
Should CMR be the default imaging modality in clinical trials for heart failure?
Heart failure (HF) is a complex syndrome with a broad heterogeneity of underlying aetiologies. Anatomically, it may result from pathologic conditions in the myocardium, pericardium, endocardium or the heart valves (1). However, HF can just as well be a maladaptive response to extra-cardiac conditions. This diagnostic ambiguity creates difficulties in matching clinical presentations and underlying aetiology once HF is clinically overt. Furthermore, pre-clinical stages of the disease often remain undiagnosed leading to dissatisfactory rates of primary prevention. The lack in understanding and revealing a potential common molecular basis of the syndrome is currently reflected by clinician’s approaches to phenotype it (2).
At present, the definition of HF according to both the European Society of Cardiology (ESC) and American College of Cardiology/American Heart Association (ACC/AHA) is based on non-physiological cut-off values of left ventricular ejection fraction (LVEF) and divides the disease in two (AHA/ACC) or three (ESC) different categories (1,3). HF with a reduced ejection fraction (HFrEF) is hereby defined as left ventricular ejection fraction ≤40% (LVEF) whereas HF with a preserved ejection fraction (HFpEF) incorporates patients with a LVEF ≥50%. The latest addendum to this group is HF with a mid-range ejection fraction (HFmrEF, LVEF 41–49%) which, thus far, exclusively appears in European guidelines, mainly to stimulate research in this frequently neglected patient group.
Guideline recommended EF cut-offs for HF are arbitrary constructs adapted from early stages of HF outcome trials and mirror patient selection for these studies rather than disease processes or underlying pathophysiology (4). Therefore, optimal reproducibility and reliability of imaging techniques used in these trials is vital. In HFrEF, there is significant progress and multiple aetiologies are now identified by enhanced imaging tests at earlier stages translating into informed care decisions and thus, improved care of patients (5-9). However, despite a declining incidence and availability of novel treatment options in HFrEF over the last 2 decades, hospital admissions due to HF are rising, 5-year mortality rates remain around 50% and survival rates in elderly populations are stagnant (10-12). Diagnosing HFpEF is significantly more time-consuming and no single test is yet available to ascertain a diagnosis (13). Adding to the confusion is the difficulty in discerning underlying pathophysiological mechanisms in patients with a high burden of multi-morbidity and concomitant conditions. Unsurprisingly, it is estimated that the currently diagnosed patients are only the “tip of the iceberg” as the syndrome often goes unrecognised (14). Furthermore, prognostic treatment is absent and current disease management is based on risk factor optimisation and symptom control.
Subsequently, there is a clear unmet need for novel medical therapies in patients with HFpEF and HFrEF.
Randomised controlled trials (RCT) provide the least biased method to assess efficacy and safety of emerging therapies in many diseases, including HF, and are therefore considered the gold standard (15,16). Nevertheless, evidence generated by different clinical trials on the same topic may be conflicting and translating results into clinical practice challenging (17). Disparities in trial designs have previously been identified as a frequent cause for the aforementioned and it has been shown that generating meta-analyses of published RCTs does not necessarily increase generalisability of results (17,18). In addition, individual trials have used distinctive imaging modalities for screening and/or outcome measures and evidence comparing the various techniques is scarce (19-23). With this in mind, the question arises whether a certain imaging test should be favoured when designing clinical trials and if so, which imaging technique offers the best overall value?
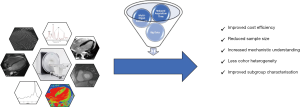
Firstly, the ideal imaging test for trials in HF patients has to have gatekeeping function for screening purposes but also reveal underlying phenocopies of a disease and thus offer best possible patient selection. In HFpEF, with its varying definitions and high phenotypic heterogeneity, insufficient exclusion of imaging phenocopies like hypertrophic cardiomyopathy, constrictive pericarditis or microvascular dysfunction might interfere with overall outcomes and could be a possible explanation for negative trial results in this population (24).
Secondly, if the imaging technique improves phenotyping and patient characterisation, it may lead to enhanced definition of subgroups within the trial cohort. As a growing number of trials report detailed subgroup analyses in various contexts, this feature could generate ideas for future dedicated outcome trials. A positive example for this approach is the success story of the anti glycaemic sodium glucose co-transporter 2 (SGLT-2) inhibitors for HF treatment, which began with a positive signal in a subgroup of the patients enrolled in the EMPAREG-OUTCOME trial and has now shown efficacy in a dedicated HF outcome trial (25,26).
Thirdly, despite available prognostic treatment options for HFrEF, it has to be noted that most phase III trials in both HFrEF and HFpEF patients were overall negative hence, not meeting their primary outcomes (27,28). This makes a trial a luxurious but equally necessary experiment with many uncertainties involved and may be one of the reasons why drug development and trial conduct in cardiovascular diseases are stagnant (29). Consequently, the imaging technique should be able to reduce costs by improving stratification of promising investigational agents in phase II trials and allow for reduced sample sizes. Furthermore, by providing a plethora of surrogate endpoints it may increase mechanistic understanding of the investigational agent at the same time (30).
Lastly, as HF incidence and prevalence increases with age, patients frequently present with one or more comorbidities. One of the most important comorbidities is renal dysfunction and many experts refer to both HFrEF and HFpEF as cardio-renal diseases as the incidence of renal dysfunction in chronic HF is increasing (31). Consequently, it is clear that a deeper understanding of mechanisms that link cardiac and renal dysfunction is required. Therefore, the imaging technique should allow multi-organ assessment during a single session.
Cardiovascular magnetic resonance (CMR) is a unique non-invasive imaging technique to assess HF patients without using ionising radiation or radionuclides and with excellent image quality independently of anatomical variations (5). Its high temporal and spatial resolution offer assessment of anatomical structures and functional cardiovascular parameters alike (32,33). It allows for comprehensive assessment of cardiac remodelling including tissue characterisation, quantification of extracellular volume content and identification of myocardial fibrosis, infarction, inflammation and oedema (7). In addition, novel sequences (4D-Flow) permit quantification of ventricular blood flow and kinetic energy in a single, short acquisition (34). As signal generation by CMR works via stimulating nuclei, when generating images these are hydrogen nuclei in water bonds within any given structure, it is not limited to image creation but with different spectroscopic techniques can also assess myocardial energy metabolism and fat composition (35-37). Furthermore, it has an excellent diagnostic accuracy, reproducibility and is less operator dependent when compared to echocardiography (5,38). Finally, it allows assessment of multiple organs in one session which are closely related to the clinical syndrome of HF and may provide improved mechanistic understanding of inter-organ links in pathophysiology.
Nevertheless, using CMR in the context of clinical trials implies certain limitations. The need for the participant to lie flat and hold their breath for a certain amount of time may reduce applicability of CMR in trials enrolling acutely decompensated HF patients. As HF is more prevalent in elderly, frail patients there is reduced tolerance to the confined space in the MR-scanner and patients may be less enthusiastic about participating in trials using this imaging technique, specifically when used at multiple timepoints. Furthermore, it has to be taken into consideration that patients with implanted cardiac devices and/or metal implants usually present with a reduced diagnostic accuracy as the implants produce artefacts. Conversely, assessment by echocardiography is more convenient and nowadays available in any hospital. It can be used in any patient group and therefore is the customary imaging technique used in clinical trials involving HF patients (39).
However, use of CMR in HF trials is rapidly increasing and although large scale data from randomised trials comparing the imaging modalities is scarce, registry data suggests that use of CMR in HF patients may be more cost-effective due to its superior diagnostic accuracy (40,41). The recent randomised controlled OUTSMART-HF trial investigating routine versus selective use of CMR in chronic HF patients confirmed these results (42,43). Use of CMR increased the diagnostic accuracy in determining HF aetiology by 16%, when compared to transthoracic echocardiography. Nonetheless, routine CMR use did not yield more specific heart failure aetiologies which is concerning as patients with a distinct aetiology were at higher risk for cardiovascular events. More than 25% of the study population in each group had HF conditions lacking characteristic imaging findings highlighting the need to improve our categorisation of HF. Follow-up trials of the IMAGE-HF project are designed to identify novel imaging biomarkers in HF patients and will hopefully lead to better stratification (43).
With the expansion of shorter, free-breathing acquisitions, new sequences to assess haemodynamic parameters, refined contrast free tissue characterization and enhanced multi-organ assessment acceptance, accuracy and cost-effectiveness of CMR in trials will further improve (44). Increasing precision of automated post-processing techniques will advance its efficiency and promote new big data algorithms to identify novel imaging targets.
All of the above makes CMR, when compared to the most frequently used echocardiography, the ideal imaging technique to be utilised in phase II and phase III HF trials (Figure 1).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-244). The series “The use of advanced cardiac MRI in heart failure and cardiac hypertrophy” was commissioned by the editorial office without any funding or sponsorship. MH reports grants from Boehringer Ingelheim International GmbH, during the conduct of the study.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Cleland JGF, Pellicori P, Clark AL. Prevention or Procrastination for Heart Failure?: Why We Need a Universal Definition of Heart Failure. J Am Coll Cardiol 2019;73:2398-400. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [Crossref] [PubMed]
- Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 2016;37:1642-50. [Crossref] [PubMed]
- American College of Cardiology Foundation Task Force on Expert Consensus D, Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 2010;55:2614-62. [Crossref] [PubMed]
- Francone M. Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution and prognostic significance. ISRN Radiol 2014;2014:365404. [Crossref] [PubMed]
- Peterzan MA, Rider OJ, Anderson LJ. The Role of Cardiovascular Magnetic Resonance Imaging in Heart Failure. Card Fail Rev 2016;2:115-22. [Crossref] [PubMed]
- Sengupta PP, Kramer CM, Narula J, et al. The Potential of Clinical Phenotyping of Heart Failure With Imaging Biomarkers for Guiding Therapies: A Focused Update. JACC Cardiovasc Imaging 2017;10:1056-71. [Crossref] [PubMed]
- Senni M, Rodeheffer RJ, Tribouilloy CM, et al. Use of echocardiography in the management of congestive heart failure in the community. J Am Coll Cardiol 1999;33:164-70. [Crossref] [PubMed]
- Jones NR, Roalfe AK, Adoki I, et al. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail 2019;21:1306-25. [Crossref] [PubMed]
- Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572-80. [Crossref] [PubMed]
- Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996-1004. [Crossref] [PubMed]
- Pieske B, Tschope C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297-317. [Crossref] [PubMed]
- Rutten FH, Cramer MJ, Grobbee DE, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 2005;26:1887-94. [Crossref] [PubMed]
- Kaptchuk TJ. The double-blind, randomized, placebo-controlled trial: gold standard or golden calf? J Clin Epidemiol 2001;54:541-9. [Crossref] [PubMed]
- Ioannidis JP, Haidich AB, Pappa M, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001;286:821-30. [Crossref] [PubMed]
- Horwitz RI. Complexity and contradiction in clinical trial research. Am J Med 1987;82:498-510. [Crossref] [PubMed]
- LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med 1997;337:536-42. [Crossref] [PubMed]
- Xie BQ, Tian YQ, Zhang J, et al. Evaluation of left and right ventricular ejection fraction and volumes from gated blood-pool SPECT in patients with dilated cardiomyopathy: comparison with cardiac MRI. J Nucl Med 2012;53:584-91. [Crossref] [PubMed]
- Pellikka PA, She L, Holly TA, et al. Variability in Ejection Fraction Measured By Echocardiography, Gated Single-Photon Emission Computed Tomography, and Cardiac Magnetic Resonance in Patients With Coronary Artery Disease and Left Ventricular Dysfunction. JAMA Netw Open 2018;1:e181456. [Crossref] [PubMed]
- Hoffmann R, von Bardeleben S, ten Cate F, et al. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J 2005;26:607-16. [Crossref] [PubMed]
- Herregods MC, De Paep G, Bijnens B, et al. Determination of left ventricular volume by two-dimensional echocardiography: comparison with magnetic resonance imaging. Eur Heart J 1994;15:1070-3. [Crossref] [PubMed]
- Gaudio C, Tanzilli G, Mazzarotto P, et al. Comparison of left ventricular ejection fraction by magnetic resonance imaging and radionuclide ventriculography in idiopathic dilated cardiomyopathy. Am J Cardiol 1991;67:411-5. [Crossref] [PubMed]
- Kanagala P, Cheng ASH, Singh A, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in heart failure with preserved ejection fraction - implications for clinical trials. J Cardiovasc Magn Reson 2018;20:4. [Crossref] [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. [Crossref] [PubMed]
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995-2008. [Crossref] [PubMed]
- Massie BM. 15 years of heart-failure trials: what have we learned? Lancet 1998;352 Suppl 1:SI29-33. [Crossref] [PubMed]
- O'Connor CM. Why Negative Trials Are Positive for Heart Failure Patients. JACC Heart Fail 2016;4:329-30. [Crossref] [PubMed]
- Fordyce CB, Roe MT, Ahmad T, et al. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol 2015;65:1567-82. [Crossref] [PubMed]
- Greene SJ, Mentz RJ, Fiuzat M, et al. Reassessing the Role of Surrogate End Points in Drug Development for Heart Failure. Circulation 2018;138:1039-53. [Crossref] [PubMed]
- Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015;36:1437-44. [Crossref] [PubMed]
- Bogaert J, Francone M. Cardiovascular magnetic resonance in pericardial diseases. J Cardiovasc Magn Reson 2009;11:14. [Crossref] [PubMed]
- Patel MR, White RD, Abbara S, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: a joint report of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Foundation Appropriate Use Criteria Task Force. J Am Coll Cardiol 2013;61:2207-31. [Crossref] [PubMed]
- Stoll VM, Hess AT, Rodgers CT, et al. Left Ventricular Flow Analysis. Circ Cardiovasc Imaging 2019;12:e008130. [Crossref] [PubMed]
- Ten Hove M, Neubauer S. MR spectroscopy in heart failure--clinical and experimental findings. Heart Fail Rev 2007;12:48-57. [Crossref] [PubMed]
- Faller KM, Lygate CA, Neubauer S, et al. (1)H-MR spectroscopy for analysis of cardiac lipid and creatine metabolism. Heart Fail Rev 2013;18:657-68. [Crossref] [PubMed]
- Bottomley PA, Panjrath GS, Lai S, et al. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death. Sci Transl Med 2013;5:215re3. [Crossref] [PubMed]
- Gandy SJ, Waugh SA, Nicholas RS, et al. Comparison of the reproducibility of quantitative cardiac left ventricular assessments in healthy volunteers using different MRI scanners: a multicenter simulation. J Magn Reson Imaging 2008;28:359-65. [Crossref] [PubMed]
- Shah SJ, Fonarow GC, Gheorghiade M, et al. Phase II trials in heart failure: the role of cardiovascular imaging. Am Heart J 2011;162:3-15.e3. [Crossref] [PubMed]
- Bruder O, Schneider S, Nothnagel D, et al. EuroCMR (European Cardiovascular Magnetic Resonance) registry: results of the German pilot phase. J Am Coll Cardiol 2009;54:1457-66. [Crossref] [PubMed]
- Francis SA, Daly C, Heydari B, et al. Cost-effectiveness analysis for imaging techniques with a focus on cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013;15:52. [Crossref] [PubMed]
- Paterson DI, Wells G, Erthal F, et al. OUTSMART HF: A Randomized Controlled Trial of Routine Versus Selective Cardiac Magnetic Resonance for Patients With Nonischemic Heart Failure (IMAGE-HF 1B). Circulation 2020;141:818-27. [Crossref] [PubMed]
- Paterson I, Wells GA, Ezekowitz JA, et al. Routine versus selective cardiac magnetic resonance in non-ischemic heart failure - OUTSMART-HF: study protocol for a randomized controlled trial (IMAGE-HF (heart failure) project 1-B). Trials 2013;14:332. [Crossref] [PubMed]
- Friedrich MG. The Future of Cardiovascular Magnetic Resonance Imaging. Eur Heart J 2017;38:1698-701. [Crossref] [PubMed]


