Imaging of intracranial atherosclerotic plaques using 3.0 T and 7.0 T magnetic resonance imaging—current trends and future perspectives
Introduction
Intracranial atherosclerotic disease (ICAD) is one of the major causes responsible for ischemic stroke, especially in Asians and Hispanics (1). The current clinical diagnosis of ICAD, however, is based on the recognition and measurement of luminal stenosis by computed tomography angiography, magnetic resonance angiography (MRA), and digital subtraction angiography (2-4). There are various mechanisms of stroke associated with ICAD. Besides hemodynamic compromise caused by significant stenosis, artery-to-artery embolization due to plaque rupture and occlusion of perforating arteries resulting from parental atheromatous lesions are also underlying mechanisms of stroke in ICAD (5). Therefore, relying solely on the assessment of the vessel lumen may have limitations in determining the pathogenesis of stroke and making targeted treatment strategies. Another challenge in these conventional lumenography-based methods is their limited ability to differentiate between an atherosclerotic lesion and nonatherosclerotic vasculopathies such as dissection, Moyamoya disease, or vasculitis (6). Intracranial high-resolution MR imaging (HRMRI) has been developed for visualizing the intracranial arterial vessel wall, allowing direct evaluation of intracranial atherosclerotic plaques. Plaque features on HRMRI such as wall thickening pattern, remodeling patterns, high signal on T1-weighted images (HST1), contrast-enhancement, and the location of plaque relative to branch artery ostia make it possible to better understand the underlying pathophysiological mechanisms of plaque formation, progression, rupture, and stroke (7-13). Besides, the diagnostic value of HRMRI in differentiating various vasculopathies has been reported in the literature (6). Although HRMRI used in clinical practice and scientific research now is mostly performed at a magnetic field of 3.0 T, this technique at ultra-high-field strength (7.0 T) may benefit from an increased signal to noise ratio (SNR), a high spatial resolution, and better cerebrospinal fluid (CSF) suppression (14,15). Thus, several studies exploring the utility of HRMRI at 7.0 T for ICAD in vivo and vitro have been conducted during the past decade (15-18). These studies published so far have chiefly focused on the ability of 7.0 T MRI: (I) to visualize walls of intracranial arterial vessels with good contrast ratio; (II) to detect the total burden of vessel wall lesions in large intracranial arteries and their branches; (III) and to distinguish different plaque components (15-18). This review will provide an overview of intracranial plaque imaging using 3.0 T and 7.0 T MRI.
Technical implementation of intracranial HRMRI
To clearly depict the inner and outer boundaries of vessel walls, adequate spatial resolution, as well as excellent blood and CSF suppression are essential for intracranial vessel wall imaging techniques. Due to small diameter of the intracranial arteries, ranging from 3 to 5 mm in proximal middle cerebral arteries (MCAs) (19), a high resolution with voxel size <1×1×1 mm3 is necessary. A 2-dimensional (2D) sequence can provide a better spatial resolution (a voxel size of 0.4×0.4×2.0 mm3) and SNR for targeted vessel wall lesions (7). However, it is unable to achieve a more global depiction of multiple vessels and detect lesions without luminal stenosis. Another disadvantage of 2D techniques is partial volume effects resulting from tortuous course of intracranial vessels. A 3D isotropic imaging technique with a larger coverage of whole-brain vessels makes it possible to perform multi-planar and curved-planar reformations, as well as assess plaque burden and distribution of major intracranial arteries on various sectional views. Most currently used 3D imaging methods in recent studies have isotropic voxel dimensions of 0.5 to 0.7 mm keeping a scan time of 7–10 minutes (20,21). Compared to 3D technique, 2D sequence provides a relatively superior “in-plane” spatial resolution focusing on more details about the vessel wall lesions. Therefore, an optimal imaging protocol for intracranial arteries should include both 2D and 3D sequences. Delineating the inner and outer margins of the intracranial artery requires effective elimination of the signal from blood flow and CSF. Spin-echo (SE) sequences exploit the outflow of blood to suppress blood signal, which requires a transverse imaging plane perpendicular to the arteries (22). A limitation of this technique is that most intracranial arteries have a very tortuous course. Thus, 2D SE and turbo SE (TSE) sequences are used to image the artery of interest, providing a high resolution within the slice and minimized imaging blurring. Currently, 3D TSE with variable refocusing flip angles sequences (SPACE, sampling perfection with application-optimized contrasts by using different flip angle evolutions, Siemens, Erlangen, Germany; VISTA, volume isotropic turbo SE acquisition, Philips Healthcare, Best, the Netherlands; and Cube, GE Healthcare, Milwaukee, Wisconsin, USA) have emerged as the most commonly used black-blood techniques, allowing excellent suppression of blood and CSF signal, and large spatial coverage in a reasonable scan time. These sequences can be employed with T1-weighted, T2-weighted, and proton density-weighted imaging. Double inversion recovery prepared T1-weighted sequence can generate additional blood and CSF suppression based on both the flow and T1 properties of blood (23). A drawback of this technique is that it takes time for spins to reach the null point, thereby increasing scan time. The delay alternating with nutation for tailored excitation (DANTE) pulse train has been used as a prepulse with 3D variable refocusing flip angles techniques, which may be complementary for blood suppression, especially in turbulent or slow flow (24). Accelerated or fast vessel wall imaging techniques recently have gained increased attention due to the possibility of shortening acquisition times while retaining adequate image quality. A recent study has demonstrated an accelerated multi−contrast 3D intracranial vessel wall MRI using cartesian undersampling with target ordering method (CUSTOM) and self-supporting tailored k-space estimation for parallel imaging reconstruction (STEP) within 30 min of scan time (25). Another method for scan acceleration is compressed sensing, which has been used to accelerate T1-weighted intracranial and extracranial plaque imaging (26,27). The application of compressed sensing for rapid intracranial vessel wall imaging provides a shorter scan time compared to conventional method while maintaining good image quality.
Currently, HRMRI for intracranial arteries is performed at 3.0 T MRI at most centers. Considering the small caliber of intracranial arteries, ultra-high-field (7.0 T) may provide an additional value for evaluating intracranial atherosclerotic plaques, as it allows for a high SNR, spatial resolution, and contrast to noise ratio. These advantages make it possible to identify the total burden of vessel wall lesions (15). However, B1+ inhomogeneities at 7.0 T cause spatially varying SNR and contrast, most prominently in the regions of temporal lobes and cerebellum (28). The use of dielectric pads may improve part of the signal loss. Though the feasibility of intracranial HRMRI at 7.0 T has been demonstrated in several vivo and vitro studies (15-18), more evidence of an additional clinical value of 7.0 T MRI is needed before it could be used in routine clinical practice.
Assessments of atherosclerotic plaques at 3.0 T and 7.0 T
Plaque burden
The advantage of a 3D vessel wall imaging protocol over a 2D sequence is the ability to simultaneously detect atherosclerotic plaques in multiple arterial segments and non-stenotic lesions (29,30). Recently, a systematic review has investigated the clinical significance of intracranial atherosclerotic plaques without substantial stenosis in recent ischemic stroke patients using HRMRI at 3.0 T or 1.5 T (31). The authors reviewed twenty-one studies, including 463 patients without stenosis of the intracranial arteries and 651 patients with stenosis <50%. Overall, 50.6% (95% confidence interval, 46.1–55.1%) of acute/subacute ischemic stroke patients without stenotic MRA were identified with intracranial plaques on HRMRI. Sensitive identification of early non-stenotic ICAD using HRMRI allows accurate recognization of stroke etiology.
Wall thickness and area at the most stenotic intracranial artery segment have been considered to be associated with risk of stroke (32,33). Plaque volume has also been demonstrated as a risk factor for cerebrovascular events in carotid arteries (34). Due to the small vessel diameter of intracranial arteries and limitation of automatic analysis software, it remains difficult to evaluate plaque volume in intracranial arteries. Qiao et al. measured wall volume on 3D HRMRI at 3.0 T by automatic segmentation of large intracranial arteries in the general population. Their study revealed that examination and reader reliability are good to excellent for the quantitative measurement of the wall volume (20). In our recent study, we measured the total plaque volume in patients with different stroke types (35). First, we manually dyed the plaques on multi-planar reformation images. Then total volumes of the plaques were automatically calculated by a semi-automatic analysis software. Finally, we found that plaque volume in ipsilateral intracranial arteries was higher in patients with non-deep perforator infarction (130.9±90.3 mm3) than patients with deep perforator infarction (82.0±45.9 mm3), suggesting that higher plaque volume may contain more vulnerable components and cause downstream infarction. Further studies are required to explore the role of intracranial plaque volume played in determining the risk of cerebrovascular events.
Reliability analysis for this novel imaging technique should be performed before it could enter routine clinical practice. Several studies have revealed that scan-rescan, and intra- and inter-observer reproducibility using HRMRI for qualitative and quantitative assessment of intracranial plaque are excellent, suggesting that it is a reliable diagnostic method for the assessment and efficacy monitoring of ICAD (36,37).
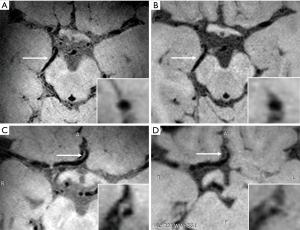
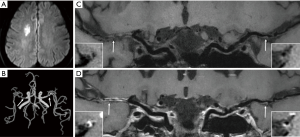
Compared to 3.0 T MRI, the increased SNR and better CSF suppression of 7.0 T lead to an overall improvement of vessel wall visibility. A previous study has compared visualization of the intracranial vessel wall and possible vessel wall lesions between 3.0 T and 7.0 MRI in 21 elderly asymptomatic volunteers (15). This study showed that 7.0 T MRI has a higher potential to identify the total burden of intracranial plaques and the greater sensitivity to detect vessel wall enhancement. Besides, the better vessel wall visibility at 7.0 T was most notably in the proximal anterior cerebral circulation and the posterior cerebral artery (Figure 1). In this study, however, inversion pulse for CSF suppression was lacking at 3.0 T.
Plaque components
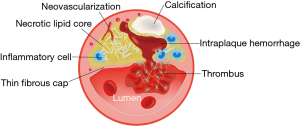
Intraplaque hemorrhage (IPH), fissured fibrous cap, lipid rich necrotic core, neovascularization, and inflammation are considered common features of vulnerable plaque found in symptomatic lesions (38) (Figure 2). Identification of plaque components by HRMRI has the potential to determine vulnerable plaque and predict the risk of rupture and events. For extracranial carotid atherosclerosis, endarterectomy specimen analyses allow the validation of plaque signal characteristics on HRMRI with histopathology (39). For intracranial atherosclerosis, however, HRMRI-pathology correlation was explored in limited studies of post-mortem artery specimens. Chen et al. reported that HST1 on vitro MRI at 1.5 T was IPH using pathologically-verified in a post-mortem case of Chinese adult (40). Then, the correlation between lipid core assessed on histology and low signal on T1-weighted fat-suppressed images (LST1) within intracranial vessel walls (76 MCA segments) has also been explored (41). This study reported a high sensitivity (81.2%) and high specificity (91.7%) of LST1 at 1.5 T MRI for identifying lipid core of area ≥0.80 mm2 within MCA atherosclerotic plaques. Another case report demonstrated the correlation between atherosclerotic plaque characteristics on T1-and T2-weighted HRMRI at 3.0 T obtained in-vivo and postmortem pathology findings (lipid and loose matrix, fibrous tissue, and calcium) in a patient with symptomatic left cavernous carotid stenosis (42). Additionally, relaxation times of intracranial plaque components were measured ex vivo at 3.0 T in a post-mortem study, which demonstrated measurement of relaxation times derived from quantitative images has the potential to identify intracranial plaque types (43).
An in vivo 3.0 T and ex vivo 7.0 T MRI-histology correlation study has been conducted in carotid artery atherosclerotic plaque, which found that the high resolution of vitro 7.0 T MRI was able to highlight greater detail in the components of carotid plaques (44). Several post-mortem studies have investigated the feasibility of 7.0 T MRI to identify intracranial plaque components compared with histopathologic analysis (16,18,45). These studies showed that 7.0 T MRI has the potential to detail plaque substructure, although they were performed in a limited number of samples. A study of 44 arterial segments from 5 specimens of the circle reported that signal heterogeneities of more advanced intracranial atherosclerotic plaques on 7.0 T MRI with different image contrast weightings enabled the spatial distinction of different plaque components, like collagen and foamy macrophages (16). Another comparative study with 7.0 T MRI and histopathology showed that the positive predictive values of 7.0 T MR imaging for identifying fibrous and attenuated calcium components were 88% and 93%, respectively (45). However, they only performed a qualitative comparison of 7.0 T MRI and histology. Regarding the advance of imaging techniques, development of quantitative MRI, like T1-, T2-, and T2*mapping, would allow for characterization of atherosclerotic plaque components in a quantitative manner. In a previous study, Harteveld et al. explored the ability of quantitative T1, T2, and T2* relaxation times and PD values at 7.0 T in identification of different intracranial atherosclerotic plaque components using histopathology for validation (18). The authors concluded that the most promising sequence for distinguishing these plaque components at 7.0 T is T1-weighted imaging. Although the above-mentioned studies with histologic validation have demonstrated the feasibility of HRMRI at 3.0 T and 7.0 T to identify intracranial plaque components, in vivo intracranial HRMRI still faces some practical challenges in assessing plaque vulnerability. Considering plaque shrinkage during fixation and histological processing, the signal intensity and area measurements of various plaque components on MRI may differ between in vivo arteries and ex vivo specimens. Therefore, the ability of in vivo MRI for detecting components of intracranial vulnerable or high-risk plaques still needs to be demonstrated with comparison to histopathology.
Plaque characteristics and ischemic infarcts
Multiple studies have investigated the relationship between plaque location, morphology, remodeling pattern, signal intensity, as well as contrast enhancement on intracranial HRMRI and acute cerebrovascular events (9-11,13). HRMRI is performed at 3.0 T field strength in the majority of studies. All these studies aimed to establish imaging biomarkers to identify patients at high risk and allow further risk stratification in ICAD. Therefore, aggressive medical management should be focused on these patients. In addition, specific biomarkers to differentiate stroke mechanisms of ICAD are also needed, because identifying the pathogenesis of ICAD could have important potential therapeutic implications.
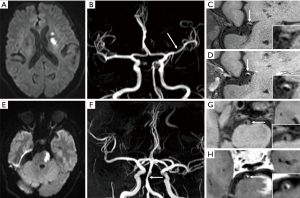
Deep perforator infarction is considered to be caused by a large artery atherosclerotic plaque that blocks ostia of a perforating artery (46). Several studies have shown that MCA plaques on HRMRI can be detected in 40–63.2% of patients with deep perforator infarction (47,48). Most of perforating arteries arise dorsally from the upper part of MCA wall (49). Location of atherosclerotic plaque close to branch vessel ostia increases the risk of branch occlusion following intracranial angioplasty and stenting, in which neighboring atheromatous material is pushed into a branch (50). By using HRMRI, determining the location of intracranial atherosclerotic plaque relative to branch artery ostia may help predict stroke occurrence and its subtypes (Figure 3), as well as estimate the risk of angioplasty. Xu et al. were the first to explore the plaque location of MCA and its clinical relevance (51). They reported a higher prevalence of plaques located at the ventral (44.8%) and inferior (31.7%) wall which is opposite to the orifices of perforation arteries. In their studies, symptomatic MCA stenosis had more superior and less inferior wall plaques than asymptomatic stenosis. Besides, patients with deep perforator infarction had more superior but less ventral and inferior plaques than those without deep perforator infarction. Similarly, a study of basilar artery plaque location on HRMRI revealed that patients with lateral pontine infarctions showed more plaques at the lateral wall of the basilar artery, where the lateral circumferential branches originate (52) (Figure 3). Paramedian infarctions were more frequently to have plaques at the posterior basilar artery wall, where the paramedian perforating branches originate.
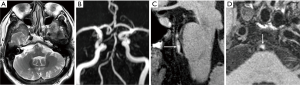
IPH is considered as a good predictor of plaque progression and ischemic events, which has been demonstrated in studies of coronary and extracranial carotid atherosclerotic diseases with pathologic validation (53,54). Increased density of microvessels and microvascular incompetence are viable sources of IPH (53,55). Heavily T1-weighted techniques with HRMRI is commonly used to identify IPH due to its higher sensitivity for detecting high-signal intensity (Figure 4). Several studies investigating the relationship between HST1 and symptoms have been published. Xu et al. reported a relatively higher frequency of HST1 in symptomatic MCA stenosis (19.6%) compared to asymptomatic MCA stenosis (3.2%) (9). A study based on 73 patients with > 50% basilar artery stenosis showed that the prevalence of HSTI was 41.1% using magnetization-prepared rapid acquisition with gradient-echo. They also found more symptomatic lesions in the MR-positive HSTI group than the MR-negative group (80.0% versus 48.8%) (12). Recently, a HRMRI study recruiting 126 patients with >30% basilar artery stenosis revealed that the incidence of HST1 was 22%, detected in both low and high grade stenotic basilar arteries (11.3% versus 16.3%). Based on their results, HST1 showed a high specificity (98.3%) but a low sensitivity (31.8%) for indicating acute/subacute symptomatic stroke (56). In the above studies, HST1 was defined as an area with signal intensity >150% of the signal from the adjacent muscle. Our recent study evaluated HST1 in ICAD patients with recent stroke using adjacent normal vessel wall as the reference (57). By using whole-brain vessel wall imaging technique, we explored the atherosclerotic plaque characteristics in different stroke subtypes. We found that HST1 were more frequently observed in patients with artery-to-artery embolic infarction than non artery-to-artery embolic infarction (75.0% versus 21.1%), suggesting a correlation between plaque rupture, formation of blood clots, and embolic stroke. A potential reason for the relatively higher prevalence of HST1 in our work is the use of different reference and improved T1 contrast weighting offered by the inversion recovery prepared 3D variable refocusing flip angles sequence. To sum up, it is still needed to define a standard threshold for HST1 as the most valuable marker for stratification of stroke risk. In other words, more sensitive and quantitative imaging biomarker to identify IPH should be developed.
A postmortem study has demonstrated that neovasculature can be found in MCA atherosclerotic plaque and was associated with ipsilateral infarction (58). Histopathologic studies of carotid artery plaques also demonstrated that an increased density of neovessels was associated with inflammation and increased risk of plaque rupture (53,55). Gadolinium enhancement of carotid plaques was proved to be associated with vulnerable plaque, neovascularization, macrophages, and loose fibrosis correlating with histopathology (59). Using HRMRI, intracranial plaque enhancement can be evaluated and the relationship between plaque enhancement and recent infarction has been established (11,60) (Figure 5). Qiao et al. categorized intracranial plaque enhancement into two grades by using the pituitary infundibulum for reference (11). They revealed that all culprit and probably culprit plaques enhanced and the culprit plaques had a higher degree of contrast enhancement compared to nonculprit plaques. Another study has evaluated enhancement of intracranial atherosclerotic plaques in 29 patients with ischemic stroke of different phases (60). It demonstrated that atherosclerotic plaques within the vascular territory of the stroke showed strong enhancement in all of the acute phase patients, whereas enhancement of the plaques in the vessel supplying the stroke territory was mild or absent in the subacute or chronic phase patients. They also found a trend of decreasing enhancement with increased time after stroke onset. Lou et al. measured plaque enhancement in different sites of basilar arterial atherosclerosis and discussed its relevance with recent stroke and subsequent ischemic events (61). In their results, both patients with recent stroke and subsequent ischemic events had a stronger wall enhancement at the section proximal to the most stenotic site. However, plaque enhancement in the section at and distal to the most stenotic site showed no significant difference. Their study suggested that wall enhancement is not equally distributed within the atherosclerotic plaque. The values of plaque characteristics on HRMRI to predict future stroke was also discussed in several studies. A recent study recruited 138 acute stroke patients with symptomatic ICAD who were followed-up after ischemic event (13). Of the 138 patients, 39 patients experienced stroke recurrence (37 with plaque enhancement and 2 without plaque enhancement). The results showed that plaque enhancement was independently associated with recurrent stroke with a hazard ratio of 7.42. Another study based on 43 symptomatic severe MCA stenosis, however, found no significant difference in plaque enhancement between patients with and without one year ischemic events (62). Therefore, further studies determining the optimal predictors of early recurrence in patients with intracranial stenosis are still needed.
Future perspectives
During the past two decades, intracranial HRMRI has been developed at 3.0 T and 7.0 T for evaluating plaque vulnerability, stroke etiology, and future stroke risk (63,64). However, several important limitations of current research should be noted. Because endarterectomy specimens are not available for ICAD, it is difficult to compare in-vivo HRMRI and postoperative histopathology images. Only few studies to date have been performed in a limited number of samples, focusing on pathological validation of intracranial HRMRI with post-mortem artery specimens (40-43). Therefore, it should be noted that the plaque signal characteristics on HRMRI underlying plaque components remain poorly understood, representing a major limitation of this approach. Future studies still need to assess the feasibility of intracranial HRMRI in identifying plaque components with histologic validation. Another issue of current studies is the diversity in the acquisitions and assessment methods of HRMRI data. The relationship between HRMRI characteristics of intracranial arteries and ischemic events has been explored in multiple studies to establish the role of evaluation of plaque vulnerability in intracranial HRMRI (9-11). However, the varied stroke phase of patients, MRI techniques, and measurement criteria of plaque features in these studies may lead to heterogeneity in results. In addition, a few studies investigating the association between HRMRI findings and stroke recurrence have demonstrated the prognostic value of HRMRI in stroke patients (13). Considering a high recurrent stroke rate of symptomatic ICAD, more prospective longitudinal studies are needed to explore optimal imaging biomarkers for predicting early deterioration after stroke in patients with ICAD. Currently available research is the first step to design focused trials on individualized treatment and prevention strategies of intracranial stenosis. Further studies are required to investigate the use of selected biomarkers in randomized control trials of secondary prevention and treatment of symptomatic ICAD. Finally, HRMRI at 7.0 T MRI has the potential to identify early lesions and total burden of ICAD, which has been demonstrated in a limited number of studies (15). More comparative studies of 3.0 T and 7.0 T HRMRI still need to address if 7.0 T MRI is superior to 3.0 T MRI for the evaluation of ICAD. Overall, HRMRI for the assessment of ICAD still faces some challenges before it could enter routine clinical practice.
Conclusions
HRMRI holds promise in improving our pathological understanding of ICAD and providing incremental information on plaque vulnerability and stroke risk stratification. Ultra-high-field strength MRI with 7.0 T has shown great potential in the quantification of total plaque burden. Further studies comparing 3.0 T and 7.0 T MRI are needed in order to verify the diagnostic and therapeutic advantages in imaging intracranial plaques on a larger scale.
Acknowledgments
Funding: This work was supported by the National Key Research and Development Program of China [2016YFC1301702, 2017YFC1307903]; the Beijing Natural Science Foundation (L172043); and the National Science Foundation of China (NSFC 91749127).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.02.03). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1-25. [Crossref] [PubMed]
- Duffis EJ, Jethwa P, Gupta G, et al. Accuracy of computed tomographic angiography compared to digital subtraction angiography in the diagnosis of intracranial stenosis and its impact on clinical decision-making. J Stroke Cerebrovasc Dis 2013;22:1013-7. [Crossref] [PubMed]
- Yaghi S, Elkind MS. Cryptogenic stroke: A diagnostic challenge. Neurol Clin Pract 2014;4:386-93. [Crossref] [PubMed]
- Cloft HJ, Lynn MJ, Feldmann E, et al. Risk of cerebral angiography in patients with symptomatic intracranial atherosclerotic stenosis. Cerebrovasc Dis 2011;31:588-91. [Crossref] [PubMed]
- Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke 2014;16:27-35. [Crossref] [PubMed]
- Mossa-Basha M, de Havenon A, Becker KJ, et al. Added Value of Vessel Wall Magnetic Resonance Imaging in the Differentiation of Moyamoya Vasculopathies in a Non-Asian Cohort. Stroke 2016;47:1782-8. [Crossref] [PubMed]
- Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218-29. [Crossref] [PubMed]
- Dieleman N, Yang W, Abrigo JM, et al. Magnetic Resonance Imaging of Plaque Morphology, Burden, and Distribution in Patients With Symptomatic Middle Cerebral Artery Stenosis. Stroke 2016;47:1797-802. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012;71:195-8. [Crossref] [PubMed]
- Ryoo S, Lee MJ, Cha J, et al. Differential Vascular Pathophysiologic Types of Intracranial Atherosclerotic Stroke: A High-Resolution Wall Magnetic Resonance Imaging Study. Stroke 2015;46:2815-21. [Crossref] [PubMed]
- Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534-42. [Crossref] [PubMed]
- Yu JH, Kwak HS, Chung GH, et al. Association of Intraplaque Hemorrhage and Acute Infarction in Patients With Basilar Artery Plaque. Stroke 2015;46:2768-72. [Crossref] [PubMed]
- Kim JM, Jung KH, Sohn CH, et al. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke 2016;11:171-9. [Crossref] [PubMed]
- De Cocker LJ, Lindenholz A, Zwanenburg JJ, et al. Clinical vascular imaging in the brain at 7T. Neuroimage 2018;168:452-8. [Crossref] [PubMed]
- Harteveld AA, van der Kolk AG, van der Worp HB, et al. High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur Radiol 2017;27:1585-95. [Crossref] [PubMed]
- van der Kolk AG, Zwanenburg JJ, Denswil NP, et al. Imaging the intracranial atherosclerotic vessel wall using 7T MRI: initial comparison with histopathology. AJNR Am J Neuroradiol 2015;36:694-701. [Crossref] [PubMed]
- van der Kolk AG, Hendrikse J, Brundel M, et al. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. Eur Radiol 2013;23:2996-3004. [Crossref] [PubMed]
- Harteveld AA, Denswil NP, Siero JC, et al. Quantitative Intracranial Atherosclerotic Plaque Characterization at 7T MRI: An Ex Vivo Study with Histologic Validation. AJNR Am J Neuroradiol 2016;37:802-10. [Crossref] [PubMed]
- Jain KK. Some Observations on the Anatomy of the Middle Cerebral Artery. Can J Surg 1964;7:134-9. [PubMed]
- Qiao Y, Guallar E, Suri FK, et al. MR Imaging Measures of Intracranial Atherosclerosis in a Population-based Study. Radiology 2016;280:860-8. [Crossref] [PubMed]
- Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging 2017;46:751-7. [Crossref] [PubMed]
- Edelman RR, Mattle HP, Wallner B, et al. Extracranial carotid arteries: evaluation with "black blood" MR angiography. Radiology 1990;177:45-50. [Crossref] [PubMed]
- Edelman RR, Chien D, Kim D. Fast selective black blood MR imaging. Radiology 1991;181:655-60. [Crossref] [PubMed]
- Xie Y, Yang Q, Xie G, et al. Improved black-blood imaging using DANTE-SPACE for simultaneous carotid and intracranial vessel wall evaluation. Magn Reson Med 2016;75:2286-94. [Crossref] [PubMed]
- Balu N, Zhou Z, Hippe DS, et al. Accelerated multi-contrast high isotropic resolution 3D intracranial vessel wall MRI using a tailored k-space undersampling and partially parallel reconstruction strategy. MAGMA 2019;32:343-57. [Crossref] [PubMed]
- Zhu C, Tian B, Chen L, et al. Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE). MAGMA 2018;31:457-67. [Crossref] [PubMed]
- Okuchi S, Fushimi Y, Okada T, et al. Visualization of carotid vessel wall and atherosclerotic plaque: T1-SPACE vs. compressed sensing T1-SPACE. Eur Radiol 2019;29:4114-22. [Crossref] [PubMed]
- van der Kolk AG, Hendrikse J, Zwanenburg JJ, et al. Clinical applications of 7 T MRI in the brain. Eur J Radiol 2013;82:708-18. [Crossref] [PubMed]
- Wu F, Ma Q, Song H, et al. Differential Features of Culprit Intracranial Atherosclerotic Lesions: A Whole-Brain Vessel Wall Imaging Study in Patients With Acute Ischemic Stroke. J Am Heart Assoc 2018;7:e009705. [Crossref] [PubMed]
- Sun LL, Li ZH, Tang WX, et al. High resolution magnetic resonance imaging in pathogenesis diagnosis of single lenticulostriate infarction with nonstenotic middle cerebral artery, a retrospective study. BMC Neurol 2018;18:51. [Crossref] [PubMed]
- Wang Y, Liu X, Wu X, et al. Culprit intracranial plaque without substantial stenosis in acute ischemic stroke on vessel wall MRI: A systematic review. Atherosclerosis 2019;287:112-21. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010;212:507-11. [Crossref] [PubMed]
- Ryu CW, Jahng GH, Kim EJ, et al. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovasc Dis 2009;27:433-42. [Crossref] [PubMed]
- Vukadinovic D, Rozie S, van Gils M, et al. Automated versus manual segmentation of atherosclerotic carotid plaque volume and components in CTA: associations with cardiovascular risk factors. Int J Cardiovasc Imaging 2012;28:877-87. [Crossref] [PubMed]
- Wu F, Zhang Q, Dong K, et al. Whole-brain magnetic resonance imaging of plaque burden and lenticulostriate arteries in patients with different types of stroke. Ther Adv Neurol Disord 2019;12:1756286419833295. [Crossref] [PubMed]
- Zhang N, Zhang F, Deng Z, et al. 3D whole-brain vessel wall cardiovascular magnetic resonance imaging: a study on the reliability in the quantification of intracranial vessel dimensions. J Cardiovasc Magn Reson 2018;20:39. [Crossref] [PubMed]
- Mossa-Basha M, Watase H, Sun J, et al. Inter-rater and scan-rescan reproducibility of the detection of intracranial atherosclerosis on contrast-enhanced 3D vessel wall MRI. Br J Radiol 2019;92:20180973. [Crossref] [PubMed]
- Altaf N, Daniels L, Morgan PS, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg 2008;47:337-42. [Crossref] [PubMed]
- Yuan C, Miller ZE, Cai J, et al. Carotid atherosclerotic wall imaging by MRI. Neuroimaging Clin N Am 2002;12:391-401. vi. [Crossref] [PubMed]
- Chen XY, Wong KS, Lam WW, et al. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke 2014;9:E19. [Crossref] [PubMed]
- Yang WJ, Chen XY, Zhao HL, et al. Postmortem Study of Validation of Low Signal on Fat-Suppressed T1-Weighted Magnetic Resonance Imaging as Marker of Lipid Core in Middle Cerebral Artery Atherosclerosis. Stroke 2016;47:2299-304. [Crossref] [PubMed]
- Turan TN, Rumboldt Z, Granholm AC, et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 2014;237:460-3. [Crossref] [PubMed]
- Jiang Y, Zhu C, Peng W, et al. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis 2016;249:10-6. [Crossref] [PubMed]
- Lopez Gonzalez MR, Foo SY, Holmes WM, et al. Atherosclerotic Carotid Plaque Composition: A 3T and 7T MRI-Histology Correlation Study. J Neuroimaging 2016;26:406-13. [Crossref] [PubMed]
- Majidi S, Sein J, Watanabe M, et al. Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol 2013;34:2259-64. [Crossref] [PubMed]
- Bang OY, Heo JH, Kim JY, et al. Middle cerebral artery stenosis is a major clinical determinant in striatocapsular small, deep infarction. Arch Neurol 2002;59:259-63. [Crossref] [PubMed]
- Chung JW, Kim BJ, Sohn CH, et al. Branch atheromatous plaque: a major cause of lacunar infarction (high-resolution MRI study). Cerebrovasc Dis Extra 2012;2:36-44. [Crossref] [PubMed]
- Yoon Y, Lee DH, Kang DW, et al. Single subcortical infarction and atherosclerotic plaques in the middle cerebral artery: high-resolution magnetic resonance imaging findings. Stroke 2013;44:2462-7. [Crossref] [PubMed]
- Umansky F, Gomes FB, Dujovny M, et al. The perforating branches of the middle cerebral artery. A microanatomical study. J Neurosurg 1985;62:261-8. [Crossref] [PubMed]
- Aliabadi D, Tilli FV, Bowers TR, et al. Incidence and angiographic predictors of side branch occlusion following high-pressure intracoronary stenting. Am J Cardiol 1997;80:994-7. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke 2011;42:2957-9. [Crossref] [PubMed]
- Kim BJ, Lee KM, Kim HY, et al. Basilar Artery Plaque and Pontine Infarction Location and Vascular Geometry. J Stroke 2018;20:92-8. [Crossref] [PubMed]
- Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054-61. [Crossref] [PubMed]
- Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 2005;111:2768-75. [Crossref] [PubMed]
- McCarthy MJ, Loftus IM, Thompson MM, et al. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg 1999;30:261-8. [Crossref] [PubMed]
- Zhu C, Tian X, Degnan AJ, et al. Clinical Significance of Intraplaque Hemorrhage in Low- and High-Grade Basilar Artery Stenosis on High-Resolution MRI. AJNR Am J Neuroradiol 2018;39:1286-92. [Crossref] [PubMed]
- Wu F, Song H, Ma Q, et al. Hyperintense Plaque on Intracranial Vessel Wall Magnetic Resonance Imaging as a Predictor of Artery-to-Artery Embolic Infarction. Stroke 2018;49:905-11. [Crossref] [PubMed]
- Chen XY, Wong KS, Lam WW, et al. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis 2008;25:74-80. [Crossref] [PubMed]
- Millon A, Boussel L, Brevet M, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012;43:3023-8. [Crossref] [PubMed]
- Skarpathiotakis M, Mandell DM, Swartz RH, et al. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol 2013;34:299-304. [Crossref] [PubMed]
- Lou X, Ma N, Ma L, et al. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR Am J Neuroradiol 2013;34:513-7. [Crossref] [PubMed]
- Lyu J, Ma N, Tian C, et al. Perfusion and plaque evaluation to predict recurrent stroke in symptomatic middle cerebral artery stenosis. Stroke Vasc Neurol 2019;4:129-34. [Crossref] [PubMed]
- de Havenon A, Mossa-Basha M, Shah L, et al. High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology 2017;59:1193-202. [Crossref] [PubMed]
- Dieleman N, van der Kolk AG, Zwanenburg JJ, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation 2014;130:192-201. [Crossref] [PubMed]