
Plaque imaging volume analysis: technique and application
The atherosclerotic plaque
Cardiovascular disease: a global threat
Atherosclerosis is an inflammatory vascular disease characterized by metabolic alterations which, via the formation of vascular plaques, can lead to severe cardiovascular complications, most importantly in the form of ischemic heart disease and cerebrovascular disease. In developed countries, carotid artery disease affects 75% of men and 62% of women in a population of over 65 years of age.
Despite reduction of the incidence and mortality rates for cardiovascular disease in Western countries over the course of the 20th century, stroke still ranks as the second most common cause of death worldwide; up to 18–25% of all strokes ensue as a complication of carotid atherosclerotic disease (1-5).
Therefore, in designing an effective stroke prevention strategy, it is of paramount importance that carotid atherosclerotic disease and high-risk determinants be identified.
Natural history of the atherosclerotic plaque
One important concept to start from in the understanding of atherosclerotic disease is that carotid artery plaques modify over time. Indeed, the composition of the plaque changes with the stage of development of atherosclerosis and, interestingly, some phases seem to be particularly prone to major cardiovascular events (6).
According to a classification proposed by the Committee on Vascular Lesions of the Council on Arteriosclerosis (American Heart Association), the natural history of the disease can be divided in 6 stages that reflect histological and clinical features, which in turn correlate to the imaging appearance of lesions observed in clinical studies. In the histological classification, Roman numerals indicate the phase of lesion progression. The initial (type I) is characterized by scattered macrophage foam cells. Type II lesions consist in macrophage foam cells and lipid-laden smooth muscle cells (the so-called “fatty streaks”). Type III lesions present with extracellular lipid droplets interposed among the intimal smooth muscle cells. Type IV lesions express a larger and more confluent disruptive core of extracellular lipidic material. Type V lesions contain a lipid core associated to thick layers of fibrous connective tissue. These lesions are subdivided into largely calcified (type Vb) and mainly fibrotic (type Vc). Type VI lesions are defined by the presence of a fissure, hematoma or thrombus (7).
With regards to the progressive modification of carotid plaques in time, Van Gils et al. conducted an interesting study based on 109 patients with a history of transient ischemic attack (TIA) or stroke. Serial Multi Detector Computed Tomographic Angiography (MDCTA) of the carotid arteries was performed for a period of around 5 years after the cardiovascular event. Semi-automated segmentation tools were employed in this study to define outer vessel walls and lumen boundaries, while plaque component volumes were measured based on HU thresholds. Annual modifications in plaque volume and specific component were reported. In their work, the Authors identified a change in plaque composition over time: the calcific component increased, accompanied by a decrease in fibrous and lipid content. In particular, larger plaques displayed faster increment in calcium content compared to smaller ones, while the more calcified plaques displayed a much larger annual increment in calcium proportion compared to the less calcified ones. Since calcified carotid plaques appear to be more stable than non-calcified ones, the Authors suggest that plaque progression is associated to a more stable profile. It remains to be elucidated whether secondary preventive medication may partly contribute to this trend. Indeed, a number of studies indicated a change in composition towards a more stable plaque phenotype in patients under statin treatment by showing a decrement in lipid content and an increase in calcium; however, larger randomized trials are needed to confirm these results. It still remains to be unravelled whether monitoring plaque progression might play a role in the definition of risk prediction in the clinical routine. The Authors conclude by stressing out that further future research is needed to evaluate the associations between temporal plaque changes and recurrent cerebrovascular events (8).
Apart from the aspects of plaque progression described above, it is noteworthy that atherosclerosis seems to express different characteristics according to the location of the affected vessel. In this regard, Saba et al. conducted a work to compare the carotid artery bifurcation and the carotid siphon in terms of plaque type and degree of stenosis. In fact, while the carotid siphon is a common location for plaque formation, some Authors put forward the questions whether carotid siphon lesions could represent a contraindication for endarterectomy of carotid artery bifurcation plaques. A total of 119 patients were retrospectively studied using MDCTA. Plaque components of the carotid artery bifurcation and siphon were defined based on HU values and each volume was calculated. The study found no significant association between carotid bifurcation and carotid siphon components. While the carotid artery bifurcation total plaque volume showed an association with previous cerebrovascular events (in particular the plaques with a lipid content), no association was found with the volume of carotid siphon plaque or its subcomponents (9).
Another factor that has been shown to affect the volume and composition of carotid plaques is neck radiation therapy (HNXRT). Anzidei et al. retrospectively observed progressive changes in a group of 62 patients over the course of a 2-year period during HNXRT, comparing them against a control group of 40 patients who underwent surgery alone. This preliminary study suggested that HNXRT promotes the volumetric progression of carotid artery plaques, particularly for what concerns the fatty component (10).
From degree of stenosis to vulnerable plaque: a paradigm shift
The degree of luminal stenosis has classically been regarded as the most important feature expressing the grade of severity of carotid atherosclerotic disease.
For several years, digital subtraction angiography has been recognized as the gold standard for the evaluation of stenosis secondary to atherosclerotic plaques. This method defines the opacified lumen with optimal spatial resolution, allowing for accurate evaluation of the degree of luminal stenosis; also, plaque-related luminal changes can be identified, such as lumen irregularity and plaque ulcerations.
Stenosis can also be quantified effectively using Computed Tomography. Recently, the introduction of dual energy CT (DECT) scanners made it possible to generate multiple datasets with different levels of kilo-electronvolt (keV), with the potential to discriminate between different components on the basis of their atomic number. In other words, this technology introduced the possibility to identify different tissue types relying on the variation of their characteristic HU attenuation values. Mannelli et al. found that an overestimation of the degree of stenosis at the level of calcified plaques occurs when low keV values are used for image reconstruction (11). As a consequence, keV values may significantly affect the evaluation of stenosis in carotid arteries. Agreeably, the Authors suggest that a standardization of the energy levels used in image reconstruction protocols would be beneficial (12,13).
A number of trials, undertaken during the 1980s to mid-1990s, quantified the benefit of carotid endarterectomy (CEA) taking the degree of luminal stenosis as the reference point. In particular, 3 multicenter randomized studies, the European Carotid Surgery Trial (ECST), NASCET, and Asymptomatic Carotid Atherosclerosis Study (ACAS), consolidated the concept that the degree of stenosis should be advocated as the primary tool for stratifying stroke risk and guide therapeutic approach (surgery against best medical management) (14-18).
However, recent literature indicates that plaque structure and composition may represent a more reliable biomarker for the development of cerebrovascular ischemic events (19-22). From this more updated body of evidence the following new concepts emerge: (I) the mere degree of stenosis is a poor indicator of the true volume and extension of a given carotid plaque; (II) a number of plaque features, which can be assessed by currently available imaging technology, are more closely linked to the development of ischemic symptoms; (III) such features show predictive value for stroke regardless of the degree of stenosis (23).
The rationale that underlies this paradigm shift is conceptually straightforward: rather than hypoperfusion secondary to a stenotic vessel, it is the generation of emboli from the heart or a carotid plaque that represents the cause of the majority of brain ischemic infarcts. After all, it is known that the Circle of Willis and cerebral collaterals are capable to compensate stenosis up to a certain degree.
Several works published in the late 1980s, based on coronary artery angiography, demonstrated that events of myocardial infarction could be observed even in the presence of a moderate degree of stenosis, consolidating the idea that mere luminal narrowing could not be deemed as the only pathogenetic factor for myocardial infarction. Further, histopathologic evidence shed light on the association between plaque erosion and myocardial infarction. Other studies which focused on carotid arteries showed similar results, with cerebrovascular events occurring even in cases of low-grade stenosis (<30%).
Therefore, moving away from the mere concept of stenosis, a number of carotid plaque features have been demonstrated to increase the risk of ischemic events. Morphological characteristics of unstable plaques include: the presence of a thin fibrous cap (FC) with a large lipid core, intraplaque hemorrhage (IPH), intraplaque active inflammation, eccentric distribution, surface irregularities and surface ulcerations with intimal exposure. These features show strong correlation to the presence of hemodynamic alterations and shear stress. In particular, plaque inflammation increases vulnerability, since chronic inflammation (characterized histopathologically by the presence of macrophages and T-cells) determines a weakening of the vasa vasorum, disruption of plaque connective-tissue and arterial wall thrombosis.
Moreover, there is agreement in the current literature that the process of remodeling, initially described in the coronary vascular bed, can also be ascribed to the carotid arteries. Plaque remodeling can be described as negative or positive: the former implies luminal stenosis while the latter refers to a dilatation of the vessel wall subsequent to a volumetric increase in the plaque component. Positive remodeling may be seen as an adaptive change through which vessels preserve sufficient luminal diameter. When this compensatory effect becomes overbalanced, negative remodeling ensues, resulting in the reduction of luminal diameter (24).
Progressively, a diverse body of literature converged into the newly introduced concept of plaque burden, which may convey more information on the extent of cardiovascular disease than mere stenosis, in accordance with the observation that mildly stenotic and asymptomatic carotid plaques are highly prevalent among the elderly.
In an effort to improve measures of cardiovascular disease prevention and management, non-invasive approaches to the determination of plaque burden have been proposed. In this regard, as part of the Rotterdam Study, which was designed to identify determinants of various diseases in the elderly population, Selwaness et al. analyzed on MRI the carotid arteries of 1,562 stroke-free patients. Implementing the use of an automated segmentation tool, the Authors quantified the inner and outer wall of the carotid arteries, lumen volume, wall volume and plaque burden (wall volume/outer wall volume). Also, plaque components were visually evaluated and the degree of luminal stenosis was measured. The Authors unveiled the associations between cardiovascular risk factors, plaque components and plaque volume. Results showed that several cardiovascular risk factors bore associations with carotid plaque burden and lumen volume. Moreover, the most relevant data regarded the presence of IPH, lipid content and calcification, all of which appeared to be strongly associated with low lumen volume and high plaque burden, especially for what concerned IPH (25).
The concept of plaque burden spurred a lot of interest in the literature with a growing number of publications focused on noninvasive measurement of plaque volume and identification of vulnerable plaque features (24,26,27). The following paragraph will offer an overview of the main features of carotid plaque vulnerability, together with the imaging modalities available for their identification, highlighting strengths and weakness in the diagnostic capability of each imaging technique.
Imaging of the carotid plaque
Features of vulnerability: diagnostic yield of different imaging methodologies
Plaque imaging offers a change of perspective in the way we look at carotid atherosclerosis disease, moving the viewpoint from the measurement of luminal stenosis to the identification of imaging biomarkers of vulnerability and, ultimately, reinforce stroke risk prediction strategies. The following paragraphs will attempt to offer a review of the most relevant evidence emerging from recent literature on the imaging of vulnerable plaques (28-30).
Intraplaque hemorrhage
Among the features that characterize carotid plaques, intraplaque hemorrhage (IPH) appears to be the strongest predictor for acute events. IPH is responsible for the enlargement of the lipid-rich necrotic core (LRNC) and rapid plaque volume progression.
A number of recently published works stress the importance of IPH in carotid plaque evaluation; for instance, Gupta et al. conducted a meta-analysis of nine MRI studies demonstrating an association between carotid intraplaque hemorrhage and ischemic stroke in patients with both symptomatic and asymptomatic stenosis (31).
Saba et al. performed the analysis of 343 patients with a recent history of anterior circulation ischemic events and bilateral intraplaque hemorrhage. In this work, the Authors demonstrated that the symptomatic side associated with the presence of ipsilateral plaques of larger volume and greater intraplaque hemorrhage component when compared to the plaques of the asymptomatic controlateral side (32).
Similarly, a study published by Liu et al. found that, in a group of symptomatic patients with carotid atherosclerotic plaques, the size of IPH was an independent risk factor for ipsilateral acute cerebral infarct, suggesting the idea that the volume of IPH might serve as a useful indicator for such events (33).
Several works indicate that MRI can effectively show intraplaque hemorrhage (34,35), thanks to its capability to detect the oxidative state of hemoglobin even using common imaging sequences, such as T1-weighted fat-saturated turbo spin echo, inversion recovery turbo field echo, or inversion recovery fast spoiled gradient recalled acquisition in the steady state. Additionally, there is evidence that intraplaque hemorrhage can also be assessed in MRI at lower spatial resolution using large field-of view (FOV) neck coils, without the need for dedicated carotid small FOV surface coils (36).
With regards to CT, a number of publications in the past years argued that via this methodology it would be difficult to differentiate between fibrous, lipid and hemorrhagic components of the plaque owing to a substantial overlap in the radiation attenuation characteristics of such tissues. However, recent literature suggests a greater potential for CT in this diagnostic area, bringing evidence that IPH can be effectively delineated in CTA. For instance, a retrospective study including 91 patients conducted by Saba et al. indicated that a threshold of 25 HU following administration of contrast medium is a highly sensitive and specific marker for the detection of IPH (37).
With regards to US, it is generally agreed that this methodology has low sensitivity in the detection of IPH, due to operator dependence and scarce specificity in the differentiation of tissue components (38).
LRNC and fibrous cap
The LRNC and the fibrous cap represent another two important features of carotid plaque vulnerability. The LRNC is a heterogeneous tissue consisting in a mixture of cholesterol crystals, apoptotic cellular debris and calcium, while the fibrous cap is a layer of connective tissue that secludes the core of the plaque from the vascular lumen.
While some reports indicate that MRI may be able to measure LRNC superior to CT it remains the case that there is no histological confirmation of this, and no clinically approved product on the market. Reports of processing CTA using older methods, lacking image restoration and/or utilizing Hounsfield units thresholding, have suggested that since both these features have attenuation values below 60 Hounsfield units, they may be difficult to distinguish using CT. Instead of indicating CTA cannot do it reliably, this simply motivates more recent processing approaches that mitigate the issues, and allow the superior spatial resolution of CTA to not only quantify LRNC specifically (39), but also provide a measure of cap thickness better than any non-invasive modality. Using this method, CTA is the only modality with specific regulatory labelling indicating performance for measuring LRNC for clinical use.
For the time being, US is not considered a methodology suitable for the detection of the LRNC since it cannot identify intraplaque hemorrhage, both features being hypoechogenic.
Inflammation and neovascularization
Another important aspect to consider is the so-called plaque activity, which is reflected by the presence of inflammation and angiogenesis. It has been shown that inflammatory cells gather in specific areas of the plaque, such as the shoulder or the connective tissue of the fibrous cap; neoangiogenesis determines an increased risk for neovessel rupture and hemorrhage.
In a number of works MRI has shown good correlation with histological markers of inflammation, suggesting for this methodology a potential role for quantitative and non-invasive plaque activity evaluation (29). In particular, the degree of plaque enhancement appears to correlate with the extent of intraplaque neovascularisation on dynamic contrast enhancement perfusion MRI, where signal intensity in tissues is measured over time (usually 5–10 minutes) after gadolinium administration. In accordance with this evidence, a study conducted by Kerwin et al. on 20 consecutive patients enrolled for CEA, demonstrated the efficacy of dynamic contrast-enhanced MRI in delineating the extent of neovasculature within the examined plaques. There are, however, a number of limitations to this technique to take into account, such as the small size of the target vessels and motion artifacts (40).
Several published works, including a meta-analysis of 20 studies (41), advocate the use of contrast-enhanced ultrasound as a reliable technique to visualize intraplaque neovascularisation.
With regards to contrast enhanced CT, a number of works show that this methodology proves effective in the detection and quantification of plaque activity, since contrast medium delineates the extent of intraplaque neovascularisation (42).
Recent literature has investigated the potential role of Positron Emission Tomography (PET) in the identification of plaque inflammation. While there is evidence that 18F-fluorodeoxyglucose (18F-FDG) accumulates in active inflammatory cells, which can be interpreted as a marker of plaque inflammation and neovascularization, a consensus is yet to be met for a standardized quantification of radiotracer uptake in the context of atherosclerosis (29).
At present, research is ongoing on innovative techniques of molecular imaging, which may emerge with novel results in the near future. A number of nanoparticles are being put to the test, such as iron oxide, sodium fluoride, or polyethylene glycol; in particular, macrophage iron labeling in MRI techniques is being tested with promising results for in vivo detection of plaque inflammation.
Carotid plaque thickness
Maximum plaque thickness is an indicator of vulnerability since it correlates with plaque size and volume. According to the Mannheim consensus, plaques are defined as a focal structure with a thickness >1.5 mm when measured from the media-adventitia interface to the intima-lumen interface.
The thickness of the carotid artery plaque is quantifiable with ultrasound, CT, and MRI.
In an attempt to standardize the measurement of carotid arterial wall thickness, Saba et al. proposed the use of a semiautomatic MRI system based on level set method. The study shows that the semiautomated method can provide measures of the lumen and outer wall boundaries with reasonable accuracy if compared to manual segmentation. However, in this method the quantification of carotid arterial wall thickness and arterial wall area showed some degree of overestimation in arterial walls with small areas when compared to the manual segmentation (43).
Surface irregularities
Another aspect to consider is the surface of carotid plaques, which can be described as smooth, irregular (surface fluctuates within a range of 0.3 to 0.9 mm), or ulcerated (cavities >1 mm). Surface irregularities, with particular regard to ulcerations, represent a risk feature for stroke.
The carotid plaque surface can be assessed by ultrasound, CT, and MRI, with varying diagnostic accuracy.
The diagnostic potential of US in the identification of ulcerations is diminished by the presence of artifacts such as the acoustic shadowing of calcified components. However, contrast-enhanced ultrasound may boost accuracy, since microbubbles help discern between intimal layer and blood flow (44).
Plaque volume
The measurement of carotid plaque volume (CPV) may serve as a useful feature to identify plaque vulnerability.
Numerous works in the past literature indicated MRI as a reliable tool to quantify total and subcomponent volumes of the plaque, thanks to its excellent soft tissue characterization capabilities.
Recent evidence demonstrated that CTA can perform as accurately or better in the quantification of total and subcomponent (e.g., fatty, mixed, calcified) CPV, based on HU voxel values, high spatial resolution and the capabilities offered by imaging algorithm analysis. A number of algorithms using HU thresholding have been reported to various levels of success, but newer approaches that relax the use of HU as a strict threshold perform better and at least one algorithm has reached the level of being approved for specific tissue labeling of LRNC for clinical use (39), avoiding more ambiguous terms such as low attenuation plaque or soft plaque but rather using pathologist annotated tissue as the gold standard against which performance is assessed and reported.
The following section will provide a more detailed review of the present literature regarding the volumetric analysis of the carotid atherosclerotic plaque, indicating advantages and drawbacks for each methodology.
Volumetric analysis: current knowledge and future scenarios
Currently, the most widely accepted indicator for CEA remains the degree of stenosis. Patients with 70–99% stenosis have an absolute risk reduction of 23% when CEA is performed within 2 weeks from the insurgence of an acute event. While it is generally agreed that recent cerebral ischemic symptoms represent a clear indication to expedite carotid surgery, as it was previously discussed there is a growing body of evidence suggesting that the mere degree of stenosis is a poor predictor for major events. Indeed, asymptomatic patients with a degree of carotid stenosis greater than 70% manifest an ipsilateral stroke risk of only 2% per year if treated with best medical care (45).
With regards to the coronary vascular bed, it is now generally accepted that the concept of overall atherosclerotic burden represents a better predictor of acute events than the degree of stenosis alone. The PROSPECT study of coronary atherosclerosis, for instance, demonstrated that acute cardiac events manifest a stronger correlation with high atherosclerotic burden rather than stenosis (45).
In this scenario, CPV, measured as the volume of atherosclerotic material within a certain length of the artery, can be regarded as a marker of the atherosclerotic burden. The importance of shifting the attention from mere stenosis to the overall atherosclerotic burden has been highlighted in several studies that evidenced substantial plaque burden in angiographically normal arteries. Indeed, severe complex lesions (American Heart Association type VI) were frequently documented in carotid arteries with stenosis lower than 50% (46,47).
In support of this evidence, a work conducted by Ball et al. on 339 patients enrolled for CEA evaluated the relationship between CPV and symptoms of cerebral ischemia. This study took into consideration the volume of the endarterectomy specimen, the time since last symptom occurred and the degree of stenosis measured via duplex ultrasonography. Additionally, middle cerebral artery emboli were counted using transcranial Doppler imaging in a subset of patients. The Authors concluded that the magnitude of the plaque volume directly correlated with recent cerebral ischemic symptoms; no significant relationship was found between the degree of stenosis and plaque volume. Also, a correlation was found between plaque volume and the number of cerebral emboli, suggesting that a plaque volumetric analysis may be employed as a measure of stroke risk. The fact that no clear correlation was found between the presence of cerebral emboli and the degree of stenosis reflects the low risk of stroke observed in asymptomatic severe carotid stenosis. The Authors argue that if such results should be confirmed in larger studies, the measurement of plaque volume could replace the severity of stenosis as the main indication for CEA. The ultimate future objective may be the institution of population screening protocols for carotid disease (48).
Along these lines, a retrospective study conducted on 72 consecutive patients by Saba et al. explored the association between CPV (considering both total volume and subcomponents) and the presence of cerebral microbleeds. Carotid arteries were studied on CT while brain was examined on MRI. The results confirmed not only that an association exists between the presence of microbleeds and symptoms, but also that higher relative volumes of fatty component reflect a greater number of cerebral microbleeds (49).
Several imaging methodologies have been so far employed in the volumetric analysis of the carotid plaque, each one having specific advantages and drawbacks. Continuous technological advances and clinical implementations are taking place making this a rapidly evolving scenario.
Two-dimensional ultrasound with color and spectral Doppler currently represents the mainstay for non-invasive assessment of carotid atherosclerosis in most clinical realities. US allows for measurements such as the carotid intima-media thickness (IMT) and the severity of carotid artery stenosis, which have long been considered a reliable estimate of the risk for ischemic cardiovascular events in patients with a history of ischemic stroke or TIA. Nonetheless, the prognostic value of IMT as a risk marker has recently been questioned in a large meta-analysis (50). Moreover, it must be considered that US assessment of stenosis is based on Doppler measurements which, by their own nature, are influenced by a number of technical and hemodynamic variables which make results from this technique, to some degree, inconsistent and highly operator-dependent. Overall, while on the one hand sonography is widely available and ideally suited for screening protocols, on the other hand it is operator-dependent and lacks spatial, temporal and contrast resolution when compared to other modalities such as MRI and CT.
One of the most promising advances in recent years has been the development of three-dimensional US imaging techniques. Recently published works have explored with good results the potential of three-dimensional US in the identification of CPV, severity of stenosis, plaque morphological aspects, composition and the capacity to monitor plaque progression over time. For instance, Kalashyan et al. analyzed the carotid plaque of 137 consecutive patients with a history of stroke or TIA employing a new automated three-dimensional ultrasound system equipped with a single-sweep volumetric transducer; their results indicate for this technique good feasibility and reproducibility in plaque volumetric measurements. Besides, good speed of acquisition and operator-friendly measurements represent additional value; the Authors suggest this method deserves further investigation (51,52).
Classically, MRI has been regarded as a technique of high accuracy and reliability in the definition of plaque components and total plaque burden (53). Nevertheless, MRI quantitative image interpretation is time-consuming, since a single sequence cannot provide tissue-specific signal intensity values; a differential analysis across multiple sequences is needed, which in turn results in longer processing times (39). Much of what has been reported with MR has also depended on the use of dedicated neck coils, which remain incompatible with the head coil often utilized in clinical protocol due to the need to document collateral perfusion, causing the multi-sequence work, however promising it may appear in research, to be inapplicable to clinical adoption. Moreover, MRI is expensive, not as widely available across institutions as other imaging modalities (34) and there appears to be a certain degree of variability among readers in terms of accuracy and reproducibility. In order to address the latter issue, large-scale multicenter studies tested automatic in vivo segmentation against manual reading in order to evaluate if automated methods may involve time-saving and a reduction of inter-reader variability. In this regard, Yoneyama et al. compared a semi-automatic approach versus expert human reading in the analysis of carotid atherosclerosis progression. Results showed that expert human review and an automatic atherosclerotic plaque segmentation algorithm performed similarly (54).
While Multidetector Computed Tomographic Angiography (MDCTA) has long been employed in the measurement of the degree of luminal stenosis, recent research indicates that MDCTA is also effective in the qualitative and quantitative evaluation of the atherosclerotic plaque in terms of its volume and subcomponents (55).
Sheahan et al. conducted a study based on thirty-one consecutive patients with atherosclerosis enrolled for endarterectomy. The Authors extracted 239 CT angiographic cross sections and matched them with the corresponding histopathological examination of the surgical specimen. Results showed a high level of correlation between the images of the plaque tissue obtained in vivo, analyzed via an image processing algorithm, and the histopathologic quantitative measures obtained ex vivo. The Authors sustain that the reliability of conventional MDCTA in the depiction of plaque tissue characteristics has so far received little attention in the literature when compared to the more classically recognized role of MRI. Conversely, as a result of the objective basis on which the voxel values are obtained in CT, material densities allow for more quantitative information than the signal intensity used with MR imaging, particularly when processed using algorithms such as described by Sheahan. Additionally, the speed of acquisition and high spatial resolution of MDCTA bring promise of more widespread use in the future clinical setting (39). This work remains at this time the only regulatory approved method, on any non-invasive modality, and with performance characteristics published in the label (56).
For what concerns the reproducibility of results in MDCTA images, a number of published works tested dedicated software for volumetric and compositional analysis of carotid arteries; results indicate that MDCTA offers reasonable observer variability (57,58).
With regards to the most significant feature of plaque vulnerability, namely intraplaque hemorrhage (IPH), recent evidence suggests that MDCTA can identify this important biomarker with reasonable consistency. In this regard, Saba et al. conducted a MDCTA study on 246 carotid arteries (123 patients) with the intent to assess the relationship between volume and percentage of IPH and acute cerebrovascular events (stroke and TIA). Subcomponent volumes were semi-automatically quantified via a dedicated software (Elucid Bioimaging, Wenham, Massachusetts) (Figures 1-4). This software allows for the identification of specific tissue types within a given volume; whereas the trained algorithm includes HU limits loosely analogous to thresholds, the algorithm does not use them as strict thresholds, instead utilizing an iterative image restoration coupled with an optimizer to more properly match the heterogeneous nature of the tissues of interest to better align with how pathologists mark the tissue when such tissue is available to them. The lumen, calcium, LRNC, IPH, and matrix components are visually differentiable in a contrast CTA, but blurring and partial volume effects reduce accurate measurement. Moreover, different focal regions of the same tissue type may vary widely in true underlying density and contrast uptake. The software uses the lumen boundary to determine the scanner blur and then optimize component densities assessed at sub-voxel boundaries in order to best fit the observed image. Descriptions of the software’s algorithms are included in the work by Sheahan et al. (39), with more specific image processing detail available in its online supplement. In particular, five classes were identified; the first three correspond to the classes already identified in the work of De Weert et al. (57), namely (reference HU values given here to facilitate continuity in the literature): lipid tissue (<60 HU), fibrous tissue (between 60 and 130 HU) and calcium (>130 HU); within the lipid range two further classes were identified in accordance with the findings of Saba et al. (37), namely: IPH (<25 HU) and lipid-IPH (between 26 and 59 HU). The Authors also calculated the relative percentage per single subcomponent and a ratio of IPH/lipid volume (no IPH: value 0; total plaque occupied by IPH: value 1). Results demonstrated a clear association between the presence of ipsilateral cerebrovascular events and the following: absolute volume of IPH, relative lipid content and ratio of IPH/lipid volume (59).
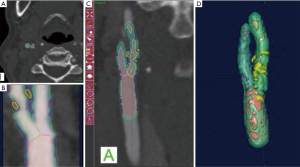
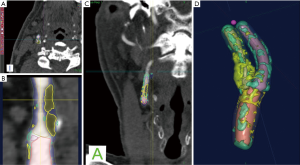

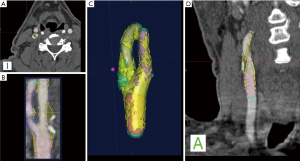
CT shows exceptional sensitivity in the evaluation of the calcific component of the plaque. In fact, the assessment of calcium content can be performed semi-quantitatively (using calcium score) or quantitatively (using direct volume analysis of plaque components). In a MDCTA study, De Weert et al. analyzed the CPV and calcification volumes of 56 patients with a history of TIA or minor stroke. Notably, this study showed no intimate relationship between the volume of carotid calcifications and atherosclerotic CPV (60). Despite the clear contrast difference, it is widely reported that CTA overestimates calcium, with the term “calcium blooming”. An important aspect of the image restoration and newer processing summarized above, it is possible to return to values for correctly aligning with a histological true calcification, but care must be taken to use appropriate algorithms and always to have defined performance characterized relative to truth so as to avoid previous artifacts.
Interestingly, calcium appears to have a complex relationship with the carotid artery plaque. A cross-sectional study of 229 carotid plaques, revealed the presence of two types of calcium in atherosclerotic plaques: hydroxyapatite and calcium oxalate. Hydroxyapatite calcification appears to have an association with vulnerable carotid plaques, while calcium oxalate calcifications were seen mostly in stable plaques. In this regard, it is interesting to note that multi-energy CT scanners have the potential to distinguish between hydroxyapatite and calcium oxalate calcifications, meaning that there may be an increased interest in this technology in the future (28).
Finally, artificial intelligence could play a fundamental role in the future of carotid disease imaging. Recent advances in this field of enquiry are paving the road towards the creation of novel predictive methods which may soon find clinical implementation. Deep learning models could be trained to identify imaging biomarkers which could aid in risk stratification, even with the ability to incorporate imaging features from different techniques (59,61-63). One particularly interesting area is the use of multi-scale in silico modeling of tissue characterized by CTA for determination of molecular mechanism (64), and as a response marker for biologic therapies in systemic inflammatory diseases (65).
Plaque imaging and clinical implications
Valuable information for clinicians
A large study carried out by Deseive et al., which included over 1,500 patients monitored over a 5.5-year period, demonstrated that MDCTA identification of coronary plaques and subsequent characterization of discrete plaque components may represent an effective tool to predict patient outcome (66).
Similar results have been shown in carotid arteries. A number of studies show that MDCTA-derived plaque features, analyzed via automated-model based algorithm, significantly improve cardiovascular events prognostication when compared to the observation of clinical risk factors alone (39,63,67).
There is abundant evidence in the literature that carotid revascularization in symptomatic patients (with a recent history of TIA or stroke) and moderate (50–69%) to severe (70–99%) stenosis, represents an effective measure against the recurrence of stroke.
However, in a longitudinal study conducted on over 800 patients with a clinical history of stroke, 44 (90%) out of the 49 people who presented with moderate to severe stenosis and remained untreated, did not have a recurrence of stroke over a 5-year span (68).
In a systematic review published by Abbot, it is argued that since the years in which large randomized trials for asymptomatic severe carotid stenosis were published (1983–2003), significant advances have been made in vascular disease medical care. The Author suggests that, in the light of such change of scenario, the indication for surgical intervention should be reconsidered. Rates of recurrent stroke with medical treatment alone have sharply fallen since the mid-1980s: recent estimates show overlapping results between patients treated surgically or medically. Such evidence is of high relevance when one considers that current medical care alone appears to be at least 3 to 8 times more cost-effective than surgical approaches. The Author concludes that medical treatment should nowadays be favored over interventional strategies for stroke prevention in the asymptomatic patient with severe carotid stenosis (69).
Nonetheless, this appears to be a topic that raises a certain degree of controversy in the literature. Interestingly, some Authors debate on what should be considered as the very endpoint of carotid revascularization nowadays. Classically, the role of carotid revascularization has been to prevent recurrence of stroke in patients with known severe stenosis. However, some Authors bring the attention to the potential virtuous effect of carotid revascularization on cognitive function, based on the hypothesis that revascularization would supposedly resolve a condition of chronic hypoperfusion to the brain tissues. While the debate is ongoing, to present date there is, however, no sufficient evidence in the literature regarding repercussions of endarterectomy on higher cognitive functions (70).
Porcu et al. conducted a prospective exploratory study on 14 asymptomatic consecutive patients with significant unilateral internal carotid artery stenosis enrolled for endarterectomy. Their analysis aimed at assessing whether CEA carried out on these patients would be followed by mid-term reorganization in brain networks connectivity. Resting state functional connectivity magnetic resonance (fc-rsMR) was employed in this study, especially focused on the Default Mode Network (DMN). The results showed a degree of reorganization of functional connectivity associated to an improvement in neurocognitive performance (mini-Mental State Examination) following endarterectomy (71).
Along the same lines, a study by Picchetto et al. tested whether the resolution of a carotid stenosis was followed by modifications in cognitive functions in 22 patients who enrolled with asymptomatic stenosis. Also in this work the Authors, albeit with some contrasting results, show findings that support the hypothesis that the resolution of a hypoperfusion state improves brain function at a cognitive level (72).
In such complex scenario, where much debate is still ongoing with regards to the appropriateness of medical rather than surgical intervention, imaging features of carotid plaque may provide clinicians with some discriminating information which, once standardized and implemented in the clinical routine, may help in situations of difficult decision making.
For instance, plaque imaging may help selecting patients in whom, regardless of the degree of stenosis, features of a stable plaque are identified, suggesting that surgical intervention may not be necessary. Conversely, plaque imaging analysis may also help identifying symptomatic patients in whom vulnerable plaque features prompt the need of surgical approaches despite only mild stenosis (28,39,73).
Overall, in the light of growing evidence that stroke is often caused by vulnerable carotid plaques even in the absence of marked stenosis (>50%), defining plaque features of vulnerability acquires significant importance. A number of ongoing prospective trials (i.e., CREST, ECST-2, ACAS-2), which focus on the influence of carotid plaque components in the development of stroke in patients with substenotic carotid arteries, will hopefully shed new light in this complex and rapidly evolving scenario (28).
Although the atherosclerotic disease has generally been considered a chronic and irreversible process, it is now widely accepted that high-dose lipid-lowering therapy can contribute to the partial regression of the disease. In recent years, imaging studies have contributed considerably to the definition and quantification of plaque regression (58). In the longitudinal Rotterdam Study, which enrolled over of 1,700 patients, carotid plaque MRI analysis were conducted on a population of subjects under high-dose statin treatment. The study documented beneficial results from therapy, in particular, demonstrated a shift in composition of the plaque, from a vulnerable LRNC to a more stable calcified plaque (34).
Finally, it has been shown that carotid plaque imaging may also serve as a surrogate marker of disease within other vascular beds. In fact, carotid atherosclerosis has been correlated to the presence of coronary artery disease and its clinical manifestations (i.e., angina, myocardial infarction and coronary atherosclerosis related death).
Cost effectiveness
Evidence shows that plaque-feature analysis may contribute towards making treatment decisions beneficial in terms of cost-effectiveness. Cost-effectiveness analysis is an approach that can help clinicians in making decisions that incorporate aspects of economic impact, weighing the advantages related to risk prevention against their related implementation costs (28).
In a model-analysis study published by Gupta et al., two competing stroke prevention strategies were compared: on one side, a best medical care approach; on the other side, an imaging-based strategy that identified a subset of patients (with asymptomatic carotid artery stenosis and IPH) treated with CEA in addition to medical therapy. Results showed that MRI of intraplaque hemorrhage proved to be a cost-effective method to identify patients most likely to benefit from endarterectomy, with subsequent benefits in terms of both life expectancy and costs (74).
Within this context, Sheahan et al. explored the potential of using clinically routine MDCTA against more time-consuming and less widely available MRI multisequence protocols, identifying for MDCTA better cost-effectiveness (39).
Conclusions
There is broad agreement in the literature focused on carotid atherosclerotic disease that a change of perspective has finally taken place in the way we look at the atherosclerotic plaque, shifting the attention from sole severity of stenosis to the identification of features of plaque vulnerability. In particular, the presence of intraplaque hemorrhage (IPH) appears to be the strongest predictor of future acute cerebrovascular events. While past literature has focused on the primary role of MRI for the in the identification of IPH, recent evidence shows that CT can reliably and accurately perform quantitative and qualitative analysis of carotid atherosclerotic plaques, including the identification of IPH. Various other imaging modalities are currently being put to the test, from contrast enhanced ultrasound to molecular imaging among others, within a scenario that is rapidly evolving; in particular, machine learning techniques may become a reality in the near future. While research is ongoing, imaging studies can already offer a viable tool in the clinical setting. In particular, there appear to be contrasting opinions in the literature with regards to the indications for endarterectomy procedures, with some Authors suggesting that NASCET guidelines should be partly reconsidered. Within this context, the identification of imaging features of plaque vulnerability may provide clinicians with additional tools to approach difficult decision making.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.03.01). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from July 2019 to June 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barquera S, Pedroza-Tobías A, Medina C, et al. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch Med Res 2015;46:328-38. [Crossref] [PubMed]
- Naghshtabrizi B, Moradi A, Amiri J, et al. An Evaluation of the Numbers and Locations of Coronary Artery Disease with Some of the Major Atherosclerotic Risk Factors in Patients with Coronary Artery Disease. J Clin Diagn Res 2017;11:OC21-4. [PubMed]
- Yanez ND, Burke GL, Manolio T, et al. Sibling history of myocardial infarction or stroke and risk of cardiovascular disease in the elderly: the Cardiovascular Health Study. Ann Epidemiol 2009;19:858-66. [Crossref] [PubMed]
- Truelsen T, Begg S, Mathers C. The global burden of cerebrovascular disease. Geneva: World Health Organization; 2000. Available online: http://www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf, Accessed October 30, 2019.
- Ooi YC, Gonzalez NR. Management of extracranial carotid artery disease. Cardiol Clin 2015;33:1-35. [Crossref] [PubMed]
- van Gils MJ, Vukadinovic D, van Dijk AC, et al. Carotid atherosclerotic plaque progression and change in plaque composition over time: a 5-year follow-up study using serial CT angiography. AJNR Am J Neuroradiol 2012;33:1267-73. [Crossref] [PubMed]
- Stary HC, Chandler AB, Dinsmore RE, et al. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995;92:1355-74. [Crossref] [PubMed]
- van Gils MJ, Vukadinovic D, van Dijk AC, et al. Carotid atherosclerotic plaque progression and change in plaque composition over time: a 5-year follow-up study using serial CT angiography. AJNR Am J Neuroradiol 2012;33:1267-73. [Crossref] [PubMed]
- Saba L, Raz E, Anzidei M, et al. Differences in Plaque Morphology and Correlation of Stenosis at the Carotid Artery Bifurcation and the Carotid Siphon. AJR Am J Roentgenol 2013;201:1108-14. [Crossref] [PubMed]
- Anzidei M, Suri JS, Saba L, et al. Longitudinal assessment of carotid atherosclerosis after Radiation Therapy using Computed Tomography: A case control Study. Eur Radiol 2016;26:72-8. [Crossref] [PubMed]
- Mannelli L, MacDonald L, Mancini M, et al. Dualvenergy computed tomography quantification of carotid plaques calcification: comparison between monochromatic and polychromatic energies with pathology correlation. Eur Radiol 2015;25:1238-46. [Crossref] [PubMed]
- Saba L, Argioas GM, Lucatelli P, et al. Variation of degree of stenosis quantification using different Energy level with dual energy CT scanner. Neuroradiology 2019;61:285-91. [Crossref] [PubMed]
- Das M, Braunschweig T, Mühlenbruch G, et al. Carotid plaque analysis: comparison of dual-source computed tomography (CT) findings and histopathological correlation. Eur J Vasc Endovasc Surg 2009;38:14-9. [Crossref] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-53. [Crossref] [PubMed]
- Barnett HJ, Taylor DW, Eliasziw M, et al. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339:1415-25. [Crossref] [PubMed]
- European Carotid Surgery Trialists' Collaborative Group. MCR European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991;337:1235-43. [Crossref] [PubMed]
- European Carotid Surgery Trialists' Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379-87. [Crossref] [PubMed]
- Mofidi R, Green BR. Carotid Plaque Morphology: Plaque Instability and Correlation with Development of Ischaemic Neurological Events. IntechOpen. Available online: http://dx.doi.org/ [Crossref]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108:1664-72. [Crossref] [PubMed]
- Saba L, Anzidei M, Sanfilippo R. at al. Imaging of the carotid artery. Atherosclerosis 2012;220:294-309. [Crossref] [PubMed]
- Schwarz F, Bayer-Karpinska A, Poppert H, et al. Serial carotid MRI identifies rupture of a vulnerable plaque resulting in amaurosis fugax. Neurology 2013;80:1171-2. [Crossref] [PubMed]
- Saba L, Sanfilippo R, Sannia S, et al. Association between carotid artery plaque volume, composition, and ulceration: a retrospective assessment with MDCT. AJR Am J Roentgenol 2012;199:151-6. [Crossref] [PubMed]
- Saba L, Yuan C, Hatsukami TS, et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018;39:E9-31. [Crossref] [PubMed]
- Saba L, Anzidei M, Marincola BC, et al. Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol 2014;37:572-85. [Crossref] [PubMed]
- Selwaness M, Hameetemanb R, Kloosterc RV, et al. Determinants of carotid atherosclerotic plaque burden in a stroke-free population. Atherosclerosis 2016;255:186-92. [Crossref] [PubMed]
- Hingwala DR, Chandrasekhakan K, Thomas B, et al. Atherosclerotic Carotid Plaques: Multimodality Imaging with Contrast-enhanced Ultrasound, Computed Tomography, and Magnetic Resonance Imaging. Ann Indian Acad Neurol 2017;20:378-86. [Crossref] [PubMed]
- Hingwala D, Kesavadas C, Sylaja P N, et al. Multimodality imaging of carotid atherosclerotic plaque: Going beyond stenosis. Indian J Radiol Imaging 2013;23:26-34. [Crossref] [PubMed]
- Saba L, Saam T, Jäger H R, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. [Crossref] [PubMed]
- Porcu M, Anzidei M, Suri JS, et al. Carotid artery imaging: The study of intra-plaque vascularization and hemorrhage in the era of the “vulnerable” plaque. J Neuroradiol 2019. [Crossref] [PubMed]
- Daghem M, Bing R, Fayad Z A, et al. Noninvasive Imaging to Assess Atherosclerotic Plaque Composition and Disease Activity: Coronary and Carotid Applications. JACC Cardiovasc Imaging 2020;13:1055-68. [Crossref] [PubMed]
- Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071-7. [Crossref] [PubMed]
- Saba L, Lanzino G, Lucatelli P, et al. Carotid Plaque CTA Analysis in Symptomatic Subjects with Bilateral Intraparenchymal Hemorrhage: A Preliminary Analysis. AJNR Am J Neuroradiol 2019;40:1538-45. [PubMed]
- Liu Y, Wang M, Zhang B, et al. Size of carotid artery intraplaque hemorrhage and acute ischemic stroke: a cardiovascular magnetic resonance Chinese atherosclerosis risk evaluation study. J Cardiovasc Magn Reson 2019;21:36. [Crossref] [PubMed]
- Singh N, Moody AR, Roifman I, et al. Advanced MRI for carotid plaque imaging. Int J Cardiovasc Imaging 2016;32:83-9. [Crossref] [PubMed]
- Kramer CM, Narula J. Atherosclerotic plaque imaging: the last frontier for cardiac magnetic resonance. JACC Cardiovasc Imaging 2009;2:916-8. [Crossref] [PubMed]
- Brinjikji W, DeMarco JK, Shih R, et al. Diagnostic accuracy of a clinical carotid plaque MR protocol using a neurovascular coil compared to a surface coil protocol. J Magn Reson Imaging 2018;48:1264-72. [Crossref] [PubMed]
- Saba L, Francone M, Bassareo PP, et al. CT Attenuation Analysis of Carotid Intraplaque Hemorrhage. AJNR Am J Neuroradiol 2018;39:131-7. [Crossref] [PubMed]
- Huibers A, de Borst GJ, Wan S, et al. Non-invasive Carotid Artery Imaging to Identify the Vulnerable Plaque: Current Status and Future Goals. Eur J Vasc Endovasc Surg 2015;50:563e572.
- Sheahan M, Ma X, Paik D, et al. Atherosclerotic plaque tissue: noninvasive quantitative assessment of characteristics with software-aided measurements from conventional CT angiography. Radiology 2018;286:622-631. [Crossref] [PubMed]
- Kerwin W, Hooker A, Spilker M, et al. Quantitative Magnetic Resonance Imaging Analysis of Neovasculature Volume in Carotid Atherosclerotic Plaque. Circulation 2003;107:851-6. [Crossref] [PubMed]
- Huang R, Abdelmoneim SS, Ball CA, et al. Detection of carotid atherosclerotic plaque neovascularization using contrast enhanced ultrasound: a systematic review and meta-analysis of diagnostic accuracy studies. J Am Soc Echocardiogr 2016;29:491-502. [Crossref] [PubMed]
- Saba L, Lai ML, Montisci R, et al. Association between carotid plaque enhancement shown by multidetector CT angiography and histologically validated microvessel density. Eur Radiol 2012;22:2237-45. [Crossref] [PubMed]
- Saba L, Gao H, Raz E, et al. Semiautomated analysis of carotid artery wall thickness in MRI. J Magn Reson Imaging 2014;39:1457-67. [Crossref] [PubMed]
- Saha SA, Gourineni V, Feinstein SB. The use of contrast-enhanced ultrasonography for imaging of carotid atherosclerotic plaques: current evidence, future directions. Neuroimaging Clin N Am 2016;26:81-96. [Crossref] [PubMed]
- Stone GW, Maehara A, Lansky AJ, et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. [Crossref] [PubMed]
- Dong L, Underhill HR, Yu W, et al. Geometric and compositional appearance of atheroma in an angiographically normal carotid artery in patients with atherosclerosis. AJNR Am J Neuroradiol 2010;31:311-6. [Crossref] [PubMed]
- Saam T, Underhill HR, Chu B, et al. Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol 2008;51:1014-21. [Crossref] [PubMed]
- Ball S, Rogers S, Kanesalingam K, et al. Carotid plaque volume in patients undergoing carotid endarterectomy. Br J Surg 2018;105:262-9. [Crossref] [PubMed]
- Saba L, Sanfilippo R, di Martino M, et al. Volumetric Analysis of Carotid Plaque Components and Cerebral Microbleeds: A Correlative Study. J Stroke Cerebrovasc Dis 2017;26:552-8. [Crossref] [PubMed]
- Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 2012;220:128-33. [Crossref] [PubMed]
- Kalashyan H, Shuaib A, Gibson P, et al. Single sweep three-dimensional carotid ultrasound: reproducibility in plaque and artery volume measurements. Atherosclerosis 2014;232:397-402. [Crossref] [PubMed]
- Kalashyan H, Saqqur M, Shuaib A, et al. Carotid plaque volume changes of stroke and aTIA patients in six months follow up period: observation a study with new three-dimensional carotid ultrasound. Can J Cardiol 2015;31:S230-1. [Crossref]
- Keenan NG, Sheppard MN, Grasso A, et al. Validation of carotid arterial wall volume measurement by cardiovascular magnetic resonance. J Magn Reson Imaging 2010;31:935-41. [Crossref] [PubMed]
- Yoneyama T, Sun J, Hippe DS, et al. In vivo semi-automatic segmentation of multicontrast cardiovascular magnetic resonance for prospective cohort studies on plaque tissue composition: initial experience. Int J Cardiovasc Imaging 2016;32:73-81. [Crossref] [PubMed]
- Saba L, Raz E, Grassi R, et al. Association between the volume of carotid artery plaque and its subcomponents and the volume of white matter lesions in patients selected for endarterectomy. AJR Am J Roentgenol 2013;201:W747-52. [Crossref] [PubMed]
- vascuCAP (Image processing system). Department of Health and Human Services - Food and Drug Administration. Available online: https://fda.report/PMN/K183012/18/K183012.pdf
- de Weert TT, de Monyé C, Meijering E, et al. Assessment of atherosclerotic carotid plaque volume with multidetector computed tomography angiography. Int J Cardiovasc Imaging 2008;24:751-9. [Crossref] [PubMed]
- Chrencik MT, Khan AA, Luther L, et al. Quantitative assessment of carotid plaque morphology (geometry and tissue composition) using computed tomography angiography. J Vasc Surg 2019;70:858-68. [Crossref] [PubMed]
- Saba L, Micheletti G, Brinjikji W, et al. Carotid Intraplaque-Hemorrhage Volume and Its Association with Cerebrovascular Events. AJNR Am J Neuroradiol 2019;40:1731-7. [PubMed]
- de Weert TT, Rozie S, Meijering E, et al. Is There a Relationship Between Carotid Calcifications and Atherosclerotic Carotid Plaque Volume? Radiological Society of North America 2006 Scientific Assembly and Annual Meeting.
- Saba L, Biswas M, Kuppili V, et al. The present and future of deep learning in radiology. Eur J Radiol 2019;114:14-24. [Crossref] [PubMed]
- Wan T, Madabhushi A, Phinikaridou A, et al. Spatio-temporal texture (SpTeT) for distinguishing vulnerable from stable atherosclerotic plaque on dynamic contrast enhancement (DCE) MRI in a rabbit model. Med Phys 2014;41:042303. [Crossref] [PubMed]
- Zhu G, Li Y, Ding V, et al. Semiautomated characterization of carotid artery plaque features from computed tomography angiography to predict atherosclerotic cardiovascular disease risk score. J Comput Assist Tomogr 2019;43:452-9. [Crossref] [PubMed]
- NHLBI Coronary CT Angiography (CTA) Summit. 2019. Available online: https://videocast.nih.gov/summary.asp?live=35292&bhcp=1
- Choi H, Aksentijevich M, Dey AK, et al. Treatment of Psoriasis With Biologic Therapy is Associated With Regression of Coronary Artery Plaque Necrotic Core and Positive Remodeling: Results From a Prospective, Observational Pilot Study. Circulation 2019;140:A11998.
- Deseive S, Straub R, Kupke M, et al. Automated Quantification of Coronary Plaque Volume From CT Angiography Improves CV Risk Prediction at Long-Term Follow-Up. JACC Cardiovasc Imaging 2018;11:280-2. [Crossref] [PubMed]
- van Assen M, Varga-Szemesa A, Schoep J, et al. Automated plaque analysis for the prognostication of major adverse cardiac events. Eur J Radiol 2019;116:76-83. [Crossref] [PubMed]
- Fairhead JF, Mehta Z, Rothwell P M. Population-based study of delays in carotid imaging and surgery and the risk of recurrent stroke. Neurology 2005;65:371-75. [Crossref] [PubMed]
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009;40:e573-83. [Crossref] [PubMed]
- De Rango P, Caso V, Leys D, et al. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke 2008;39:3116-27. [Crossref] [PubMed]
- Porcu M, Craboledda D, Garofalo P, et al. Reorganization of brain networks following carotid endarterectomy: an exploratory study using resting state functional connectivity with a focus on the changes in Default Mode Network connectivity. Eur J Radiol 2019;110:233-41. [Crossref] [PubMed]
- Picchetto L, Spalletta G, Casolla B, et al. Cognitive Performance following Carotid Endarterectomy or Stenting in Asymptomatic Patients with Severe ICA Stenosis. Curr Neurovasc Res 2016;13:45-9. [Crossref] [PubMed]
- Naim C, Douziech M, Therasse E, et al. Vulnerable atherosclerotic carotid plaque evaluation by ultrasound, computed tomography angiography, and magnetic resonance imaging: an overview. Can Assoc Radiol J 2014;65:275-86. [Crossref] [PubMed]
- Gupta A, Mushlin AI, Kamel H, et al. Cost-effectiveness of carotid plaque MR imaging as a stroke risk stratification tool in asymptomatic carotid artery stenosis. Radiology 2015;277:763-72. [Crossref] [PubMed]


