
Obesity-related ventricular remodelling is exacerbated in dilated and hypertrophic cardiomyopathy
Introduction
Obesity is a clear risk factor for the development of heart failure (1), and weight loss can reduce this risk significantly (2). This has been attributed, in part, to the characteristic left ventricular (LV) cavity dilatation and additional concentric LV hypertrophy (LVH) that occur in obesity (3-5), are linked to poor outcome (6,7) and are reversed with weight loss (8). However, the effects of obesity in established cardiac diseases appear to be less predictable and currently are far from clear. While obesity apparently improves outcomes in systolic heart failure (9,10), it also impacts negatively on the clinical course in both hypertrophic cardiomyopathy (11) and aortic stenosis (12). The reasons for these differences are unknown but this raises the question whether cardiac diseases that are characterised by concentric LV remodelling respond differently to obesity than those that exhibit a more dilated phenotype. If the response of the heart to obesity is modified by the underlying disease, this might explain the variations in obesity-related outcomes across differing cardiac pathologies.
The response of the normal heart to obesity is complex. Excess adiposity imposes an increased metabolic demand on the body, and thus both cardiac output and total blood volume are elevated in obesity (13). This hyperdynamic circulation causes LV cavity dilatation and elevated LV mass (5). In addition, the hormonal milieu in obesity contributes, with insulin and leptin both stimulating hypertrophy. The effects of obesity on the clinical course of established cardiac diseases are not well understood, are not uniform, and are often paradoxical. The response of the LV to obesity in established disease are also sparsely investigated. Studies have shown increased ventricular remodelling in both aortic stenosis (14) and hypertrophic cardiomyopathy (11), diseases where hypertrophy has traditionally been regarded as independent from environmental influences. This suggests not only that obesity could alter the clinical course of established cardiac diseases, but also that it should itself be considered a modifiable risk factor for disease progression. Understanding how obesity affects the heart in established cardiac diseases will further our understanding of whether weight loss should be employed as an additional therapeutic strategy.
Therefore, the aim of this study was to use CMR to investigate (I) whether LV adaptation to obesity occurs in established cardiac disease, (II) if present, whether the effect is similar in magnitude to that seen without established cardiac disease and, (III) whether the changes are similar between a disease characterised by hypertrophy in the absence of cavity dilatation (hypertrophic cardiomyopathy, HCM) and one by dilatation (dilated cardiomyopathy, DCM).
Methods
We examined all cardiac magnetic resonance imaging (CMR) scans undertaken for clinical purposes between 2009 and 2015 (n=9,068) in our tertiary-referral CMR unit, in Oxford, United Kingdom. For this analysis we selected the scans of patients with a clear diagnosis of DCM or HCM, or those which were reported unequivocally as a normal cardiac study, with normal biventricular mass, volumes and systolic function, with no evidence of valve disease or late gadolinium enhancement. This work was compiled from several studies with National Research Ethics Committee approval, in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. Informed written consent was obtained from all patients.
In total, 1,570 scans (17%) were included in the analysis, separated into the following cohorts as determined by at least one consultant cardiologist with significant CMR expertise (>5 years): (I) normal CMR scan (n=728; BMI range, 16–57 kg/m2), (II) typical DCM with LVEF under 57% (determined as the lower limit of normal LVEF in the Oxfordshire population) with no evidence of myocardial infarction on CMR imaging, or history of ischaemic heart disease, (n=529, BMI range 16–61 kg/m2), and 3) hypertrophic cardiomyopathy, with a typical pattern of asymmetrical LV hypertrophy (>15 mm wall thickness) and/or typical gadolinium enhancement pattern (n=297, BMI range: 18–50 kg/m2). Scans were excluded if more than mild valvular heart disease or any myocardial infarction was present. Any scan where more than one likely pathology was identified was also excluded. Patients aged under 16 were also excluded.
Magnetic resonance imaging of the left ventricle
All MR scans for the assessment of myocardial mass, volumes and ejection fraction were performed on a 1.5 Tesla MR system (Siemens Medical Solutions, Erlangen, Germany). All imaging was cardiac gated with a precordial ECG and acquired during end expiration breath-hold. Images were acquired using a steady state free precession (SSFP) sequence with an echo time (TE) of 1.5 ms, a repetition time (TR) of 3.0 ms, temporal resolution 47.84 ms and a flip angle of 60° as previously described (15). SSFP cine sequences were used to acquire localisation images followed by a SSFP left and right ventricular short axis stack of contiguous images with a slice thickness of 7mm and an interslice gap of 3 mm.
Data analysis
Image analysis for left ventricular volumes and mass was performed either using Siemens analytical software (ARGUS©, 2009 to 2011) or cmr42© (Circle Cardiovascular Imaging Inc, Calgary, Canada, 2011 to 2015) (16). The short axis stack was analysed manually, contouring the endocardial borders from base to apex at end-diastole and end-systole, excluding papillary muscles. The epicardial border was contoured at end-diastole to yield myocardial mass and wall thickness. Left ventricular mass (g) was calculated as the epicardial volume minus the endocardial volume multiplied by 1.05 (specific gravity of myocardium) as previously reported (4).
Statistical analysis
All statistics were analysed using a commercial software package (SPSS 22; SPSS, Chicago, Ill). ANOVA analysis was performed, with Bonferroni correction to compare baseline variables. Linear regression analysis was used to assess the effect of BMI on LV mass, EDV and LV mass to volume ratio (LV MVR) for each of the groups. Residuals were used to confirm normality of distribution and homoscedasticity. To compare coefficient of regression between disease groups and the normal heart, dummy variable regression analysis was performed. All regression models were adjusted to account for the effects of age. Values of P<0.05 were considered as statistically significant.
Results
Anthropomorphic data
Table 1 and Figure 1 indicates anthropomorphic and left ventricular data from the patients, separated into groups according to cardiac disease. Although body mass index was statistically different between some groups, the difference in absolute range between groups (lowest BMI group normal heart 27±6 kg/m2; highest BMI group HCM 29±5 kg/m2) was small.
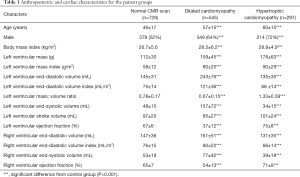
Full table
Disease differences in ventricular geometric remodelling in obesity
Dilated cardiomyopathy
Although LV stroke volume was positively correlated with BMI in both DCM (r=0.17, P<0.001) and normal hearts (r=0.17, P<0.001), there was no difference between the groups in the relationship between increasing stroke volume and increasing BMI (DCM +0.7 mL per BMI point increase, normal hearts +0.6 mL per BMI point increase, P=0.55). This suggests that the additional haemodynamic burden of obesity is independent from underlying cardiac status.
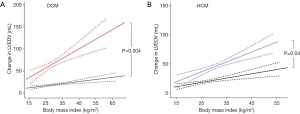
When examining the effect of this additional burden on LV geometry, increasing BMI was related to increased LV EDV in both dilated cardiomyopathy (r=0.18, P<0.001) and in normal hearts (r=0.14, P<0.001). However, when comparing the coefficient of regression between BMI and LV EDV in DCM and normal hearts, patients with DCM exhibited a 3-fold greater LV dilatation in response to increasing BMI (DCM +2.2 mL vs. normal heart +0.7 mL per BMI point increase, P=0.004, Figure 2). Interestingly, whereas in normal heart the dilatation associated with BMI was only seen in women (+1.1 mL vs. males +0.3 mL per unit increase in BMI, P=0.03), the increase in cavity size with BMI in DCM was seen in both men and women (male +2.7 mL vs. female 2.1 mL per unit increase in BMI, P=0.51).
Increasing BMI was also related to increased LV mass in both DCM (r=0.30, P<0.001) and normal hearts (r=0.26, P<0.001, Figure 3). Patients with DCM also exhibited a greater LV hypertrophic response to the same increase in BMI (DCM +2.2 g vs. normal heart 1.3 g per BMI point increase, P=0.01, Figure 3).
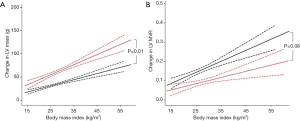
In order to establish whether this excess LV mass increase in DCM was proportional to the LV cavity dilation, we compared LV mass:volume ratio (LV MVR) between the two cohorts. LVMVR was positively correlated to increased BMI in both DCM (r=0.13, P<0.001) and normal hearts (r=0.20, P<0.001), suggesting an element of concentric remodelling occurs in both groups. However, in contrast to both EDV and LV mass, there was no significant difference in the degree of concentric remodelling between the groups (DCM +0.003 vs. normal heart +0.006 change in LVMVR per BMI point increase, P=0.08, Figure 3), albeit with a suggestion of a lesser degree of remodelling in DCM than might be expected. Taken together these data suggest that, although the stroke volume increase that accompanies obesity is consistent, and is accommodated by LV cavity dilatation, in established DCM a three-fold greater LV cavity dilatation occurs to generate the stroke volume increase than that seen in normal hearts. The increase in hypertrophy appears to be proportional to the increase in cavity size in the two groups.
Hypertrophic cardiomyopathy
LV stroke volume was positively correlated with BMI in both HCM (r=0.26, P<0.001) and normal hearts (r=0.17, P<0.001), and again no difference in the increase in LV stroke volume with increased BMI was observed between the groups (P=0.18).
Increasing BMI was again related to increased LV EDV in both HCM (r=0.27, P<0.001) and in normal hearts (r=0.14, P<0.001). However, when comparing the coefficient of regression between BMI and LV EDV in HCM and normal hearts, patients with HCM exhibited a more than 2-fold greater LV dilatation in response to increasing BMI (HCM +1.9 mL vs. normal heart +0.7 mL per BMI point increase, P=0.04, Figure 2).
Increasing BMI was also related to increased LV mass in both HCM (r=0.17, P=0.003) and normal hearts (r=0.26, P<0.001, Figure 4). When comparing the coefficient of regression between BMI and LV mass in HCM and normal hearts, although patients with HCM exhibited numerically a greater LV hypertrophic response to increasing BMI (HCM +2.3 g vs. normal heart +1.3 g per BMI point increase) this was not statistically significant when adjusted for age (P=0.10, Figure 4).
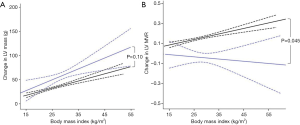
LV mass-to-volume ratio (LVMVR) was positively correlated to increased BMI in normal hearts (r=0.20, P<0.001) although only in men (MVR +0.01 per unit increase in BMI, women +0.002, P=0.051). In contrast to DCM, LVMVR was not related to BMI in patients with HCM (r=−0.03, P=0.63) irrespective of sex (P=0.69). This indicates that the degree of concentric remodelling, represented by increasing LVMVR, in response to increased BMI was greater in normal hearts than in HCM (HCM −0.002 vs. normal heart +0.006 per BMI point increase, P=0.045, Figure 4). In keeping with this finding, maximal LV wall thickness was not associated with increasing BMI in the HCM cohort (P=0.069).
Taken together these data suggest again that, despite a similar increase in LV stroke volume with increasing BMI, the LV cavity dilatation required to accommodate this in HCM was 2-fold greater than that seen in normal hearts. In addition, it suggests that in contrast to DCM and normal hearts, obesity does not appear to be the primary driver behind the LV hypertrophy seen in HCM.
Discussion
In the absence of overt cardiac disease, the cardiac effects of obesity and weight loss have been previously demonstrated and likely contribute to both the increased risk of developing heart failure and the risk reduction seen with weight loss (1-4,8). The response of the LV to obesity in coexisting disease is also sparsely investigated. This study has shown that the extent of LV remodelling can be significantly greater in established cardiac diseases, with LV cavity dilation in response to increasing BMI being three-fold greater in DCM, and over 2-fold greater in HCM than that observed in the normal heart. In addition, the concentric LV remodelling normally observed in response to obesity, although seen in DCM, was not seen in HCM. Given the link between LV remodelling patterns and mortality, these observations support the notion that obesity is a modifiable risk factor in established cardiac disease, and potentially of greater importance than in those with a normal heart.
Obesity and dilated cardiomyopathy
The combination of the fat expansion and increased skeletal muscle (15) in obesity results in a need for increased circulating volume (17) and as a result, LV stroke volume is increased to meet these demands (15). Assuming that the changes in body composition with increasing BMI remain similar across groups, it would be expected that this increase in demand for stroke volume would be a fixed haemodynamic load. This study supports this assumption, demonstrating that the LV stroke volume increase that accompanies increased BMI is not different between patients with and without DCM, and equates to around 6–7 mL per 10 kg/m2 increase in BMI. However, the means by which the LV accommodates the need for this increased stroke volume appear to be very different in DCM than in the normal heart.
In principal, the heart could augment stroke volume by either increasing end-diastolic volume or by decreasing end-systolic volume. In the normal heart, this increase in LV stroke volume is achieved solely by increasing end diastolic volume (+7 mL per 10 kg/m2 increase), with no change in end-systolic volume (P=0.24). This cavity dilatation would be in keeping with a compensatory response that allows the heart to increase LV stroke volume with a lesser degree of circumferential fibre shortening than would be required if end systolic volume was reduced as a means to augment stroke volume (18). However, the mechanical advantage conferred by this ventricular dilatation is offset by the co-existing increase in LV wall stress that occurs (in line with LaPlace’s law) and is associated with increased myocardial oxygen consumption (19). As LV dilatation is a known predictor for developing heart failure (20), this may in part explain why obesity is an independent risk factor for developing systolic dysfunction.
In contrast to the normal heart, the LV cavity dilatation that accompanies the increase in stroke volume with BMI was 3-fold larger in DCM (21 mL per 10 kg/m2) than in normal (7 mL per 10 kg/m2), and was three times that necessary to generate the change in LV stroke volume by itself.
End-diastolic volume increased more than end-systolic volume (EDV +2.1 mL vs. ESV +1.4 mL per BMI point increase, P=0.001). We postulate that, in order to gain the necessary mechanical advantage to further increase stroke volume in the already dilated and impaired heart in DCM, a greater cavity dilatation is needed. In other words, greater LV dilatation is needed to achieve the same stroke volume increase, and as greater end-diastolic dilatation occurs than is needed to increase stroke volume, this explains why end-systolic volume was also seen to increase with obesity in DCM.
Given that LV end-diastolic cavity size is a determinant of worse clinical outcomes in heart failure (21), this finding of disproportionate LV cavity dilation in obesity in DCM would be expected to be detrimental. However, on face value the opposite appears to be the case and obesity itself is paradoxically associated with better survival in non-ischaemic as well as ischaemic cardiomyopathies (9). The reasons for this are as yet unestablished, and indeed the findings of this study would indicate that only adverse remodelling occurs as a result of increasing body weight. Treatment strategies that reduce LV cavity size in heart failure have been shown to be beneficial (22), and intentional weight loss in obesity is known to reduce LV cavity size (7) in otherwise healthy individuals—therefore, intentional weight loss in DCM would be expected to be beneficial. The evidence for this, however, is not established, with no dedicated prospective trials being performed for intentional weight loss in DCM. This question is especially important given the current ESC guideline that in patients with HF with moderate degrees of obesity, weight loss cannot be recommended. It is clear that dedicated trials of intentional weight loss in heart failure are needed.
Obesity and hypertrophic cardiomyopathy
The impact of obesity on hypertrophic cardiomyopathy was recently assessed in an observational study of 275 patients (11) using CMR, and showed that obesity was associated with an increase in LV mass. As a result, the authors concluded that in HCM patients, obesity may dictate progression of heart failure symptoms. This study has reiterated that obesity is related to LV mass, but has identified further that the LV mass increase seen (>2 g per kg/m2) was only proportional to the LV cavity size increase (LV mass:volume ratio and BMI not being related in HCM, r=−0.02, P=0.63). This contrasts with normal hearts and DCM where additional concentric LV remodelling, with increased LV mass:volume ratio was seen. Additionally, we have demonstrated that maximal LV wall thickness in HCM was not related to BMI (P=0.069), as was previously shown but not explained in the aforementioned study (11). This would suggest that in HCM the distinct and specific processes that drive increasing wall thickness such as cardiomyocyte hypertrophy, myofibre disarray and fibrosis are independent from those reflecting the supraphysiological demand of obesity.
The finding that the LV cavity dilatation response to obesity was greater in HCM than that seen in normal hearts again suggests that, in order to gain the necessary mechanical advantage to increase stroke volume in a diseased heart, a greater increase in cavity dilation is needed than in the normal heart. Whilst overt systolic dysfunction is not common in the natural history (23), there are more subtle subclinical abnormalities of systolic function (24), whereas risk of sudden death is more related to wall thickness. Whether the increase in LV mass in HCM, which appears to be proportional to LV cavity size increase, predicts risk of developing heart failure, sudden cardiac death or is modifiable with intentional weight loss is unknown, but again dedicated trials are needed to answer these questions.
Study limitations
This is retrospective analysis of clinical registry data, in which detailed clinical review of individual patients’ medical history was not possible. However, this disadvantage is outweighed by the numbers of CMR scans involved; additionally, the potential clinical implications of these findings need to be analysed in further prospective trials.
Conclusions
Obesity is associated with greater LV remodelling in established cardiac disease than in the normal heart, with obesity-related LV dilatation increasing 3-fold in DCM and 2-fold in HCM. In addition, while the obesity-related increase in LV mass seen in HCM is only proportional to the LV cavity increase, there is further hypertrophy/remodelling in DCM. Given the link between LV cavity size and mortality in heart failure, and the evidence that weight loss in obesity results in beneficial reverse remodelling, this raises the exciting possibility that obesity may be a modifiable risk factor with particular importance in underlying cardiomyopathy.
Acknowledgments
Funding: SN, OR and HW acknowledge support from the Oxford British Heart Foundation Centre of Research Excellence and the Oxford National Institute for Health Research Biomedical Research Centre. JR is funded by a British Heart Foundation Clinical Research Training Fellowship (ref FS/14/54/30946).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Oliver Rider and Andrew J. Lewis) for the series “The Use of Advanced Cardiac MRI in Heart Failure and Cardiac Hypertrophy” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-19-587
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-19-587). The series “The Use of Advanced Cardiac MRI in Heart Failure and Cardiac Hypertrophy” was commissioned by the editorial office without any funding or sponsorship. OJR served as the unpaid Guest Editor of the series. SN reports grants and personal fees from Cytokinetcs, grants from Boerhringer Ingelheim, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki. The studies involved were approved by National Research Ethics Committees and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305-13. [Crossref] [PubMed]
- Persson CE, Bjorck L, Lagergren J, et al. Risk of Heart Failure in Obese Patients with and Without Bariatric Surgery in Sweden - a Registry-Based Study. J Card Fail 2017;23:530-7. [Crossref] [PubMed]
- Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging 2010;3:266-74. [Crossref] [PubMed]
- Rider OJ, Lewandowski A, Nethononda R, et al. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J 2013;34:292-9. [Crossref] [PubMed]
- Rider OJ, Petersen SE, Francis JM, et al. Ventricular hypertrophy and cavity dilatation in relation to body mass index in women with uncomplicated obesity. Heart 2011;97:203-8. [Crossref] [PubMed]
- Milani RV, Drazner MH, Lavie CJ, et al. Progression from concentric left ventricular hypertrophy and normal ejection fraction to left ventricular dysfunction. Am J Cardiol 2011;108:992-6. [Crossref] [PubMed]
- Krishnamoorthy A, Brown T, Ayers CR, et al. Progression from normal to reduced left ventricular ejection fraction in patients with concentric left ventricular hypertrophy after long-term follow-up. Am J Cardiol 2011;108:997-1001. [Crossref] [PubMed]
- Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol 2009;54:718-26. [Crossref] [PubMed]
- Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;116:627-36. [Crossref] [PubMed]
- Wojciechowska C, Jachec W, Romuk E, et al. The effect of BMI, serum leptin, and adiponectin levels on prognosis in patients with non-ischaemic dilated cardiomyopathy. Endokrynologia Polska 2017;68:26-34. [Crossref] [PubMed]
- Olivotto I, Maron BJ, Tomberli B, et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol 2013;62:449-57. [Crossref] [PubMed]
- Rogge BP, Cramariuc D, Lonnebakken MT, et al. Effect of overweight and obesity on cardiovascular events in asymptomatic aortic stenosis: a SEAS substudy (Simvastatin Ezetimibe in Aortic Stenosis). J Am Coll Cardiol 2013;62:1683-90. [Crossref] [PubMed]
- Rider OJ, Lewis AJ, Neubauer S. Structural and Metabolic Effects of Obesity on the Myocardium and the Aorta. Obes Facts 2014;7:329-38. [Crossref] [PubMed]
- Ávila-Vanzzini N, Fritche-Salazar JF, Vazquez-Castro NM, et al. Echocardiographic and Histologic Correlations in Patients with Severe Aortic Stenosis: Influence of Overweight and Obesity. J Cardiovasc Ultrasound 2016;24:303-11. [Crossref] [PubMed]
- Rider OJ, Francis JM, Ali MK, et al. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2009;11:9. [Crossref] [PubMed]
- Rider OJ, Nethononda R, Petersen SE, et al. Concentric left ventricular remodeling and aortic stiffness: a comparison of obesity and hypertension. Int J Cardiol 2013;167:2989-94. [Crossref] [PubMed]
- Alexander JK, Dennis EW, Smith WG, et al. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull 1962-1963;1:39-44. [PubMed]
- Gaudron P, Eilles C, Ertl G, et al. Compensatory and noncompensatory left ventricular dilatation after myocardial infarction: time course and hemodynamic consequences at rest and during exercise. Am Heart J 1992;123:377-85. [Crossref] [PubMed]
- Jacob R, Gulch RW. Functional significance of ventricular dilatation. Reconsideration of Linzbach's concept of chronic heart failure. Basic Res Cardiol 1988;83:461-75. [Crossref] [PubMed]
- Vasan RS, Larson MG, Benjamin EJ, et al. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med 1997;336:1350-5. [Crossref] [PubMed]
- Lee TH, Hamilton MA, Stevenson LW, et al. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol 1993;72:672-6. [Crossref] [PubMed]
- St John Sutton M, Pfeffer MA, Plappert T, et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation 1994;89:68-75. [Crossref] [PubMed]
- Olivotto I, Cecchi F, Poggesi C, et al. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail 2012;5:535-46. [Crossref] [PubMed]
- Urbano-Moral JA, Rowin EJ, Maron MS, et al. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2014;7:11-9. [Crossref] [PubMed]


