
MR imaging of vulnerable carotid plaque
Introduction
Stroke remains a leading cause of morbidity and mortality, with current risk stratification still based upon percentage of carotid stenosis. Randomized clinical trials (RCT) have demonstrated that carotid endarterectomies (CEA) reduce the risk of future stroke in recently symptomatic patients with ≥70% ipsilateral carotid stenosis (1,2) and to a lesser extent in asymptomatic patients (3,4). However, simple carotid stenosis measurement provides minimal patient-specific information on the actual risk of stroke for symptomatic individuals with less significant carotid artery disease and asymptomatic carotid stenosis patients. In addition, the risk of stroke has decreased since these landmark RCTs due to improvement in medical therapy, and in some carotid stenosis patients CEA may no longer offer additional benefit (5). MR imaging of unstable carotid plaque after guideline-based medical therapy may therefore provide this needed additional risk stratification to better select an appropriate treatment for the individual patient. Within the last decade, a growing body of literature suggests that the presence of certain carotid plaque characteristics provide a superior means of predicting future stroke when compared with percentage of carotid stenosis. These carotid plaque characteristics, including lipid-rich necrotic core (LRNC), thin/ruptured fibrous cap (FC), and intraplaque hemorrhage (IPH), are detectable via magnetic resonance imaging (MRI), with most features detectable using commercially available coils and sequences utilized in routine clinical practice in as little as 4 minutes. An even more detailed, comprehensive evaluation of carotid plaque using dedicated carotid surface coils and specialized MR sequences is also available, requiring approximately 40 minutes of scanner table time.
Definition of vulnerable plaque
Histological studies have demonstrated that coronary artery plaques with large LRNC and a thin overlying FC are associated with sudden cardiac death, leading to the concept of “vulnerable plaque (6).” The key features of the vulnerable plaque were defined in two multidisciplinary consensus review articles (7,8). The authors stressed that rupture-prone plaques are not the only vulnerable plaques and that all types of plaque with a high likelihood of thrombotic complications and rapid progression should be considered as vulnerable. The original concept of the coronary vulnerable plaque was then extended to the carotid arteries (9). Many of these features, including carotid plaque volume, maximal wall thickness, large LRNC with a thin or ruptured FC, and IPH were found to be detectable and quantifiable via MRI, each with specific imaging characteristics (10).
MR findings of vulnerable plaque
Morphology
Plaque thickness/volume
Carotid plaque is best found at or near the carotid bifurcation and may or not contribute to a significant level of stenosis. Multicontrast MRI of the carotid arteries using dedicated carotid coils and research MR sequences has been extensively tested, with initial repeatability studies of plaque morphology from a multi-institution study showing that plaque measurement error based on multiple locations [e.g., total plaque volume (TPV)] was, in general, lower than a single location (e.g., maximal wall thickness), making TPV an attractive measurand for future prospective imaging-guided therapeutic drug trials (11).
Ulcerations/surface irregularity
In the North American Symptomatic Carotid Endarterectomy Trial (NASCET), a plaque was defined as ulcerated either if an ulcer niche was seen in profile as a crater from the lumen into a stenotic plaque or if seen en face as a double density (12). The plaque was considered irregular if there were multiple possible small craters. In a separate study of 128 symptomatic severely stenotic patients, a very strong association was seen between detailed histology from CEA specimens and preoperative intra-arterial angiography. The angiographic ulcerations were associated with IPH and large LRNC (13). These intra-arterial angiographic ulceration definitions have been extended to MR, using both the luminal evaluation on bright-blood MR angiograms as well as direct visualization of the > 1 mm ulceration niche extending into the carotid plaque on cross-sectional black-blood MR series (14,15).
Composition
LRNC
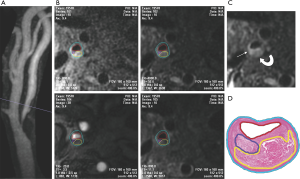
LRNC is a heterogeneous tissue composed of cholesterol crystal, apoptotic cellular debris, and particles of calcium (14). As described previously, a large LRNC occupying more than 40% of the plaque with a thin overlying FC infiltrated by macrophages was prone to rupture (7). Multicontrast carotid artery MRI using black-blood T2 weighted (T2W), T1 weighted (T1W), and bright-blood time-of-flight (TOF) MR angiography (MRA) has been histologically validated to accurately identify and quantify LRNC (9,16), with contrast-enhanced (CE) T1W images improving the differentiation between nonenhancing LRNC and the surrounding fibrous plaque tissue (17) (Figure 1). LRNC is hypointense on T2W images and will not enhance on CE-T1W images. It may or may not be associated with IPH.
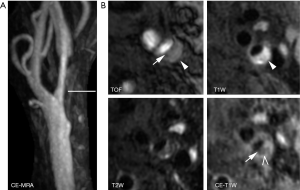
FC is most accurately characterized with 3D TOF MRA and CE-T1W images. Utilizing dedicated carotid coils, research MR sequences, and either noncontrast 3D TOF MRA or CE-T1W MRI, multiple authors have demonstrated the ability to differentiate between a thick, intact FC and a thin or ruptured FC. Using 3D TOF MRA, Hatsukami et al. demonstrated a high level of agreement (89%) between MRI and histological findings (18), with Cai et al. instead utilizing CE-T1W to demonstrate FC with moderate-to-good correlation between carotid MRI findings and the excised histological specimens (16). The overlying FC is described as intact if there is an enhancing band adjacent to the dark lumen on CE-T1W, with a smooth luminal surface on TOF and CE-T1W images. A thin but still intact FC will demonstrate a smooth luminal surface, but will lose the enhancing band on CE-T1W images (Figure 2). A ruptured FC will demonstrate a disrupted, dark band on CE-T1W with an irregular luminal surface on all images. Ultimately, the distinction between thin vs. ruptured is less important than thick vs not thick (i.e., thin or ruptured), as thin and ruptured FCs are both associated with increased risk of future stroke/TIA.
IPH
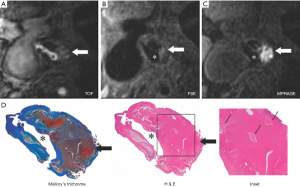
With IPH there is extravasation of proinflammatory lipid-rich membranes of red blood cells and iron into the arterial wall, which results in plaque destabilization (19). IPH will demonstrate high signal on all T1W imaging sequences, including magnetization-prepared rapid acquisition gradient-echo (MPRAGE), TOF, and fast spin-echo (FSE). It is typically seen within LRNC, but it may be seen elsewhere within the plaque. While identification of IPH is field-strength dependent (20), with 3 T outclassing 1.5 T, IPH is still readily detectable at 1.5 T. IPH was found to be detectable with both T1W and TOF MRA images at 1.5 T with a sensitivity of 82% and specificity of 77% by Saam et al. (9), whereas 3T MPRAGE depicted IPH with a similar sensitivity (80%) but a much higher specificity (97%) (21). MPRAGE, as compared with FSE and TOF, demonstrated higher diagnostic capability for the detection and quantification of IPH (21) (Figure 3). MPRAGE is clinically available on most MR manufactures at either 1.5 T or 3 T.
IPH has been further characterized as type I or type II based on the appearance on T2W (22,23). Type I IPH is dark on T2W with short T2 and has been shown to correlate with a history of recent ipsilateral stroke/TIA. The original MPRAGE sequence was modified to include multiple echoes to not only identify IPH but to measure the IPH T2*. This Sequence for Hemorrhage assessment using INversion recovery and multiple Echoes (3D SHINE) allowed automatic detection of IPH and characterization of type I and type II IPH with a single 4-minute scan, replacing the more time consuming multicontrast approach (24). Simultaneous Noncontrast angiography and intraPlaque hemorrhage (SNAP) is a recently developed sequence that uses a phase sensitive inversion recovery technique with the phase sensitive reconstruction separating the high T1 signal intensity of IPH from negative signal corresponding to flowing blood within the lumen. A noncontrast MRA is generated by displaying only the negative signals, whereas displaying only the high signals yields a highly T1 weighted image suitable for IPH detection during the same 4–6 minute acquisition (25). It is important to note that neither 3D SHINE nor SNAP are currently clinically available.
Summary of single/multi-contrast carotid plaque MRI
In 2015, Moody and Singh made a compelling case for incorporating carotid plaque imaging into routine clinical carotid MRA, demonstrating that IPH can be reliably detected at either 1.5T or 3T with a variety of IPH-detecting vessel wall sequences, including widely-available MPRAGE, while only adding 4 to 5 minutes of scanner table time (26). As more data emerges upon the clinical relevance of IPH and its potential as a biomarker for future stroke/TIA (detailed in a later section), these additional few minutes may prove to be highly beneficial and cost-effective.
Recent data has demonstrated that the characterization of vulnerable plaque features need not be limited to research coils and sequences. A head-to-head comparison between dedicated carotid research coils/sequences versus large field of view, clinically available MR plaque sequences with a clinical neurovascular coil demonstrated high sensitivity, specificity, and accuracy of LRNC and IPH detection with the clinically-available coils/sequences, albeit with limited assessment for FC (27). Nevertheless, the ability to identify, characterize, and quantify most vulnerable plaque features using only clinically available coils and sequences represents a crucial step forward in shifting the paradigm from percentage stenosis to vulnerable plaque identification and quantification by decreasing the barriers to entry.
In a recent consensus statement, the Vessel Wall Imaging study group of the American Society of Neuroradiology presented its perspective on the current status of arterial wall imaging of the carotid artery. They stated that current carotid vessel wall imaging techniques can be informative, and made specific recommendations about imaging parameters for non-contrast and CE carotid plaque MR imaging (28).
Newer MR imaging techniques to detect vulnerable plaque
Single MR acquisition to identify/quantify multiple carotid plaque components
One difficulty with multicontrast carotid plaque MRI is the need to co-register multiple sequences. A newly developed 3D sequence to obtain three different contrast weightings during a single 5-minute acquisition can streamline carotid plaque imaging and analysis. The Multicontrast Atherosclerosis CHarterization (MATCH) sequence was used in 53 consecutive patients undergoing conventional multicontrast carotid plaque MRI. MATCH was comparable, if not superior, to conventional multicontrast carotid plaque MRI in identifying and quantifying major carotid plaque components (29). In another effort to streamline carotid plaque compositional analysis, a study using machine learning to identify LRNC, IPH, calcification, and fibrous tissue from a single SNAP acquisition reported promising initial results (30).
Parametric MR mapping to characterize carotid plaque
Although carotid plaque morphology and compositional analysis using multi-contrast sequences have been the mainstay of vulnerable plaque imaging, a push for more quantitative analysis using recently developed apparent diffusion coefficient (ADC), T1, and T2 parametric maps have revolutionized the field of MRI. The main benefit of quantitative ADC, T1, and T2 imaging is improved reproducibility, facilitating longitudinal monitoring of change in carotid plaque composition with little if any “learning curve” to interpret qualitative images (31). Early results demonstrate that the combination of ADC and longitudinal relaxation rate (R1) values measured in vivo enabled differentiation among LRNC, IPH, and fibrous tissue within carotid plaque endarterectomy specimens (32), with R1 defined as the reciprocal of the T1 value.
Risk stratification with vulnerable plaque MR features
LRNC with thin/ruptured FC
Recent studies have demonstrated that characterization of carotid plaque features offers far more information and superior risk stratification for patients in comparison to percentage stenosis and other cardiovascular risk factors. A sentinel paper by Takaya et al. found that the presence of a thin or ruptured FC and larger maximum percentage of LRNC as well as IPH and larger maximal wall thickness were predictors of future stroke/TIA in a group of asymptomatic moderate carotid stenosis patients (33). A prospective study of 120 patients with carotid stenosis demonstrated that increasing LRNC size was associated with the development of new ulceration or FC rupture and increasing plaque burden (34), with percentage carotid stenosis showing no association with either the development of new ulceration/FC rupture or increasing plaque burden at follow up. A meta-analysis of multiple single-center studies by Gupta et al. confirmed that the presence of LRNC, a thin/ruptured FC, and IPH is associated with increased risk of future stroke/TIA (35). Furthermore, there was no statistically significant difference in the hazard ratios (HR) between various plaque components, with LRNC showing the lowest HR, IPH demonstrating intermediate HR, and thin/ruptured FC having the highest HR. This rank ordering of risk is consistent with the original vulnerable plaque classification scheme. Lastly, in a prospective RCT of 2,323 patients with atherosclerotic disease, increasing size of LRNC and the presence of thin/rupture FC in carotid plaques were strongly associated with fatal and nonfatal myocardial infarction, ischemic stroke, hospitalization for ACS, and symptom-driven revascularization (36). Importantly, MR biomarkers of carotid plaque vulnerability can be used for systemic athero-thrombotic risk and not just stroke/TIA.
IPH
IPH as predictor of future stoke
The presence of IPH is a known predictor of future ipsilateral stroke. Carotid plaques with IPH represent a more advanced stage of atherosclerotic disease compared with plaque showing only LRNC with a thick and intact FC. In a recently published meta-analysis, Schindler et al. demonstrated that the presence of IPH increased the risk of future stroke both in 560 symptomatic and 136 asymptomatic patients, with multivariate analysis identifying IPH as an independent predictor of stroke with an adjusted HR of 11.0, independent of percent stenosis, with no statistical difference in men vs. women (37). This further demonstrates that simple carotid stenosis measurements and traditional risk factor analysis may be inadequate in identifying patients at the highest risk for adverse cerebrovascular events.
IPH as predictor of plaque progression
IPH is associated with plaque progression, with new IPH deposits suggesting a transition from stable to unstable carotid plaque morphology (38). IPH detected using large field-of-view neurovascular coils with widely available MPRAGE sequence and IPH detected as part of a multi-contrast carotid plaque MR protocol using research surface coils and carotid MRI sequences were both similar in their correlation with future stroke/TIA in a meta-analysis (39). Further, there was no correlation of future stroke/TIA with simple carotid stenosis measurements in this meta-analysis. Thus, adding a commercially available MPRAGE sequence to routine carotid MRA examination will add value in determining future cerebrovascular risk over and above simple carotid stenosis measurements.
IPH as predictor of stroke after TIA
In the 1990’s, patients with TIA or minor stroke were found to be at increased risk of future stroke and to a lesser extent future ACS (40). Since then, major changes in management of TIA or minor stroke have significantly decreased the risk of future stroke/ACS (41). The TIAregistry.com project enrolled 4,789 patients between 2009 and 2011 with acute TIA/minor stroke to determine their 1-year and 5-year risk of stroke and ACS using modern medical therapy. At one year, the composite cardiovascular outcome (stroke, ACS, death from cardiovascular causes) was 6.2% with most events related to recurrent stroke (5.1% stroke rate at one year) (42). The five-year analysis of this group demonstrated a composite cardiovascular outcome event rate of 12.9% with 50% of the events occurring in years 2–5. At five years, strokes occurred in 345 patients or 9.5% occurrence rate with 43% of the strokes in the second through fifth years (43). Despite improvements in medical therapy of TIA/minor strokes, many patients are still at high risk of future stroke/ACS. An important question is whether carotid plaque MR imaging can improve risk stratification in recently symptomatic carotid stenosis patients. In a study of 179 symptomatic >50% carotid stenosis patients, MPRAGE identified IPH in 114 patients at the time of initial symptom onset. During follow-up, there were 62 recurrent strokes, TIA, or amaurosis fugax with 57 events (92%) occurring in patients with IPH. When evaluating only recurrent stroke in recently symptomatic patients with >50% carotid stenosis, the estimated annual stroke risk is 23.2% in IPH+ patients and only 0.6% in IPH− patients. The very low stroke risk in symptomatic >50% stenosis IPH- patients calls into question current risk-benefit assessment for CEA (44).
IPH in symptomatic <50% carotid stenosis patients
Most stroke classification systems only consider carotid bifurcation atherosclerosis as the causative lesion if the stenosis is ≥50%. Embolic stroke of undetermined source (ESUS) represents 17% (9–25%) of all ischemic strokes (45). Ipsilateral <50% stenosis is seen in up to 40% of ESUS patients. Vascular imaging including IPH seen on MPRAGE demonstrates plaques with high-risk features that are five times more prevalent in the carotid artery ipsilateral to the stroke compared with the contralateral carotid artery (46). This meta-analysis suggests that vulnerable plaque in patients with symptomatic <50% carotid stenosis may be the cause of the embolic strokes previously labeled as ESUS. Correct identification of stroke etiology is critical to designing optimal therapy.
A summary of the MR characteristics and clinical significance of vulnerable plaque features can be found in Table 1.
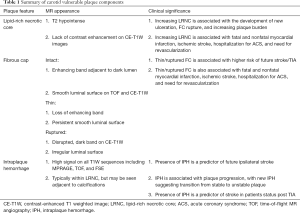
Full table
Treatment of vulnerable plaque
LRNC
The population based Multi-Ethnic Study of Atherosclerosis (MESA) of subclinical atherosclerosis in asymptomatic patients showed that LRNC was strongly associated with higher levels of plasma cholesterol (47). In the Rotterdam Study, there was a similar strong association between higher levels of cholesterol and the development of new LRNC over four years in a group of asymptomatic community-dwelling patients with carotid wall thickness ≥2.5 mm on baseline duplex ultrasonography (48). There is ample data demonstrating the response of LRNC to HMG-CoA reductase inhibitor (statin) therapy. A study by Zhao et al. in 2011 demonstrated that there is a stepwise response to LRNC from intensive lipid lowering therapy which proceeds in a predictable pattern, first by depletion of carotid plaque lipid and then subsequently by regression of TPV (49). This study illustrated the expected time course of response to lipid lowering therapy, with substantial plaque lipid depletion after 1 year on intensive therapy, continuing with a similar rate of change during year 2, and plateauing during year 3. Regression in overall plaque volume was observed after 2 to 3 years of therapy. In a modern phase II RCT (clinical trial no. NCT00851500), the placebo group of asymptomatic, stable atherosclerotic cardiovascular disease (ASCVD) patients with 16% - 79% carotid stenosis whose modifiable cardiovascular risk factors were well controlled, there was a mean regression of LRNC volume of –5.2 mm3/year seen at 6.9 months in the absence of IPH at baseline (50). Many of these patients had been on statin therapy for years before the study, suggesting continued decrease in LRNC beyond the three years noted by Zhao et al. (49). Interestingly, 7% of the patients in this study demonstrated major (>30 mm3/year) LRNC progression despite “adequate” statin therapy with rosuvastatin. The progression of LRNC was noted in 6.9 months. While some of these patients’ LRNC harbored IPH, most did not, and no other known cardiovascular risks predicted LRNC progression in this study. The authors concluded that the opportunity to reduce the residual risk associated with current medical therapy may be in patients with major progression seen on serial MRI (50). The subgroup of patients with residual large LRNC after 3 years of treatment or major progression of LRNC on “appropriate” medical therapy may therefore benefit from further intensification of their lipid-lowering therapy (51). One avenue to further intensify lipid-lowering therapy is to add proprotein convertase subtilisin kexin 9 inhibitors (PCSK9i) on top of maximally tolerated statin therapy. Typically, PCSK9i therapy can lower LDL-C to less than 50 mg/dL. In a recent study, the PCSK9i evolocumab induced further regression of coronary atheroma volume (52). No RCT evaluating carotid plaque volume with PCSK9i is currently available. There are no RCT demonstrating a clinical reduction in cerebrovascular and/or cardiovascular events using carotid plaque MRI to guide medical therapy. Hopefully, imaging substudies of two ongoing large RCTs [Carotid Revascularization Endarterectomy versus Stenting Trial (CREST-2) and Asymptomatic Carotid Surgery Trial (ACST-2)] will help evaluate the role of vulnerable plaque imaging in carotid stenosis patients.
IPH
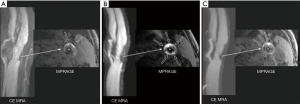
IPH is thought to form when there is compromise of the endothelial integrity of intraplaque vessels (19). In the Rotterdam Study, a subgroup of community-dwelling asymptomatic patients with carotid wall thickness ≥2.5 mm on screening carotid duplex ultrasound underwent two carotid plaque MR studies four years apart, which demonstrated new IPH statistically more often in patients with severe hypertension. It is likely that well-controlled hypertension is important to decreasing the incidence of new IPH. IPH is associated with plaque progression despite statin therapy, which suggests that IPH represents an important transition point from stable to unstable plaque morphology (53). In a modern phase II RCT (clinical trial no. NCT00851500), in the placebo group of asymptomatic, stable ASCVD patients with 16–79% carotid stenosis whose modifiable cardiovascular risk factors were well controlled, the presence of IPH at baseline was associated with fast LRNC progression seen at mean of 6.9 months (50). There are no prospective drug trials testing the ability of any lipid-lowering therapies to decrease IPH and/or TPV. There is some anecdotal evidence demonstrating that IPH and TPV can decrease with very intensive lipid lowering therapy, i.e., high intensity statin without or with PCSK9i (51,54) (Figure 4). Given the continuously increasing evidence of IPH as a significant predictor of carotid plaque progression and future adverse vascular events, trials aimed at targeted therapy for IPH represents a significant need.
Conclusions
We have reviewed the mounting evidence that various plaque features such as LRNC, thin/ruptured FC and IPH are readily identifiable on MRI and provide improved risk stratification information beyond simple carotid stenosis measurements. In particular, IPH is relatively simple to detect and measure with routine clinically available MRI; it is associated with continued plaque growth despite statin therapy; and it is associated with increased risk of future strokes. There exists a subpopulation of clinically stable ASCVD patients whose carotid plaque contains IPH despite maximum tolerated intensive statin therapy. MRI can reclassify these stable ASCVD patients without clinical very high-risk features into an imaging-defined very-high risk group who may benefit from very intensive lipid-lowering therapy including PCSK9i to lower LDL-C <50 mg/dL. There is an urgent need to design and implement a blinded imaging endpoint RCT to compare guideline-based lipid-lowering treatment with very intensive lipid-lowering treatment including PCSK9i in a population of clinically stable ASCVD patients who harbor very-high risk MR imaging features of IPH. Both arms of this study will also need to meet current ACC/AHA guidelines for management of hypertension with blood pressure <130/90 mmHg. MR imaging may be able to identify a group of stable ASCVD patients who are being undertreated medically by current guideline-based therapy. The prospective study could use a simple, rapid, robust, non-contrast MPRAGE MRI to identify IPH, with primary imaging outcome defined as regression in TPV. This prospective imaging surrogate RCT would test the hypothesis that the deleterious plaque progression effects of IPH could be slowed or reversed by very-intensive lipid-lowering therapy. The long-term goal is to use IPH imaging to inform treatment and decrease major adverse cerebrovascular events.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.03.12). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army/Navy/Air Force, Department of Defense, or U.S. Government.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415-25. [Crossref] [PubMed]
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379-87. [Crossref] [PubMed]
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421-8. [Crossref] [PubMed]
- Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491-502. [Crossref] [PubMed]
- Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke 2010;41:1193-9. [Crossref] [PubMed]
- Virmani R, Burke AP, Kolodgie FD, et al. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol 2002;15:439-46. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation 2003;108:1772-8. [Crossref] [PubMed]
- Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005;25:234-9. [Crossref] [PubMed]
- Saam T, Hatsukami TS, Takaya N, et al. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology 2007;244:64-77. [Crossref] [PubMed]
- Saam T, Kerwin WS, Chu B, et al. Sample size calculation for clinical trials using magnetic resonance imaging for the quantitative assessment of carotid atherosclerosis. J Cardiovasc Magn Reson 2005;7:799-808. [Crossref] [PubMed]
- Eliasziw M, Streifler JY, Fox AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25:304-8. [Crossref] [PubMed]
- Lovett JK, Gallagher PJ, Hands LJ, et al. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation 2004;110:2190-7. [Crossref] [PubMed]
- Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. [Crossref] [PubMed]
- Demarco JK, Ota H, Underhill HR, et al. MR carotid plaque imaging and contrast-enhanced MR angiography identifies lesions associated with recent ipsilateral thromboembolic symptoms: an in vivo study at 3T. AJNR Am J Neuroradiol 2010;31:1395-402. [Crossref] [PubMed]
- Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005;112:3437-44. [Crossref] [PubMed]
- Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging 2002;15:62-7. [Crossref] [PubMed]
- Hatsukami TS, Ross R, Polissar NL, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959-64. [Crossref] [PubMed]
- Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316-25. [Crossref] [PubMed]
- Underhill HR, Yarnykh VL, Hatsukami TS, et al. Carotid plaque morphology and composition: initial comparison between 1.5- and 3.0-T magnetic field strengths. Radiology 2008;248:550-60. [Crossref] [PubMed]
- Ota H, Yarnykh VL, Ferguson MS, et al. Carotid Intraplaque Hemorrhage Imaging at 3.0-T MR Imaging: Comparison of the Diagnostic Performance of Three T1-weighted Sequences. Radiology 2010;254:551-63. [Crossref] [PubMed]
- Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 2004;35:1079-84. [Crossref] [PubMed]
- Kampschulte A, Ferguson MS, Kerwin WS, et al. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation 2004;110:3239-44. [Crossref] [PubMed]
- Zhu DC, Vu AT, Ota H, et al. An optimized 3D spoiled gradient recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D SHINE) for carotid plaque imaging. Magn Reson Med 2010;64:1341-51. [Crossref] [PubMed]
- Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med 2013;69:337-45. [Crossref] [PubMed]
- Moody AR, Singh N. Incorporating Carotid Plaque Imaging into Routine Clinical Carotid Magnetic Resonance Angiography. Neuroimaging Clin N Am 2016;26:29-44. [Crossref] [PubMed]
- Brinjikji W, DeMarco JK, Shih R, et al. Diagnostic accuracy of a clinical carotid plaque MR protocol using a neurovascular coil compared to a surface coil protocol. J Magn Reson Imaging 2018;48:1264-72. [Crossref] [PubMed]
- Saba L, Yuan C, Hatsukami TS, et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018;39:E9-31. [Crossref] [PubMed]
- Dai Y, Lv P, Lin J, et al. Comparison study between multicontrast atherosclerosis characterization (MATCH) and conventional multicontrast MRI of carotid plaque with histology validation. J Magn Reson Imaging JMRI 2017;45:764-70. [Crossref] [PubMed]
- Zhang Q, Qiao H, Dou J, et al. Plaque components segmentation in carotid artery on simultaneous non-contrast angiography and intraplaque hemorrhage imaging using machine learning. Magn Reson Imaging 2019;60:93-100. [Crossref] [PubMed]
- Coolen BF, Calcagno C, van Ooij P, et al. Vessel wall characterization using quantitative MRI: what’s in a number? MAGMA 2018;31:201-22. [Crossref] [PubMed]
- Ota H, Tamura H, Itabashi R, et al. Quantitative Characterization of Carotid Plaque Components Using MR Apparent Diffusion Coefficients and Longitudinal Relaxation Rates at 3T: A Comparison With Histology. J Magn Reson Imaging JMRI 2018;48:1657-67. [Crossref] [PubMed]
- Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke 2006;37:818-23. [Crossref] [PubMed]
- Xu D, Hippe DS, Underhill HR, et al. Prediction of high-risk plaque development and plaque progression with the carotid atherosclerosis score. JACC Cardiovasc Imaging 2014;7:366-73. [Crossref] [PubMed]
- Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071-7. [Crossref] [PubMed]
- Sun J, Zhao XQ, Balu N, et al. Carotid Plaque Lipid Content and Fibrous Cap Status Predict Systemic CV Outcomes: The MRI Substudy in AIM-HIGH. JACC Cardiovasc Imaging 2017;10:241-9. [Crossref] [PubMed]
- Schindler A, Schinner R, Altaf N, et al. Prediction of Stroke Risk by Detection of Hemorrhage in Carotid Plaques: Meta-Analysis of Individual Patient Data. JACC Cardiovasc Imaging 2020;13:395-406. [Crossref] [PubMed]
- Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 2005;111:2768-75. [Crossref] [PubMed]
- Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013;62:1081-91. [Crossref] [PubMed]
- Johnston SC, Gress DR, Browner WS, et al. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-6. [Crossref] [PubMed]
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007;370:1432-42. [Crossref] [PubMed]
- Amarenco P, Lavallée PC, Labreuche J, et al. One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N Engl J Med 2016;374:1533-42. [Crossref] [PubMed]
- Amarenco P, Lavallée PC, Monteiro Tavares L, et al. Five-Year Risk of Stroke after TIA or Minor Ischemic Stroke. N Engl J Med 2018;378:2182-90. [Crossref] [PubMed]
- Hosseini AA, Kandiyil N, Macsweeney STS, et al. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol 2013;73:774-84. [Crossref] [PubMed]
- Hart RG, Catanese L, Perera KS, et al. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke 2017;48:867-72. [Crossref] [PubMed]
- Kamtchum-Tatuene J, Wilman A, Saqqur M, et al. Carotid Plaque With High-Risk Features in Embolic Stroke of Undetermined Source: Systematic Review and Meta-Analysis. Stroke 2020;51:311-314. [Crossref] [PubMed]
- Wasserman BA, Sharrett AR, Lai S, et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA). Stroke 2008;39:329-35. [Crossref] [PubMed]
- Pletsch-Borba L, Selwaness M, van der Lugt A, et al. Change in Carotid Plaque Components: A 4-Year Follow-Up Study With Serial MR Imaging. JACC Cardiovasc Imaging 2018;11:184-92. [Crossref] [PubMed]
- Zhao XQ, Dong L, Hatsukami T, et al. MR Imaging of Carotid Plaque Composition During Lipid-Lowering Therapy A Prospective Assessment of Effect and Time Course. JACC Cardiovasc Imaging 2011;4:977-86. [Crossref] [PubMed]
- Sun J, Balu N, Hippe DS, et al. Subclinical Carotid Atherosclerosis: Short-term Natural History of Lipid-rich Necrotic Core--A Multicenter Study with MR Imaging. Radiology 2013;268:61-8. [Crossref] [PubMed]
- DeMarco JK, Spence JD. Plaque Assessment in the Management of Patients with Asymptomatic Carotid Stenosis. Neuroimaging Clin N Am 2016;26:111-27. [Crossref] [PubMed]
- Nicholls SJ, Puri R, Anderson T, et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016;316:2373-84. [Crossref] [PubMed]
- Sun J, Underhill HR, Hippe DS, et al. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging 2012;5:798-804. [Crossref] [PubMed]
- Ogata A, Oho K, Matsumoto N, et al. Stabilization of vulnerable carotid plaques with proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab. Acta Neurochir (Wien) 2019;161:597-600. [Crossref] [PubMed]


