
Cardiac CT perfusion and FFRCTA: pathophysiological features in ischemic heart disease
Introduction
Cardiac computed tomography (CCT) has undergone an exponential growth during the last two decades, from the first multi-detector CT scanners up to the latest machines characterized by significantly higher contrast, temporal and spatial resolution. CCT role in the cardiovascular field is unchallenged in specific settings, such as measuring coronary artery calcium score (CACS) in the intermediate-risk asymptomatic subjects (1), CT coronary angiography (CTCA) in symptomatic patients with low-to-intermediate pretest probability of coronary artery disease (CAD) (2-5) or in patients with acute chest pain and low [0 to 2] Thrombolysis in Myocardial Infarction (TIMI) risk score (6), and CTCA for pre-procedural planning of structural heart disease (7). In particular, in the setting of ischemic heart disease, CTCA has shown high sensitivity and very high negative predictive value (≥95%) for significant CAD (8,9). CTCA also carries an important prognostic value, being the only technique able to measure atheroma burden along the entire length of the coronary arteries (10-14) and assess its composition (15). The large multi-center SCOT-HEART (16) and PROMISE (17) trials have supported a role for CTCA in diagnosis of significant CAD in stable patients, due to a significantly lower rates of mortality from CAD and nonfatal myocardial infarction in the CTCA arm as compared to standard care (16) or functional testing (17). Furthermore, in the 5-year follow-up of the SCOT-HEART, CTCA, in addition to standard care in patients with stable chest pain, resulted in lower cardiovascular death without an increase in coronary angiography (CA) or revascularization (18). In consideration of this strong evidence, latest European guidelines for the diagnosis and management of chronic coronary syndromes recommend CTCA as first tool to rule out CAD in patients in whom ischemic disease cannot be excluded by clinical assessment alone (class I LoE B) (19).
Despite CTCA being useful to detect coronary lumen stenoses, its main limitation relies on the impossibility to assess their functional significance, especially in the intermediate range (40–80%), a consequence of its, at best, moderate positive predictive value (about 50%) (20). In contrast, other non-invasive techniques such as stress echocardiography, stress single-photon emission computed tomography (SPECT), stress cardiac magnetic resonance imaging (MRI) and positron emission tomography (PET) are better suited to evaluate myocardial perfusion and/or ischemia, but provide limited information regarding anatomy (21-23). Thus, none of these techniques is able to provide a comprehensive anatomical-functional assessment of a coronary stenosis-inducing plaque within the same study. Of note, current practice guidelines support the clinical benefit of ischemia-guided selective revascularization based on non-invasive and invasive functional evaluation, which reduces the risk of major acute cardiovascular events including myocardial infarction and cardiovascular death (19,24).
In the last decade, rapid technological improvements of CCT technology resulting in reduction of the scan time, motion artefacts and radiation dose exposure, while yielding higher spatial and temporal resolution (25-27), have widened CCT field of application, from anatomical detection of CAD to physiological assessment of myocardial ischemia. The first human report of stress myocardial CCT perfusion (CCTP) by Kurata et al. was published in 2005 with a 16-slice CT scanner and using adenosine triphosphate stress (28). Currently, feasibility of CCTP imaging with modern multi-detector row (≥64 slices) CT systems at rest and during pharmacologic stress has been demonstrated by clinical studies and recent multicenter trials (29-40). Moreover, by applying principle of computational fluid dynamics (CFD) algorithms, a non-invasive approximation of fractional flow reserve (FFR) from resting CTCA dataset (FFRCTA) may be derived to assess lesion-specific ischemia and guide revascularization (33).
The purpose of this narrative review is to describe the principles, clinical applications, and current state of the art of these new CCT technologies, comparing them with FFR and non-invasive techniques.
Myocardial ischemia
Pathophysiology of myocardial ischemia
The term myocardial ischemia refers to an imbalance between myocardial oxygen demand and supply. Myocardial oxygen consumption (MVO2) is determined by fixed (myocardial mass) and modifiable (heart rate, systemic blood pressure, wall stress in terms of pre- and post-load and inotropic status) parameters. Heart rate is the main MVO2 influencing factor, as an increase in contraction velocity needs an increase of oxygen delivery. Wall stress is influenced by pre-load, which is the diastolic blood volume linked to venous return, and post-load, which is the tension that myocardial muscle needs to develop for starting and maintaining systolic ejection. The latter can be approximated with systemic arterial pressure, which represents the second determinant of MVO2 in the order of importance. In clinical practice a good approximation of MVO2 can be obtained by calculating the so-called “double-product” (systolic pressure x heart rate). Inotropic status refers to the strength of myocardial contraction and has a more complex relation to MVO2 because of adaptive mechanisms and neurohormonal state; according to Frank-Starling law, within physiological limits, an increase in diastolic volume (pre-load) is associated with an increase in the force of contraction (41).
Myocardial oxygen delivery depends upon oxygen blood concentration and coronary blood flow. Oxygen carriage by the blood can be disrupted by a fall in haemoglobin (anemia) or by a reduced oxygen binding capacity. In basal conditions, O2 extraction by myocytes is very high (around 70%); thus, the only mechanism to increase O2 delivery is a proportional augmentation in blood supply. In normal haemodynamic conditions, coronary auto-regulation (the ability of coronary arteries to keep blood flow constant over a wide reduction in aortic pressure) allows to maintain normal regional perfusion if determinants of myocardial oxygen consumption do not change (42). This is due to vasodilation in response to intrinsic factors (local metabolites, endothelial factors and neural tone) or extrinsic stimulation (i.e., adenosine).
The ability to increase flow above resting values is termed coronary flow reserve (CFR). When pressure falls beyond the lower limit of auto-regulation, coronary resistance vessels are maximally vasodilated due to intrinsic stimuli, and flow becomes pressure-dependent, resulting in the onset of sub-endocardial ischemia (42,43).
Causes of myocardial ischemia
The main cause of myocardial ischemia is coronary atherosclerosis, which leads to artery wall thickening and atheroma accumulation in epicardial vessels with reduction in its lumen diameter. Due to coronary autoregulation, myocardial perfusion at rest is generally normal until the luminal diameter narrowing exceeds 85–90%. However, stenosis >50% in condition of increased O2 requirement, i.e., physical exertion, can induce a reduction in CFR, leading to tissue ischemia (23,44). This physiological phenomenon is the basis of the stress tests commonly used to diagnose myocardial ischemia. Other possible causes of ischemia are microvascular disease (which escape the resolution of angiographic techniques) and vasospastic angina; they are typically associated with either no stenoses or mild-to-moderate stenoses that are deemed functionally non-relevant (19).
Although ischemia is often indicative of a coronary artery stenosis, this cause-effect relationship is not perfect. In fact, some severe stenotic lesions do not produce significant ischemia, whereas other mild/moderate stenosis may cause both ischemia and cardiac events (19).
This poor correlation has been reassessed in a sub-study of the FAME (Fractional Flow Reserve Versus Angiography in Multivessel Evaluation) trial, which assessed 1,329 lesions by FFR in 509 patients with multivessel CAD. In stenoses categorized as 50–70% by visual angiographic assessment, 35% of the cases were functionally significant (FFR ≤0.80) and 65% were not (FFR >0.80). Among stenoses categorized as 71–90%, 80% were functionally significant (FFR ≤0.80) and 20% were not (FFR >0.80). In the category of subtotal stenoses (91–99%), only 4% of the cases were not significant (FFR was >0.8), and the remaining 96% were functionally significant stenoses (FFR ≤0.80) (45).
Ischemia detection
Non-invasive imaging is essential in the diagnosis and management of ischemic heart disease from its earliest manifestations of endothelial dysfunction to myocardial infarction (46). The preferred method to induce ischemia is physical exercise, albeit pharmacological stress agents can be used with similar (but by no means identical) accuracy. Drugs frequently employed are adenosine, dipyridamole, dobutamine or regadenoson. Adenosine provides a nonselective activation of four distinct subtypes (A1, A2A, A2B, and A3) receptors. Compared to dipyridamole, adenosine has a more rapid onset of action and a shorter half-life (around 30 s); therefore, most side effects resolve in a few seconds after discontinuation of the adenosine infusion. Side effects could be AV block, peripheral vasodilation, and bronchospasm, but the most common are flushing, chest pain, dyspnea, dizziness, or nausea.
Dipyridamole inhibits cellular uptake of adenosine, indirectly leading to coronary arteriolar vasodilation. Due to its longer half-life of approximately 30 minutes, patients given this drug may require administration of aminophylline for reversal of persistent symptoms (33,47).
The synthetic catecholamine dobutamine is primarily a β1-adrenergic receptors agonist, with mild effect on α1- and β2-receptors. At low doses (≤10 µg/kg/min), dobutamine improves myocardial contractility and induces coronary vasodilation; at higher doses (20–40 µg/kg/min), it causes systemic vasodilation and exerts a positive chronotrope effect (48). Dobutamine is used for stress echocardiography or stress MRI to detect myocardial ischemia by identifying regional wall motion abnormalities (RWMA), with accuracy and sensitivity similar to dipyridamole-stress imaging (48).
Finally, regadenoson, an A2A selective agonist has been recently introduced as a vasodilator. It has a safer side effect profile in comparison to adenosine and dipyridamole, especially for patients with asthma or severe chronic obstructive pulmonary disease. Its use is mainly limited by cost and it is also not widely available. Regadenoson has been shown to be accurate for the detection of obstructive CAD in nuclear perfusion imaging, stress echocardiography, and, more recently, stress CCTP studies (49,50).
Haemodynamic significance of coronary stenosis: evaluation in the catheterization laboratory
Numerous invasive physiological indices of ischemia have been developed in the last two decades, with the aim of overcoming the obvious limitations of CA (in effect, a bidimensional “luminogram”) in guiding percutaneous coronary intervention (PCI) (51).
The pathophysiological assumption is that, during maximal hyperaemia, when arteriolar regulation is blunted, the flow across a stenosis exclusively hinges on the pressure gradient. In this scenario, the pressure drop from the upstream to the downstream portion of the vessel linearly approximates blood flow reduction attributable to the stenosis, unveiling its ischemic potential (52).
FFR was hence defined as the ratio between the pressure measured distally from a given stenosis (Pd) and that achieved in the proximal part of the vessel (Pa) under maximal hyperaemia, with the latter obtained through a transient pharmacological stimulus (e.g., intravenous adenosine) (Figure 1). Of note, being a ratio of two pressures, FFR is a dimensionless number.

The accepted FFR value to detect haemodynamically relevant stenoses has been initially set to 0.75 (e.g., a 25% reduction in the maximum achievable flow induced by the stenosis), under which revascularization was recommended in the initial physiology-guided PCI trials (52). However, the so-called “grey zone” ranging from 0.75–0.80 has been subsequently included in the FFR values prompting revascularization (52), mainly due to the appreciation that FFR values distribution is effectively a spectrum, with no clear cut-off.
Randomized clinical trials (RCTs) supported the superiority of FFR compared to standard CA in guiding PCI of angiographically intermediate lesions of both stable patients (53) and non-infarct related arteries of acute coronary syndromes (54). The use of FFR was shown to modify the treatment strategy (medical therapy, PCI or coronary bypass graft) in one out of four patients (55). Yet, real-life data point out a linear relationship between FFR values and cardiovascular events (56). On the other hand, concerns have arisen on the reliability of FFR in certain clinical settings (e.g., aortic stenosis or chronic total occlusion) (57) and the safety and tolerability of vasodilators (58). Moreover, it is a rather expensive procedure (around 1,000 USD) (59). These factors have prompted the search for further non-hyperaemia dependent or “resting” indices of ischemia.
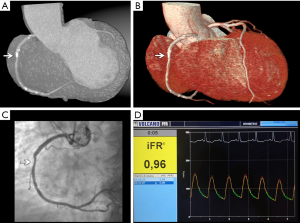
During the cardiac cycle, microvascular resistance sways, mirroring the alternation between systolic squeezing and diastolic relaxation and causing a succession of compression and suction waves, which propagate from the proximal (aortic) and distal (microcirculatory) parts of the vessel. Wave-intensity analysis allows the identification of a specific time-frame, occurring during diastole, in which no further waves are generated. In this “wave-free period” (WFP) microvascular resistance drops and becomes constant (as after maximal pharmacological vasodilation), and the distal to proximal pressure ratio across a stenosis provides an instantaneous wave-free ratio (iFR), potentially enabling the assessment of ischemia at rest and tightly correlating with FFR (60). In fact, according to two recent large-scale RCTs, iFR was non-inferior compared to FFR, showing moreover a reduction of patient’s discomfort and procedure length (61,62). Both these studies used an iFR cut-off at 0.89, which matches with an FFR ischemic cut-point of 0.80 (62) (Figure 2).
More recently, further attempts have been made in search of quicker, safer and equally performing invasive indices. For instance, unlike iFR, the resting full-cycle ratio (RFR) measures the lowest distal to proximal pressure ratio (Pd/Pa) during the entire coronary cycle (63), whereas the diastolic pressure ratio (dPR) is the average diastolic (not restricted to the WFP) Pd/Pa (64). Both of them showed a high agreement with iFR and FFR and were correlated with the risk of vessel-oriented outcomes (65).
Finally, advances in CFD have laid the foundation for angiography-based indices. In a nutshell, these are based on a three-dimensional reconstruction of the coronary tree and calculated by specific software using CFD algorithms. Among them, quantitative flow ratio (QFR) derives from single-vessel 3D-recontructions and utilizes TIMI frame count as a surrogate of blood flow (66), whereas coronary angiography-derived FFR (FFRangio) is based on a three-vessel reconstruction and subsequent estimation of resistance and flow through scaling-laws (67).
Cardiac CT perfusion: technical prerequisites and imaging protocols
The first attempts of myocardial perfusion analysis by CCT date back to the late 1970’s and early 1980’s, with experimental animal studies in infarct imaging which demonstrated the potential of the early generation head CT scanners to delineate differences in contrast media attenuation between normal and infarcted myocardium of various ages (68,69). During the last decade, important technical developments have rediscovered multi-slice CCT for cardiac perfusion imaging, transforming CCT from purely anatomical analysis of coronary stenosis and atherosclerotic plaques to functional myocardial perfusion analysis. Ongoing advances in scanner technology include higher temporal resolution (up to 66 ms with third generation dual-source CT scanner), higher spatial resolution (due to increase in detector sensitivity and efficiency), improved scan speed, wider detectors array (with a z-axis coverage as high as 16 cm with the 320-slice CT system), and improved image quality due to advanced iterative reconstruction algorithms (23). Currently, a 64 slices CT scanner is considered the minimum technology requirement that meets the basic needs for CCTP imaging (23).
CCTP is performed in a similar way to other imaging perfusion techniques, such as stress SPECT or MRI, by means of rest and stress imaging acquisitions during continuous monitoring of heart rate, blood pressure, and ECG. A comprehensive CCTP protocol typically involves: (I) a rest scan, which is used to evaluate both coronary stenosis and myocardial perfusion at rest, (II) a stress scan during administration of intravenous vasodilator drugs (e.g., adenosine, dipyridamole or regadenoson) to assess inducible myocardial perfusion defects, (III) an optional late-enhancement scan recommended in selected cases performed approximately 5–10 minutes after the last contrast injection, which is used to asses myocardial viability (34). A stress-first or rest-first protocols have been reported, with its own advantages and disadvantages (Table 1).

Full table
Two main techniques for pharmacological stress CCTP imaging have been developed: the static and the dynamic CCTP scan acquisitions.
Static cardiac CT perfusion imaging
The static CCTP acquisition, using single-energy or dual- and multi-energy techniques, relies on the acquisition of a single imaging dataset during the first-pass of contrast media thorough the myocardium providing a snapshot of myocardial enhancement (iodine distribution) at one stationary time point (23). Therefore, the timing of the scan acquisition needs to be carefully optimized in relation to the injection protocol, in order to acquire the images at the highest contrast-to-noise ratio difference between the normal and ischemic myocardium (23). Interpretation of static CCTP imaging is based on qualitative visual analysis of differences in contrast attenuation within the myocardium. Similar to nuclear imaging, “reversible” subendocardial and transmural perfusion defects are consistent with inducible myocardial ischemia, whereas “fixed” hypo-attenuated defects seen at both rest and stress phases suggest scar tissue, i.e., a myocardial infarct (34). The presence of other signs of myocardial necrosis/fibrosis (myocardial thinning; calcifications; lipomatous metaplasia; aneurysmal/pseudoaneurysmal dilation; mural thrombus; or wall motion abnormalities) on rest imaging further confirms prior infarct (34). Finally, automated software applications providing 3D modelling and 17-segment bull’s-eye plot of the left ventricular perfusion are available (23) (Figure 3).

Dual- or multi-energy CT consists in image acquisition at different energy levels with several technical approaches by various vendors, which include dual X-ray sources working at two different energy level (70–80–90/140–150 kVp), two sequential scans (not used for cardiac imaging), rapid switching (less than 1 ms) between the low- and high-energy of X-ray tube potential, and multilayer detector. Dual- or multi-energy CCTP enables differentiation by analyzing material-dependent photo-electric effect and Compton scattering processes (35,70). Dual- or multi-energy CCTP imaging may offer some advantages over single-energy CCTP by identification of iodine voxels within the myocardium, providing an estimate of contrast distribution across the myocardium at one point in time. Dual- or multi-energy CCTP allows accurate iodine quantification and distribution, which has a direct relation to myocardial blood flow (MBF), and thus provides a semi-quantitative marker of myocardial perfusion (35,71). Dual- or multi-energy datasets are post-processed using dedicated vendor-specific softwares which generate color-coded maps (iodine material density images) highlighting myocardial perfusion defects or late-enhancement of iodinated areas.
Dynamic cardiac CT perfusion imaging
Unlike static CCTP scan, dynamic CCTP imaging allows the acquisition of several imaging dataset at multiple time-points following contrast injections during a 20–30 seconds inspiratory breath-hold. Currently, there are two CT scanner techniques that enable dynamic CCTP, the prospective ECG-gated dynamic acquisition mode with a large coverage CT scanner along the z-axis (256- or 320-detector-row CT scanners) or, in the case of narrow detectors, the ECG-triggered axial shuttle mode technique implemented with the second- and third-generation dual-source CT scanners.
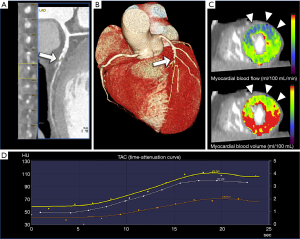
A high flow rate of injection of contrast media (at least 5 mL/s) is indicated to ensure a compact bolus and accurately defines arterial and myocardial peak enhancement for modelling. The multi-phasic dataset is used to generate time-attenuation curves (TACs) for each voxel of left ventricular myocardium and the reference arterial input function (AIF, derived from the left ventricle or the thoracic aorta). Using a dedicated parametric deconvolution based on 2-compartment model of intra- and extravascular space to fit the TACs, dynamic CCTP imaging enables absolute quantification of myocardial perfusion haemodynamic parameters such as MBF (mL/100 mL/min), MBF ratio, and myocardial blood volume (MBV, mL/100 mL) according to the formulas: MBF = MaxSlope(TissueTAC)/Maximum(AIF); MBV = Maximum(TissueTAC)/Maximum(AIF) (23,33). Furthermore, semi-quantitative parameters such as the peak enhancement, time to peak (TTP), up-slope, tissue transit time (TTT), and area under the curve (AUC) are derived (in a similar manner than for semi-quantitative dynamic MRI perfusion imaging). Finally, by comparing MBF during stress and rest phases, an assessment of absolute CFR may be obtained (72).
Similar to visual dynamic MRI, time-resolved CCTP acquisition may be evaluated for relative areas of hypo-perfusion over time (34). Further post-processing analysis of dynamic CCTP images are based on fully automatic and semiautomatic method with generation of color-coded polar maps and overlay images for each perfusion parameter (73,74) (Figure 4).
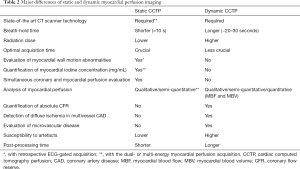
Major differences between the static and the dynamic CCTP imaging are reported in Table 2.
Full table
Accuracy of cardiac CT perfusion
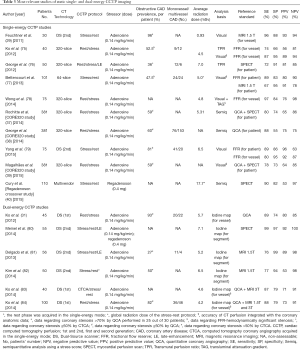
At present, various single-center experiences and first multi-center trials have evaluated the diagnostic performance of CCTP using invasive FFR, MRI, SPECT or even PET imaging as the reference standard (Tables 3,4). The advantage of CCTP imaging is an improvement in detecting the haemodynamic significance of CAD, adding incremental predictive value to CTCA-based coronary stenosis evaluation. The CORE320 trial, a prospective multicenter multinational diagnostic study including a total of 381 participants, demonstrated that combining stress CCTP imaging to anatomical evaluation of luminal stenosis substantially increased the overall diagnostic accuracy and specificity to identify flow-limiting stenosis, defined as ≥50% stenosis by CA and causing perfusion defect by SPECT (37,39). This improvement has been demonstrated in patients with and without known CAD, at both patient and vessel levels (37,39).
Full table
Full table
Furthermore, the overall performance of CCTP imaging in the diagnosis of anatomic CAD (stenosis ≥50% at CA as determined with quantitative methods) was higher than that of SPECT, and was driven in part by a higher sensitivity for left main and multivessel disease (38).
More recently, a two-center prospective sub-study of the CORE320 trial demonstrated that the diagnostic performance of CCTP with whole-heart coverage and single-beat acquisition was comparable to that of dynamic myocardial MRI perfusion imaging (98).
Similar to the CORE320 trial, the regadenoson cross-over study, a randomized, multivendor, multicenter study including 110 patients comparing the accuracy of CCTP for the detection of myocardial ischemia against SPECT, demonstrated that regadenoson CCTP was non-inferior to SPECT for detecting reversible perfusion defect with an agreement rate of 0.87. By adding regadenoson CCTP imaging to CTCA evaluation, diagnostic accuracy improved from 69% to 85% with high sensitivity and specificity of 90% and 84%, respectively, which was mainly explained by a reduction in false positive rate (40).
Moreover, stress CCTP has been shown to improve the diagnostic performance of CTCA even in patients with high pre-test probability of CAD (99), heavily calcified coronary arteries or prior coronary revascularization (100,101).
Of note, according to a recent pooled analysis on a per-patient basis, dual-energy and dynamic quantitative CCTP tends to have a slightly higher sensitivity than static CCTP imaging (36). This may be related to a higher detection of subtle perfusion defects. Finally, semi-quantitative parameters such as the transmural perfusion ratio (TPR), determined as the ratio of the subendocardial to the mean subepicardial contrast attenuation, and myocardial reserve index (difference in attenuation between the stress and rest phases) have been proposed for static myocardial CCTP. They, however, have demonstrated lower diagnostic accuracy than standard visual qualitative analysis (75,79).
Recent meta-analyses have shown an additional value of functional CCTP over anatomic CTCA alone for the assessment of haemodynamically significant CAD. Takx et al. demonstrated that, at the patient level, stress CCTP was similar to stress MRI and PET, and better than SPECT and echocardiography for the detection of myocardial ischemia defined by FFR (102). In another larger metanalysis including 5,330 patients, Celeng et al. demonstrated that both CCTP imaging and FFRCTA yielded higher diagnostic performance than CTCA in detecting haemodynamically significant CAD, with FFR as a reference (103). High to excellent sensitivity for both CCTP and FFRCTA and high specificity, especially for CCTP, have been demonstrated (103).
Dynamic CCTP has been initially validated in animal studies, showing a good correlation between quantitative dynamic-CT MBF values and invasive measurements of coronary blood flow, FFR, histopathology, and fluorescent microsphere (Table 4).
In recent clinical study, CT myocardial attenuation density was correlated with 15O-water PET MBF, the established clinical standard for MBF quantification (104). Several studies demonstrated improved diagnostic performance using indexed MBF normalized against remote myocardium, i.e., the ratio between the absolute MBF of ischemic coronary territories and segmental values of the remote myocardium (74,97,105-107).
Recently, a meta-analysis including a total of 482 patients demonstrated high diagnostic performance of dynamic CCTP to diagnose myocardial ischemia compared with clinically established reference standard (MRI, SPECT, PET perfusion and FFR). The pooled sensitivity and specificity of MBF were 83% and 90% at segment level and 93% and 83% (compared to 91% and 49% by CTCA) at patient level, respectively (108).
Prognostic value of cardiac CT perfusion
Initial evidence pointing to improved prognostic value and better risk stratification for CCTP has been recently reported. Combining CTCA and CCTP was shown to confidently predict all-cause mortality, myocardial infarction, or coronary revascularization over a 2-year period (109). Moreover, stress dynamic CCTP provides a predictive value that is incremental over clinical risk factors for major adverse cardiac events (MACE) and for the detection of coronary stenosis at CTCA (110-113). van Assen et al. (113) showed that quantification of index MBF (the ratio between territory and global MBF) was a better independent predictor of MACE, as compared to CTCA and FFRCTA.
Radiation burden of cardiac CT perfusion
Currently, it is possible to achieve a low-dose radiation exposure in clinical practice with the latest dose-reduction strategies of modern CT scanner, e.g., prospective ECG-triggered sequential scanning, wider anatomical coverage up to 320-slice, high pitch spiral acquisition, dose modulation, automated tube voltage selection, iterative image reconstruction software, and noise reduction filters. The effective radiation dose for a static single-phase CCTP scan generally is less than 5 mSv, which can be lower than the dose from CA (27,29,114) and approaches the average background radiation exposure in the United States of 3 mSv/y (27). Furthermore, an ultra-low dose protocol with an effective radiation dose as low as less than 1 mSv has been recently reported using prospectively ECG-triggered turbo high pitch spiral acquisition, which allows the acquisition of the entire heart within a single heartbeat in approximately one quarter of second (115).
A major concern is the higher radiation burden inherent to the dynamic CCTP protocols, which require a time-resolved acquisition of multiple phases. According to a recent meta-analysis, dynamic CCTP is associated with a median ionizing radiation of 9.45 mSv (range, 5.3–10.5 mSv) for a single scan, which is nonetheless comparable with that of traditional nuclear imaging techniques (108).
To overcome such limitations, the use of a low tube voltage protocol (80-kV/370-mA) instead of a conventional protocol (100-kV/300-mAs) enables 40% dose reduction without affecting image quality and MBF quantification (116). Moreover, other methods such as half-scan acquisition, new first-pass analysis using only two first-pass scans, tube current modulation, and statistical iterative reconstruction technique could substantially reduce effective radiation dose (23).
CTCA-derived FFR (FFRCTA)
Overview of FFRCTA technology
Due to the non-invasive nature of CTCA, the application of CFD algorithms on CTCA-derived 3D arterial models has received wide clinical interest. According to this approach, haemodynamic factors such as flow and pressure are not known a priori, thus parameter models regarding the cardiac output, the resistance of the coronary microcirculation and the pressure of the systemic circulation are coupled with the flow domain of the aortic root and the epicardial arteries, where the governing equations of flow dynamics are solved and can consequently provide FFRCTA calculations.
There are four approaches to non-invasive, in-silico CTCA-derived FFR estimation: (I) full-order modeling of haemodynamics; (II) reduced-order/steady-state modeling; (III) hybrid models; and (IV) deep machine learning algorithms, including both commercially available solutions and technologies still in development phase (117,118). All these techniques are based on a patient-specific anatomic coronary artery 3D model, obtained via a preliminary semiautomated process of segmentation and contouring. The full-order approaches require a complete model of the entire coronary tree, and an additional physiology model of the coronary microcirculation fluid dynamics (derived from patient-specific boundary conditions), from which a coronary blood flow model is derived. This process is computationally demanding, requiring off-site supercomputers in core laboratories. For this reason, simpler models have been introduced, that are either segment-specific and/or rely on a generalized (non-patient-specific) haemodynamic models. This allows near real time FFR estimation using workstations at the point of care, but lacks accuracy in small segments, near side branches and in eccentric lesions (119).
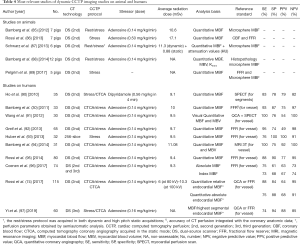
To date, the only commercially available FFR computation from CTCA solution known as FFRCT is available as a web-based service marketed by HeartFlow, Inc. (Redwood City, California, USA). According to the National Institute for Health and Care Excellence (NICE) guidance on the subject (120), HeartFlow provides an end-to-end service that includes anonymization at the source and human intervention (Figures 5,6).
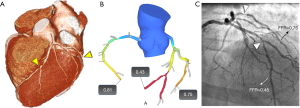
Some standalone computational methodology has been proposed for non-invasive calculation of FFR (119,121-124). SmartFFR is based on a transient blood flow simulation and the calculation of the pressure-flow curve between the distal and proximal region of the coronary artery. The novelty of SmartFFR lies in the fact that it can be effectively applied on arterial bifurcations and it can be calculated in just a few minutes, without the need of clinical tests besides a good quality CTCA. There is both a cloud-based platform and a standalone version (119,121,122) (Figures 6,7).
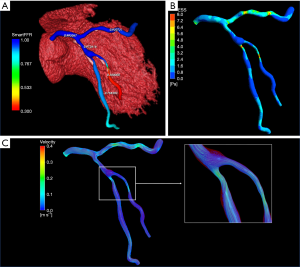
The specific value of systems that can be added to a normal server or laptop concerns direct management by a single operator during the evaluation process of the CT images. Another important advantage is rapid analysis, necessitating no more than a few minutes (121).
The main limitation for all existing 3D methodologies is that, if an error is generated, this can be propagated along the entire workflow of the reconstruction process, significantly altering the calculated blood flow simulation results (125).
Diagnostic accuracy of FFRCTA
Three major multi-center studies (DISCOVER-FLOW, DeFACTO and HeartFlow NXT) have provided clinical validation of FFRCTA, directly comparing their computational results to the measured invasive FFR values, and producing promising results that can be applied in clinical settings (126-128).
The DISCOVER-FLOW study exhibited a good correlation between FFRCTA and standard FFR (r=0.68) with respective diagnostic accuracy, sensitivity, specificity, positive predictive value and negative predictive value for predicting hemodynamically significant stenoses (FFR ≤0.8) being 84%, 88%, 82%, 74% and 92%, respectively (126).
Furthermore, when compared to cases of ≥50% stenosis detected solely by CTCA, FFRCTA showed superior discrimination (AUC: 0.90 vs. 0.75, P=0.001). In the DeFACTO study, stable CAD patients underwent CTCA, FFRCTA and CA with FFR measurement (127). The per patient diagnostic accuracy, sensitivity, specificity, positive predictive value and negative predictive value for predicting an FFR ≤0.8 were 73%, 90%, 54%, 67%, and 84%, respectively. Good correlation was also found between the two methods (r=0.68). The most recent HeartFlow NXT, further validated FFRCTA, using updated proprietary software which included refined mathematical models, further increasing automation, image quality assessment, and image segmentation (128). Diagnostic accuracy, sensitivity, specificity, positive predictive value and negative predictive value for predicting an FFR ≤0.8 were 81%, 86%, 79%, 65%, and 93%, respectively on a per-patient basis and 86%, 84%, 86%, 61%, and 95%, respectively, on a per-vessel basis. Finally, a good correlation was found between FFRCTA and FFR (r=0.82) (128). The PLATFORM study focused on the clinical outcomes of FFR by CTCA-guided diagnostic strategies compared to the standard care in CAD-suspected patients, providing insight on the clinical utilization of FFRCTA (129). Following the findings of the PLATFORM trial, the PROMISE study concluded that if CA is performed only in patients with FFRCTA ≤0.8, the rate of finding unobstructed coronaries at CA could decrease by 44% and, at the same time, the rate of CA leading to appropriate revascularization would increase by 24% (130).
Sensitivity and specificity of these techniques, however, have been shown to vary in different cohorts, due to differences in sample sizes and study population characteristics (Table 5).

Full table
In a recent meta-analysis including 908 vessels in 5 studies, FFRCTA showed an overall per-vessel diagnostic accuracy of 82% against invasive FFR (134). However, high variability of the diagnostic accuracy of FFRCTA has been observed across the entire spectrum of the disease. Regarding vessels with FFRCTA >0.90, the vast majority (97.9%) met the guideline FFR criterion for deferral (FFR >0.80), whereas for vessels with FFRCTA <0.60, 86.4% met the FFR criterion (FFR ≤0.8) for PCI. Regarding the FFRCTA values laying in between the thresholds, there was less certainty on whether invasive FFR would actually meet the criteria for stenosis deferral or revascularization (134).
Finally, FFRCTA exhibits an acceptable accuracy for the detection of haemodynamically relevant lesions without an additional radiation exposure (127), and being associated with equivalent clinical outcomes, similar quality-of-life and lower costs, when compared with usual care over 1-year follow-up (129).
Real-world role of cardiac CT perfusion and FFRCTA: limits and perspectives
Compared to other non-invasive functional imaging techniques, both static and dynamic CCTP offer the unique advantage to assess myocardial perfusion defects together with coronary anatomy in the same examination. Perfusion defects are matched with patient-specific coronary artery tree reconstruction, taking into account individual vessel anatomy, coronary artery distribution, presence, extent and type of coronary atherosclerotic plaques, and with specific identification of coronary stenoses. Therefore, the main strength of CT-based functional methods is a fully integrated anatomical/functional analysis at individual level. The knowledge of atherosclerotic plaque characteristics and plaque burden when assessing myocardial perfusion adds clinical value. In fact, it has been demonstrated that specific plaque features (e.g., positive remodeling, low attenuation, spotty calcification, as well as low-density noncalcified plaque volume), which are also associated with the risk of future coronary syndromes, reflect an intrinsic propensity to ischemia independent of stenosis severity when compared to FFR (15,135-137). Although not fully elucidated, the main mechanisms underlying the interactions between multiple plaque characteristics and lesion-specific ischemia include endothelial dysfunction, vascular inflammation, and altered shear stress patterns, which cause a shift in the balance of endothelial vasodilators and vasoconstrictors, thereby promoting ischemia during physiological stress (138).
Iodinated contrast materials used for CT express a pharmacodynamics similar to gadolinium-based extracellular media used for MRI. However, dynamic CCTP is not affected by the non-linear relationship between the measured myocardial signal intensity and myocardial contrast concentration, which may challenge accurate quantification of MBF in gadolinium MRI perfusion imaging (23). Other advantages of stress CCTP compared to MRI is the possibility to safely scan patients with active implanted medical devices, its wider availability, lower cost and quicker acquisition time. Furthermore, CCTP allows for a physiological noncorrupted 12-lead ECG monitoring during stress imaging, which is challenging in MRI.
CCTP offers several advantages in respect to nuclear imaging SPECT due to its higher temporal (up to 66 ms) and spatial resolution (≤0.3 mm), allowing detection of even small subendocardial area of ischemic or necrotic myocardium (23).
Despite these potential advantages, a significant drawback for both static and dynamic CCTP imaging is a low contrast-to-noise ratio (CNR) and the possibility of imaging artifacts linked to beam-hardening, partial volume effects, cardiac motion, and breathing. However, several dedicated reconstruction and motion correction algorithms are available (23,34).
Quantitative dynamic CCTP may offer some important advantages compared to static CCTP and SPECT in assessing balanced ischemia in the context of diffuse coronary atherosclerosis or multivessel obstructive CAD with global left ventricular impairment of MBF and CFR. Another clinical scenario worthy of quantitative CCTP is the evaluation of myocardial ischemia in patients with microvascular dysfunction and absence of obstructive CAD, where reduced hyperaemic MBF can be demonstrated, similar to PET imaging. Moreover, quantitative evaluation of MBF and MBV by dynamic CCTP may evaluate the functional importance of collateral circulation in coronary chronic total occlusions.
It should be noted, however, that differences in the optimal cut-off value of impaired MBF for detecting myocardial ischemia by dynamic CCTP have been reported, related to study design, scanner technology, acquisition protocol, post-processing elaboration, standard of reference applied, and, last but not least, individual coronary risk profile (23,33,36). Moreover, underestimation of absolute MBF and CFR values derived from dynamic CCTP imaging has been demonstrated, although in the documented range by PET studies (23,33,72). Sub-optimal temporal sampling frequencies of dynamic CCTP of the intracapillary first pass of contrast during hyperaemia and low extraction of iodine into the normal myocardium may partly explain these differences. Individual difference in vasodilator response and the known vasodilatory effect of iodinated contrast agents may be other possible explanations (72,74).
Of note, the more significant advantage of FFRCTA over static and dynamic CCTP imaging is that only one scan at rest is required for evaluation of both coronary anatomy and lesion-specific ischemia, with no additional contrast or medications, whereas a stepwise approach with combination of the rest and stress phases is obligatory for the CCTP imaging.
Major limitations of FFRCTA are the off-site and rather time-consuming core-laboratory analysis using a super-computer and the high computational costs of about £700 for a single test (120). Moreover, the large variation in study outcomes is also another point of concern. Finally, heavy calcifications, arrhythmia and tachycardia may affect both CTCA and FFRCTA image quality. In one study based on 116 patients with acute chest pain, 48 (41.4%) CTCA datasets were not included for FFRCTA analysis due to motion artifacts, severe calcium blooming artifacts or excessive image noise (139). In another clinical study conducted in real clinical setting, FFRCTA was measured in 43 out of 48 patients (89.6%) and the technical reasons were the image artifacts, transmission error and severe calcifications (140). The same reasons of unsuccessful FFRCTA calculation was found in another study (92% inclusion of patients—187 out of 204) (141). Thus, the main conclusion about FFRCTA feasibility is that it is strongly affected by image artifacts such as coronary motion or misalignment artifacts, transmission and registration errors, and finally blooming artifacts caused by severe calcifications. These factors may limit the applicability of non-invasive calculation of FFR in low-to-intermediate risk population than in high-risk patients with severe calcifications.
Conclusions
Both qualitative and quantitative CCTP imaging and FFRCTA have widened the clinical usefulness of CCT by improving its specificity and positive predictive value for evaluating the functional significance of coronary stenoses, with a concomitant reduction in false positives and unnecessary angiograms. Considerable variation in techniques and reference standards for CCTP to diagnose myocardial ischemia has emerged. Larger studies are necessary to standardize imaging protocols and interpretation, and to validate adequate databases of normal values of dynamic perfusion data, including separate metrics for men and women, similarly to nuclear imaging.
Finally, FFRCTA has gathered a very large validation dataset and could potentially alter clinical practice by providing a non-invasive approach to the functional assessment of coronary stenoses. FFRCTA might be used to screen patients with suspected obstructive CAD and to provide time and contrast-sparing information to the interventional cardiologist, allowing focused and simplified PCI in appropriate patients.
CCTP and FFRCTA will continue to receive attention from the cardiovascular research community in the near future, evolving from experimental imaging modalities to a core technology for clinical decision making of patients with suspected or known CAD.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Filippo Cademartiri) for the series “Clinical Impact of Cardiac CT in Clinical Practice” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-414). The series “Clinical Impact of Cardiac CT in Clinical Practice” was commissioned by the editorial office without any funding or sponsorship. AIS reports grants from FORTH during the conduct of the study. FC serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from Jul 2019 to Jun 2021. FC served as the unpaid Guest Editors of the series. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mantini C, Maffei E, Toia P, et al. Influence of image reconstruction parameters on cardiovascular risk reclassification by Computed Tomography Coronary Artery Calcium Score. Eur J Radiol 2018;101:1-7. [Crossref] [PubMed]
- Neglia D, Rovai D, Caselli C, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 2015;8:e002179. [Crossref] [PubMed]
- Cademartiri F, Romano M, Seitun S, et al. Prevalence and characteristics of coronary artery disease in a population with suspected ischemic heart disease using CT coronary angiography: correlations with cardiovascular risk factors and clinical presentation. Radiol Med 2008;113:363-72. [Crossref] [PubMed]
- Maffei E, Seitun S, Martini C, et al. CT coronary angiography and exercise ECG in a population with chest pain and low-to-intermediate pre-test likelihood of coronary artery disease. Heart 2010;96:1973-9. [Crossref] [PubMed]
- Cademartiri F, La Grutta L, Palumbo A, et al. Computed tomography coronary angiography vs. stress ECG in patients with stable angina. Radiol Med 2009;114:513-23. [Crossref] [PubMed]
- Maffei E, Seitun S, Guaricci AI, et al. Chest pain: coronary CT in the ER. Br J Radiol 2016;89:20150954. [Crossref] [PubMed]
- Blanke P, Weir-McCall JR, Achenbach S, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc Imaging 2019;12:1-24. [Crossref] [PubMed]
- Maffei E, Palumbo A, Martini C, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography in a large population of patients without revascularisation: registry data and review of multicentre trials. Radiol Med 2010;115:368-84. [Crossref] [PubMed]
- Yang L, Zhou T, Zhang R, et al. Meta-analysis: diagnostic accuracy of coronary CT angiography with prospective ECG gating based on step-and-shoot, Flash and volume modes for detection of coronary artery disease. Eur Radiol 2014;24:2345-52. [Crossref] [PubMed]
- Maffei E, Seitun S, Palumbo A, et al. Prognostic value of Morise clinical score, calcium score and computed tomography coronary angiography in patients with suspected or known coronary artery disease. Radiol Med 2011;116:1188-202. [Crossref] [PubMed]
- Cademartiri F, Seitun S, Romano M, et al. Prognostic value of 64-slice coronary angiography in diabetes mellitus patients with known or suspected coronary artery disease compared with a nondiabetic population. Radiol Med. 2008;113:627-43. [Crossref] [PubMed]
- Maffei E, Seitun S, Martini C, et al. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. Radiol Med 2011;116:690-705. [Crossref] [PubMed]
- Aldrovandi A, Maffei E, Palumbo A, et al. Prognostic value of computed tomography coronary angiography in patients with suspected coronary artery disease: a 24-month follow-up study. Eur Radiol 2009;19:1653-60. [Crossref] [PubMed]
- Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc imaging 2012;5:690-701. [Crossref] [PubMed]
- Motoyama S, Ito H, Sarai M, et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337-46. [Crossref] [PubMed]
- SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383-91. Erratum in: Lancet 2015;385:2354. [Crossref] [PubMed]
- Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291-300. [Crossref] [PubMed]
- Newby DE, Adamson PD, Berry C, et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med 2018;379:924-33. [Crossref] [PubMed]
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. [Crossref] [PubMed]
- Kim HL, Kim YJ, Lee SP, et al. Incremental prognostic value of sequential imaging of single-photon emission computed tomography and coronary computed tomography angiography in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging 2014;15:878-85. [Crossref] [PubMed]
- Gimelli A, Liga R, Duce V, et al. Accuracy of myocardial perfusion imaging in detecting multivessel coronary artery disease: A cardiac CZT study. J Nucl Cardiol 2017;24:687-95. [Crossref] [PubMed]
- Gimelli A, Liga R, Clemente A, et al. Appropriate choice of stress modality in patients undergoing myocardial perfusion scintigraphy with a cardiac camera equipped with solid-state detectors: the role of diabetes mellitus. Eur Heart J Cardiovasc Imaging 2018;19:1268-75. [Crossref] [PubMed]
- Seitun S, De Lorenzi C, Cademartiri F, et al. CT Myocardial Perfusion Imaging: A New Frontier in Cardiac Imaging. Biomed Res Int 2018;2018:7295460. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Maffei E, Martini C, Rossi A, et al. Diagnostic accuracy of second-generation dual-source computed tomography coronary angiography with iterative reconstructions: a real-world experience. Radiol Med 2012;117:725-38. [Crossref] [PubMed]
- Maffei E, Martini C, Tedeschi C, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography in a large population of patients without revascularisation: registry data on the comparison between male and female population. Radiol Med 2012;117:6-18. [Crossref] [PubMed]
- Maffei E, Martini C, De Crescenzo S, et al. Low dose CT of the heart: a quantum leap into a new era of cardiovascular imaging. Radiol Med 2010;115:1179-207. [Crossref] [PubMed]
- Kurata A, Mochizuki T, Koyama Y, et al. Myocardial perfusion imaging using adenosine triphosphate stress multi-slice spiral computed tomography: alternative to stress myocardial perfusion scintigraphy. Circ J 2005;69:550-7. [Crossref] [PubMed]
- Feuchtner G, Goetti R, Plass A, et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ Cardiovasc Imaging 2011;4:540-9. [Crossref] [PubMed]
- Bamberg F, Becker A, Schwarz F, et al. Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology 2011;260:689-98. [Crossref] [PubMed]
- Ko SM, Choi JW, Hwang HK, et al. Diagnostic performance of combined noninvasive anatomic and functional assessment with dual-source CT and adenosine-induced stress dual-energy CT for detection of significant coronary stenosis. AJR Am J Roentgenol 2012;198:512-20. [Crossref] [PubMed]
- Seitun S, Castiglione Morelli M, Budaj I, et al. Stress Computed Tomography Myocardial Perfusion Imaging: A New Topic in Cardiology. Rev Esp Cardiol (Engl Ed) 2016;69:188-200. [Crossref] [PubMed]
- Cademartiri F, Seitun S, Clemente A, et al. Myocardial blood flow quantification for evaluation of coronary artery disease by computed tomography. Cardiovasc Diagn Ther 2017;7:129-50. [Crossref] [PubMed]
- Mehra VC, Valdiviezo C, Arbab-Zadeh A, et al. A stepwise approach to the visual interpretation of CT-based myocardial perfusion. J Cardiovasc Comput Tomogr 2011;5:357-69. [Crossref] [PubMed]
- Danad I, Fayad ZA, Willemink MJ, et al. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. JACC Cardiovasc Imaging 2015;8:710-23. [Crossref] [PubMed]
- Danad I, Szymonifka J, Schulman-Marcus J, et al. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging 2016;17:836-44. [Crossref] [PubMed]
- Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 2014;35:1120-30. [Crossref] [PubMed]
- George RT, Mehra VC, Chen MY, et al. Myocardial CT Perfusion Imaging and SPECT for the Diagnosis of Coronary Artery Disease: A Head-to-Head Comparison from the CORE320 Multicenter Diagnostic Performance Study. Radiology 2014;272:407-16. Erratum in: Radiology 2015;274:626. [Crossref] [PubMed]
- Magalhães TA, Kishi S, George RT, et al. Combined coronary angiography and myocardial perfusion by computed tomography in the identification of flow-limiting stenosis - The CORE320 study: An integrated analysis of CT coronary angiography and myocardial perfusion. J Cardiovasc Comput Tomogr 2015;9:438-45. [Crossref] [PubMed]
- Cury RC, Kitt TM, Feaheny K, et al. A randomized, multicenter, multivendor study of myocardial perfusion imaging with regadenoson CT perfusion vs single photon emission CT. J Cardiovasc Comput Tomogr 2015;9:103-12.e1-2.
- Fukuda N, Terui T, Ohtsuki I, et al. Titin and troponin: central players in the frank-starling mechanism of the heart. Curr Cardiol Rev 2009;5:119-24. [Crossref] [PubMed]
- Berne RM. Regulation of coronary blood flow. Physiol Rev 1964;44:1-29. [Crossref] [PubMed]
- Krasnow N, Gorlin R. Myocardial lactate metabolism in coronary insufficiency. Ann Intern Med 1963;59:781-7. [Crossref] [PubMed]
- Uren NG, Melin JA, De Bruyne B, et al. Relation between Myocardial Blood Flow and the Severity of Coronary-Artery Stenosis. N Engl J Med 1994;330:1782-8. [Crossref] [PubMed]
- Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816-21. [Crossref] [PubMed]
- Stillman AE, Oudkerk M, Bluemke DA, et al. Imaging the myocardial ischemic cascade. Int J Cardiovasc Imaging 2018;34:1249-63. [Crossref] [PubMed]
- Picano E, Lattanzi F, Masini M, et al. Usefulness of the dipyridamole-exercise echocardiography test for diagnosis of coronary artery disease. Am J Cardiol 1988;62:67-70. [Crossref] [PubMed]
- Charoenpanichkit C, Hundley WG. The 20 year evolution of dobutamine stress cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:59. [Crossref] [PubMed]
- Cury RC, Kitt TM, Feaheny K, et al. Regadenoson-stress myocardial CT perfusion and single-photon emission CT: rationale, design, and acquisition methods of a prospective, multicenter, multivendor comparison. J Cardiovasc Comput Tomogr 2014;8:2-12. [Crossref] [PubMed]
- Reyes E. Regadenoson stress for myocardial perfusion imaging. Future Cardiol 2016;12:59-67. [Crossref] [PubMed]
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology: The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995;92:2333-42. [Crossref] [PubMed]
- van de Hoef TP, Meuwissen M, Escaned J, et al. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat Rev Cardiol 2013;10:439-52. [Crossref] [PubMed]
- De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208-17. [Crossref] [PubMed]
- Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med 2017;377:397-8. [Crossref] [PubMed]
- Curzen N, Rana O, Nicholas Z, et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? the ripcord study. Circ Cardiovasc Interv 2014;7:248-55. [Crossref] [PubMed]
- Ahn JM, Park DW, Shin ES, et al. Fractional Flow Reserve and Cardiac Events in Coronary Artery Disease: Data from a Prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation 2017;135:2241-51. [Crossref] [PubMed]
- Benenati S, De Maria GL, Scarsini R, et al. Invasive “in the cath-lab” assessment of myocardial ischemia in patients with coronary artery disease: When does the gold standard not apply? Cardiovasc Revasc Med 2018;19:362-72. [Crossref] [PubMed]
- Pijls NHJ, Tonino PAL. The crux of maximum hyperemia: The last remaining barrier for routine use of fractional flow reserve. JACC Cardiovasc Interv 2011;4:1093-5. [Crossref] [PubMed]
- Fearon WF, Bornschein B, Tonino PA, et al. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 2010;122:2545-50. [Crossref] [PubMed]
- Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol 2012;59:1392-402. [Crossref] [PubMed]
- Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med 2017;376:1813-23. [Crossref] [PubMed]
- Davies JE, Sen S, Dehbi HM, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med 2017;376:1824-34. [Crossref] [PubMed]
- Svanerud J, Ahn JM, Jeremias A, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: The Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention 2018;14:806-14. [Crossref] [PubMed]
- Van't Veer M, Pijls NHJ, Hennigan B, et al. Comparison of Different Diastolic Resting Indexes to iFR: Are They All Equal? J Am Coll Cardiol 2017;70:3088-96. [Crossref] [PubMed]
- Lee JM, Choi KH, Park J, et al. Physiological and Clinical Assessment of Resting Physiological Indexes. Circulation 2019;139:889-900. Erratum in: Circulation 2019;139:e41. [Crossref] [PubMed]
- Xu B, Tu S, Qiao S, et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J Am Coll Cardiol 2017;70:3077-87. [Crossref] [PubMed]
- Pellicano M, Lavi I, De Bruyne B, et al. Validation Study of Image-Based Fractional Flow Reserve During Coronary Angiography. Circ Cardiovasc Interv 2017;10:e005259. [Crossref] [PubMed]
- Adams DF, Hessel SJ, Judy PF, et al. Computed tomography of the normal and infarcted myocardium. AJR Am J Roentgenol 1976;126:786-91. [Crossref] [PubMed]
- Higgins CB, Siemers PT, Newell JD, et al. Role of iodinated contrast material in the evaluation of myocardial infarction by computerized transmission tomography. Invest Radiol 1980;15:S176-82. [Crossref] [PubMed]
- McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015;276:637-53. [Crossref] [PubMed]
- Pelgrim GJ, van Hamersvelt RW, Willemink MJ, et al. Accuracy of iodine quantification using dual energy CT in latest generation dual source and dual layer CT. Eur Radiol 2017;27:3904-12. [Crossref] [PubMed]
- Marini C, Seitun S, Zawaideh C, et al. Comparison of coronary flow reserve estimated by dynamic radionuclide SPECT and multi-detector x-ray CT. J Nucl Cardiol 2017;24:1712-21. [Crossref] [PubMed]
- Ebersberger U, Marcus RP, Schoepf UJ, et al. Dynamic CT myocardial perfusion imaging: performance of 3D semi-automated evaluation software. Eur Radiol 2014;24:191-9. [Crossref] [PubMed]
- Rossi A, Wragg A, Klotz E, et al. Dynamic Computed Tomography Myocardial Perfusion Imaging: Comparison of Clinical Analysis Methods for the Detection of Vessel-Specific Ischemia. Circ Cardiovasc Imaging 2017;10:e005505. [Crossref] [PubMed]
- Ko BS, Cameron JD, Leung M, et al. Combined CT coronary angiography and stress myocardial perfusion imaging for hemodynamically significant stenoses in patients with suspected coronary artery disease: a comparison with fractional flow reserve. JACC Cardiovasc Imaging 2012;5:1097-111. [Crossref] [PubMed]
- George RT, Arbab-Zadeh A, Miller JM, et al. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ Cardiovasc Imaging 2012;5:333-40. [Crossref] [PubMed]
- Bettencourt N, Ferreira ND, Leite D, et al. CAD detection in patients with intermediate-high pre-test probability: low-dose CT delayed enhancement detects ischemic myocardial scar with moderate accuracy but does not improve performance of a stress-rest CT perfusion protocol. JACC Cardiovasc Imaging 2013;6:1062-71. [Crossref] [PubMed]
- Wong DT, Ko BS, Cameron JD, et al. Comparison of diagnostic accuracy of combined assessment using adenosine stress computed tomography perfusion + computed tomography angiography with transluminal attenuation gradient + computed tomography angiography against invasive fractional flow reserve. J Am Coll Cardiol 2014;63:1904-12. [Crossref] [PubMed]
- Yang DH, Kim YH, Roh JH, et al. Stress Myocardial Perfusion CT in Patients Suspected of Having Coronary Artery Disease: Visual and Quantitative Analysis-Validation by Using Fractional Flow Reserve. Radiology 2015;276:715-23. [Crossref] [PubMed]
- Meinel FG, De Cecco CN, Schoepf UJ, et al. First-arterial-pass dual-energy CT for assessment of myocardial blood supply: do we need rest, stress, and delayed acquisition? Comparison with SPECT. Radiology 2014;270:708-16. [Crossref] [PubMed]
- Delgado C, Vázquez M, Oca R, et al. Myocardial ischemia evaluation with dual-source computed tomography: comparison with magnetic resonance imaging. Rev Esp Cardiol (Engl Ed) 2013;66:864-70. [Crossref] [PubMed]
- Kim SM, Chang SA, Shin W, et al. Dual-energy CT perfusion during pharmacologic stress for the assessment of myocardial perfusion defects using a second-generation dual-source CT: a comparison with cardiac magnetic resonance imaging. J Comput Assist Tomogr 2014;38:44-52. [Crossref] [PubMed]
- Ko SM, Park JH, Hwang HK, et al. Direct comparison of stress- and rest-dual-energy computed tomography for detection of myocardial perfusion defect. Int J Cardiovasc Imaging 2014;30 Suppl 1:41-53. [Crossref] [PubMed]
- Ko SM, Song MG, Chee HK, et al. Diagnostic performance of dual-energy CT stress myocardial perfusion imaging: direct comparison with cardiovascular MRI. AJR Am J Roentgenol 2014;203:W605-13. [Crossref] [PubMed]
- Bamberg F, Hinkel R, Schwarz F, et al. Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Invest Radiol 2012;47:71-7. [Crossref] [PubMed]
- Rossi A, Uitterdijk A, Dijkshoorn M, et al. Quantification of myocardial blood flow by adenosine-stress CT perfusion imaging in pigs during various degrees of stenosis correlates well with coronary artery blood flow and fractional flow reserve. Eur Heart J Cardiovasc Imaging 2013;14:331-8. [Crossref] [PubMed]
- Schwarz F, Hinkel R, Baloch E, et al. Myocardial CT perfusion imaging in a large animal model: comparison of dynamic versus single-phase acquisitions. JACC Cardiovasc Imaging 2013;6:1229-38. [Crossref] [PubMed]
- Bamberg F, Hinkel R, Marcus RP, et al. Feasibility of dynamic CT-based adenosine stress myocardial perfusion imaging to detect and differentiate ischemic and infarcted myocardium in an large experimental porcine animal model. Int J Cardiovasc Imaging 2014;30:803-12. [Crossref] [PubMed]
- Pelgrim GJ, Das M, van Tuijl S, et al. Validation of myocardial perfusion quantification by dynamic CT in an ex-vivo porcine heart model. Int J Cardiovasc Imaging 2017;33:1821-30. [Crossref] [PubMed]
- Ho KT, Chua KC, Klotz E, et al. Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source CT. JACC Cardiovasc Imaging 2010;3:811-20. [Crossref] [PubMed]
- Wang Y, Qin L, Shi X, et al. Adenosine-stress dynamic myocardial perfusion imaging with second-generation dual-source CT: comparison with conventional catheter coronary angiography and SPECT nuclear myocardial perfusion imaging. AJR Am J Roentgenol 2012;198:521-9. [Crossref] [PubMed]
- Greif M, von Ziegler F, Bamberg F, et al. CT stress perfusion imaging for detection of haemodynamically relevant coronary stenosis as defined by FFR. Heart 2013;99:1004-11. [Crossref] [PubMed]
- Huber AM, Leber V, Gramer BM, et al. Myocardium: dynamic versus single-shot CT perfusion imaging. Radiology 2013;269:378-86. [Crossref] [PubMed]
- Bamberg F, Marcus RP, Becker A, et al. Dynamic myocardial CT perfusion imaging for evaluation of myocardial ischemia as determined by MR imaging. JACC Cardiovasc Imaging 2014;7:267-77. [Crossref] [PubMed]
- Rossi A, Dharampal A, Wragg A, et al. Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: does it predict functionally significant coronary lesions? Eur Heart J Cardiovasc Imaging 2014;15:85-94. [Crossref] [PubMed]
- Coenen A, Rossi A, Lubbers MM, et al. Integrating CT Myocardial Perfusion and CT-FFR in the Work-Up of Coronary Artery Disease. JACC Cardiovasc Imaging 2017;10:760-70. [Crossref] [PubMed]
- Yi Y, Xu C, Wu W, et al. Myocardial blood flow analysis of stress dynamic myocardial CT perfusion for hemodynamically significant coronary artery disease diagnosis: The clinical value of relative parameter optimization. J Cardiovasc Comput Tomogr 2020;14:314-21. [Crossref] [PubMed]
- Rief M, Chen MY, Vavere AL, et al. Coronary Artery Disease: Analysis of Diagnostic Performance of CT Perfusion and MR Perfusion Imaging in Comparison with Quantitative Coronary Angiography and SPECT-Multicenter Prospective Trial. Radiology 2018;286:461-70. [Crossref] [PubMed]
- Sharma RK, Arbab-Zadeh A, Kishi S, et al. Incremental diagnostic accuracy of computed tomography myocardial perfusion imaging over coronary angiography stratified by pre-test probability of coronary artery disease and severity of coronary artery calcification: The CORE320 study. Int J Cardiol 2015;201:570-7. [Crossref] [PubMed]
- Bischoff B, Deseive S, Rampp M, et al. Myocardial ischemia detection with single-phase CT perfusion in symptomatic patients using high-pitch helical image acquisition technique. Int J Cardiovasc Imaging 2017;33:569-76. [Crossref] [PubMed]
- Rief M, Zimmermann E, Stenzel F, et al. Computed tomography angiography and myocardial computed tomography perfusion in patients with coronary stents: prospective intraindividual comparison with conventional coronary angiography. J Am Coll Cardiol 2013;62:1476-85. [Crossref] [PubMed]
- Takx RA, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8:e002666. [Crossref] [PubMed]
- Celeng C, Leiner T, Maurovich-Horvat P, et al. Anatomical and Functional Computed Tomography for Diagnosing Hemodynamically Significant Coronary Artery Disease: A Meta-Analysis. JACC Cardiovasc Imaging 2019;12:1316-25. [Crossref] [PubMed]
- Williams MC, Mirsadraee S, Dweck MR, et al. Computed tomography myocardial perfusion vs (15)O-water positron emission tomography and fractional flow reserve. Eur Radiol 2017;27:1114-24. [Crossref] [PubMed]
- Wichmann JL, Meinel FG, Schoepf UJ, et al. Absolute Versus Relative Myocardial Blood Flow by Dynamic CT Myocardial Perfusion Imaging in Patients With Anatomic Coronary Artery Disease. AJR Am J Roentgenol 2015;205:W67-72. [Crossref] [PubMed]
- Kono AK, Coenen A, Lubbers M, et al. Relative myocardial blood flow by dynamic computed tomographic perfusion imaging predicts hemodynamic significance of coronary stenosis better than absolute blood flow. Invest Radiol 2014;49:801-7. [Crossref] [PubMed]
- Coenen A, Lubbers MM, Kurata A, et al. Diagnostic value of transmural perfusion ratio derived from dynamic CT-based myocardial perfusion imaging for the detection of haemodynamically relevant coronary artery stenosis. Eur Radiol 2017;27:2309-16. [Crossref] [PubMed]
- Lu M, Wang S, Sirajuddin A, et al. Dynamic stress computed tomography myocardial perfusion for detecting myocardial ischemia: A systematic review and meta-analysis. Int J Cardiol 2018;258:325-31. [Crossref] [PubMed]
- Chen MY, Rochitte CE, Arbab-Zadeh A, et al. Prognostic value of combined CT angiography and myocardial perfusion imaging versus invasive coronary angiography and nuclear stress perfusion imaging in the prediction of major adverse cardiovascular events: the CORE320 multicenter study. Radiology 2017;284:55-65. [Crossref] [PubMed]
- Meinel FG, Pugliese F, Schoepf UJ, et al. Prognostic Value of Stress Dynamic Myocardial Perfusion CT in a Multicenter Population With Known or Suspected Coronary Artery Disease. AJR Am J Roentgenol 2017;208:761-9. [Crossref] [PubMed]
- Meinel FG, Wichmann JL, Schoepf UJ, et al. Global quantification of left ventricular myocardial perfusion at dynamic CT imaging: Prognostic value. J Cardiovasc Comput Tomogr 2017;11:16-24. [Crossref] [PubMed]
- Nakamura S, Kitagawa K, Goto Y, et al. Incremental Prognostic Value of Myocardial Blood Flow Quantified With Stress Dynamic Computed Tomography Perfusion Imaging. JACC Cardiovasc Imaging 2019;12:1379-87. [Crossref] [PubMed]
- van Assen M, De Cecco CN, Eid M, et al. Prognostic value of CT myocardial perfusion imaging and CT-derived fractional flow reserve for major adverse cardiac events in patients with coronary artery disease. J Cardiovasc Comput Tomogr 2019;13:26-33. [Crossref] [PubMed]
- Song YB, Arbab-Zadeh A, Matheson MB, et al. Contemporary Discrepancies of Stenosis Assessment by Computed Tomography and Invasive Coronary Angiography. Circ Cardiovasc Imaging 2019;12:e007720. [Crossref] [PubMed]
- Jia CF, Zhong J, Meng XY, et al. Image quality and diagnostic value of ultra low-voltage, ultra low-contrast coronary CT angiography. Eur Radiol 2019;29:3678-85. [Crossref] [PubMed]
- Fujita M, Kitagawa K, Ito T, et al. Dose reduction in dynamic CT stress myocardial perfusion imaging: comparison of 80-kV/370-mAs and 100-kV/300-mAs protocols. Eur Radiol 2014;24:748-55. [Crossref] [PubMed]
- Tesche C, De Cecco CN, Albrecht MH, et al. Coronary CT Angiography-derived Fractional Flow Reserve. Radiology 2017;285:17-33. [Crossref] [PubMed]
- Mastrodicasa D, Albrecht MH, Schoepf UJ, et al. Artificial intelligence machine learning-based coronary CT fractional flow reserve (CT-FFR(ML)): Impact of iterative and filtered back projection reconstruction techniques. J Cardiovasc Comput Tomogr 2019;13:331-5. [Crossref] [PubMed]
- Siogkas PK, Anagnostopoulos CD, Liga R, et al. Noninvasive CT-based hemodynamic assessment of coronary lesions derived from fast computational analysis: a comparison against fractional flow reserve. Eur Radiol 2019;29:2117-26. [Crossref] [PubMed]
- National Institute for Health and Care Excellence (NICE) HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography. Medical Technologies Guidance 32. February, 2017. Available online: https://www.nice.org.uk/guidance/mtg32/resources
- Sakellarios AI, Rigas G, Kigka V, et al. SMARTool: A tool for clinical decision support for the management of patients with coronary artery disease based on modeling of atherosclerotic plaque process. Annu Int Conf IEEE Eng Med Biol Soc 2017;2017:96-9. [Crossref] [PubMed]
- Sakellarios A, Correia J, Kyriakidis S, et al. A cloud-based platform for the non-invasive management of coronary artery disease. Enterprise Information Systems 2020. [Epub ahead of print]. [Crossref]
- Kruk M, Wardziak Ł, Demkow M, et al. Workstation-Based Calculation of CTA-Based FFR for Intermediate Stenosis. JACC Cardiovasc Imaging 2016;9:690-9. [Crossref] [PubMed]
- Ko BS, Cameron JD, Munnur RK, et al. Noninvasive CT-Derived FFR Based on Structural and Fluid Analysis: A Comparison With Invasive FFR for Detection of Functionally Significant Stenosis. JACC Cardiovasc Imaging 2017;10:663-73. [Crossref] [PubMed]
- Siogkas PK, Sakellarios AI, Kyriakidis SK, et al. The effect of error propagation in the 3D reconstruction of coronary segments using CTCA images on crucial hemodynamic parameters. Annu Int Conf IEEE Eng Med Biol Soc 2019;2019:5006-9. [Crossref] [PubMed]
- Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve)study. J Am Coll Cardiol 2011;58:1989-97. [Crossref] [PubMed]
- Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237-45. [Crossref] [PubMed]
- Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145-55. [Crossref] [PubMed]
- Douglas PS, De Bruyne B, Pontone G, et al. 1-Year Outcomes of FFRCT-Guided Care in Patients With Suspected Coronary Disease: The PLATFORM Study. J Am Coll Cardiol 2016;68:435-45. [Crossref] [PubMed]
- Lu MT, Ferencik M, Roberts RS, et al. Noninvasive FFR Derived From Coronary CT Angiography: Management and Outcomes in the PROMISE Trial. JACC Cardiovasc Imaging 2017;10:1350-8. [Crossref] [PubMed]
- Kim KH, Doh JH, Koo BK, et al. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv 2014;7:72-8. [Crossref] [PubMed]
- Renker M, Schoepf UJ, Wang R, et al. Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol 2014;114:1303-8. [Crossref] [PubMed]
- Coenen A, Lubbers MM, Kurata A, et al. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology 2015;274:674-83. [Crossref] [PubMed]
- Cook CM, Petraco R, Shun-Shin MJ, et al. Diagnostic Accuracy of Computed Tomography-Derived Fractional Flow Reserve: A Systematic Review. JAMA Cardiol 2017;2:803-10. Erratum in: JAMA Cardiol 2017;2:1284. [Crossref] [PubMed]
- Rosa GM, Bauckneht M, Masoero G, et al. The vulnerable coronary plaque: update on imaging technologies. Thromb Haemost 2013;110:706-22. [Crossref] [PubMed]
- Driessen RS, Stuijfzand WJ, Raijmakers PG, et al. Effect of Plaque Burden and Morphology on Myocardial Blood Flow and Fractional Flow Reserve. J Am Coll Cardiol 2018;71:499-509. [Crossref] [PubMed]
- Gaur S, Øvrehus KA, Dey D, et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J 2016;37:1220-7. [Crossref] [PubMed]
- Ahmadi A, Kini A, Narula J. Discordance between ischemia and stenosis, or PINSS and NIPSS: are we ready for new vocabulary? JACC Cardiovasc Imaging 2015;8:111-4. [Crossref] [PubMed]
- Ferencik M, Lu MT, Mayrhofer T, et al. Non-invasive fractional flow reserve derived from coronary computed tomography angiography in patients with acute chest pain: Subgroup analysis of the ROMICAT II trial. J Cardiovasc Comput Tomogr 2019;13:196-202. [Crossref] [PubMed]
- Kawaji T, Shiomi H, Morishita H, et al. Feasibility and diagnostic performance of fractional flow reserve measurement derived from coronary computed tomography angiography in real clinical practice. Int J Cardiovasc Imaging 2017;33:271-81. [Crossref] [PubMed]
- Rabbat M, Leipsic J, Bax J, et al. Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease. J Clin Med 2020;9:604. [Crossref] [PubMed]



