et al. Demographics of carotid atherosclerotic plaque features imaged by computed tomography. J Neuroradiol 2013;40:1-10. [Crossref] [PubMed]
- Lu M, Yuan F, Zhang L, et al. Segment-specific progression of carotid artery atherosclerosis: a magnetic resonance vessel wall imaging study. Neuroradiology 2020;62:211-20. [Crossref] [PubMed]
- Rawshani A, Rawshani A, Franzén S, et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2018;379:633-44. [Crossref] [PubMed]
- Kawamori R, Yamasaki Y, Matsushima H, et al. Prevalence of carotid atherosclerosis in diabetic patients. Ultrasound high-resolution B-mode imaging on carotid arteries. Diabetes Care 1992;15:1290-4. [Crossref] [PubMed]
- He C, Gu M, Jiang R, et al. Noninvasive assessment of the carotid and cerebrovascular atherosclerotic plaques by multidetector CT in type-2 diabetes mellitus patients with transient ischemic attack or stroke. Diabetol Metab Syndr 2013;5:9. [Crossref] [PubMed]
- Esposito L, Saam T, Heider P, et al. MRI plaque imaging reveals high-risk carotid plaques especially in diabetic patients irrespective of the degree of stenosis. BMC Med Imaging 2010;10:27. [Crossref] [PubMed]
- Menegazzo L, Poncina N, Albiero M, et al. Diabetes modifies the relationships among carotid plaque calcification, composition and inflammation. Atherosclerosis 2015;241:533-8. [Crossref] [PubMed]
- Laugesen E, Høyem P, Thrysoe S, et al. Negative Carotid Artery Remodeling in Early Type 2 Diabetes Mellitus and Increased Carotid Plaque Vulnerability in Obesity as Assessed by Magnetic Resonance Imaging. J Am Heart Assoc 2018;7:e008677. [Crossref] [PubMed]
- Jeevarethinam A, Venuraju S, Dumo A, et al. Relationship between carotid atherosclerosis and coronary artery calcification in asymptomatic diabetic patients: A prospective multicenter study. Clin Cardiol 2017;40:752-8. [Crossref] [PubMed]
- Hoke M, Schillinger M, Minar E, et al. Carotid ultrasound investigation as a prognostic tool for patients with diabetes mellitus. Cardiovasc Diabetol 2019;18:90. [Crossref] [PubMed]
- Ramanathan R, Dey D, Nørgaard BL, et al. Carotid plaque composition by CT angiography in asymptomatic subjects: a head-to-head comparison to ultrasound. Eur Radiol 2019;29:5920-31. [Crossref] [PubMed]


