
Robotic-assisted intervention strategy to minimize air exposure during the procedure: a case report of myocardial infarction and COVID-19
Introduction
Infection by the novel SARS-CoV-2 virus causes the Coronavirus Disease 2019 (COVID-19), an outbreak that has recently reached pandemic, worldwide, proportions (1). As the disease wave spreads across the countries, medical and scientific knowledge expand rapidly, results of a never-seen-before planetary task force aiming at developing preventive measures to reduce the rate of infected persons as well as validating effective therapeutic strategies (2).
Since many years, percutaneous coronary intervention (PCI) has been the most commonly applied invasive method to treat coronary disease (3,4). Particularly in the context of acute coronary syndromes, PCI is considered the treatment of choice to reduce short- and long-term cardiovascular morbi-mortality (5). Traditionally, PCI is a totally manual procedure, executed by one or more operators positioned at a close distance from the patient, typically taking between one to three hours to be accomplished. The COVID-19 pandemic has imposed severe restrictions to such an interventional environment (6). The SARS-CoV2 spreads mainly through respiratory particles expelled from infected persons, which are known to travel approximately 3-6 feet away (7). In traditional PCI procedures, that contamination range obligatorily poses the team and the patient to direct air exposure.
In this context, we herein present a case report of patient treated with PCI following a minimum-contact strategy with the main objective of minimizing interpersonal air exposure during the procedure.
We present the following article in accordance with the Case Report Guidelines (CARE) reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-521).
Case presentation
A 60-year-old male, with a medical history of systemic hypertension, non-insulin dependent diabetes and current smoking (50 pack-years), presented to the emergency department of a secondary hospital with complaints of chest pain and dyspnea. A close acquaintance had been ill and had tested positive for acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Blood pressure was 108/72 mmHg, heart rate 60 beats/min, respiratory rate 20 breaths/min, and body temperature normal. His physical examination was normal except for bilateral fine crackles on lung auscultation.
The admission EKG showed ST segment depression (I and aVL) and Q waves (III and aVF). Shortly after presentation he developed a monomorphic ventricular tachycardia with hemodynamic instability. Immediate electric cardioversion restored sinus rhythm. He had no recurrence of the thoracic pain but maintained hemodynamic instability needing vasoactive drugs for four days. He was kept on double anti-platelets and therapeutic dose of enoxaparin. High-sensitivity serum troponin was positive (peak of 3.75 ng/mL; upper limit of normality 0.034 ng/mL). Thus, non-ST elevation myocardial infarction was diagnosed. Echocardiogram showed inferior akinesia and systolic disfunction (left ventricular ejection fraction =31%).
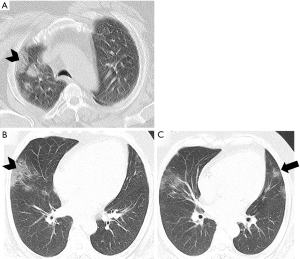
He subsequently developed fever and non-productive cough. On the 4th day, a chest non-contrast computed tomography revealed multiple, mainly subpleural ground-glass opacities in right upper lobe (Figure 1A). As fever did not subside, a new scan was obtained (12th day) and showed increased extent of ground-glass opacity on the right and new foci on the left lung (Figure 1B,C). He was managed with broad-spectrum antibiotics and did not require intubation or high-flow oxygen therapy.
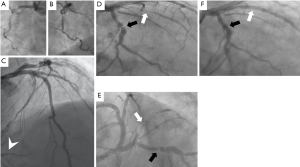
The patient was transferred to our tertiary hospital 18 days after the initial admission. A nasal swab real-time polymerase chain reaction test was still positive for COVID-19 on that same day. Cardiac catheterization was performed on the day of the transfer and revealed an occluded right coronary artery (possibly chronic total occlusion), with a significant lesion in the left circumflex artery and a TIMI II sub-occlusive lesion in the obtuse marginal branch (culprit lesion) (Figure 2).
We decided to perform ad hoc percutaneous coronary intervention (PCI) following a multiple-step strategy, designed to minimize proximity between the patient and the healthcare team during the procedure:
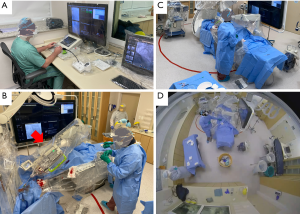
- Percutaneous coronary intervention performed through robotic assistance (CorPath GRX System. Corindus, A Siemens Healthineers Company, Waltham, MA, USA). The robotic platform is specifically developed for cardiovascular intervention and enables the manipulation of guide-catheters, 0.014” guidewires, and rapid-exchange interventional devices through a robotic arm (8-16). Robotic assistance provides accurate measurements with submilimmetric accuracy in the positioning of interventional materials. The system is operated by unscrubbed cardiac interventionalists from a control cockpit located outside the catheterization suite (Figure 3A). The physician uses joysticks and touchscreens to translate his movements of the devices (Figure 3B). The system has U.S. Food and Drug Administration approval for remote manipulation of interventional devices during percutaneous coronary and vascular procedures.
If scrubbed manual operation was required at any time during the procedure, the physician was directed to stay in that position for the minimum time possible, returning to the cockpit as soon as the need for manual maneuvering was over. - To delineate the potential zone of respiratory particle spread, a circle measuring 4 meters (13.1 feet) in diameter was traced on the floor of the cath lab with red tape, centered on the patient's mouth and nose (Figure 3). The team was rigorously trained and advised to minimize time spent within the 4-meter perimeter as much as possible during the procedure.
- To help minimize proximity, contrast administration was performed through a pump injector (ACIST CVi™ Contrast Delivery System, ACIST Corporate, Eden Prairie, Minnesota, USA) managed by a scrubbed nurse, who was positioned outside of the 4-meter circular zone (Figure 3A,B,C).
- During the procedure, the patient was kept awake, wearing a surgical mask. All personnel wore appropriate personal protective equipment while in the cath lab suite, which included a non-permeable gown, gloves, goggles and a face shield, and an N95 respirator (Figure 3A,B,C).
Robotic-assisted PCI was successfully accomplished with implantation of two stents in the obtuse marginal branch and one stent in the circumflex artery (Figure 2).
The procedure was filmed and analyzed offline to quantify the time each member of the team spent inside the 4-meter-diameter zone. The total duration of the procedure was 103 minutes and 22 seconds. During most of the procedure, the 4-meter spread zone was not entered by any personnel (Table 1). For each individual team member, the proposed strategy was effective in ensuring that they stayed outside of the 4-meter area for the majority of their work time, ranging from 96.9% to 59.7% of their respective participation.

Full table
After the procedure, the patient stayed uneventfully in the hospital for 72 hours and was discharged home 48 hours after the last fever episode.
All procedures performed were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Discussion
Several reports suggested that SARS-CoV2 per se may trigger acute cardiac injury, either due to type 2 myocardial infarction or myocarditis (17). This adds to the normal volume of patients with atherosclerotic coronary disease and presages a potential intensification in the burden of patients presenting with acute syndromes and confirmed or suspected virus infection. The perspective of diagnostic uncertainty associated with the risk of professional contamination is directing experts to advocate deferral, or even avoidance, of invasive management for selected patients with coronary disease (18). Importantly, the risk of infection to healthcare workers, along with the time needed to recover from the disease or to satisfy quarantine requirements, carries with it the potential for staffing shortages in the cath-lab, or the inability to perform interventional procedures at all from time to time. Therefore, preventive approaches to reduce the risk of contamination of health care professionals, such as the one currently presented, are timely and needed without delay.
On the other side, evidence increasingly shows that patients are now avoiding hospital care even when experiencing typical features of a heart attack, possibly due to the fear of viral exposure. In many places, out-of-hospital cardiovascular deaths due to spontaneous coronary disease have risen steeply in recent weeks (19). Going forward, public pressures will necessitate a clean hospital milieu, with care pathways designed to avoid COVID-19 exposure. The approach utilized in our case is in line with that paradigm. Obviously, to be more generally adopted, such a strategy must be confirmed by formal clinical trials. Accordingly, a pilot study with the proposed strategy is to be initiated soon (ClinicalTrials.gov number, NCT04379453).
Conclusions
This case report illustrates the potential of robotic-assisted percutaneous coronary intervention, coupled with a thoughtful strategy to reduce proximity, to provide successful invasive treatment while reducing physical proximity between the team and the patient during the procedure.
Acknowledgments
Funding: This case was partially supported by the Brazilian Program for Institutional Development of the Unified Healthcare System (PROADI-SUS) (grant number NUP: 25000.047193/2018-21).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-521
Peer Review File: Available at http://dx.doi.org/10.21037/cdt-20-521
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-521). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Photographs were cropped sufficiently to prevent human subjects from being recognized and the eyes and eyebrows were masked using Coarse Pixilation to make the individual unrecognizable. All procedures performed were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zylke JW, Bauchner H. Mortality and Morbidity: The Measure of a Pandemic. JAMA 2020;324:458-9. [Crossref] [PubMed]
- Moradian N, Ochs HD, Sedikies C, et al. The urgent need for integrated science to fight COVID-19 pandemic and beyond. J Transl Med 2020;18:205. [Crossref] [PubMed]
- Alkhouli M, Alqahtani F, Kalra A, et al. Trends in Characteristics and Outcomes of Patients Undergoing Coronary Revascularization in the United States, 2003-2016. JAMA Netw Open 2020;3:e1921326. [Crossref] [PubMed]
- Kataruka A, Maynard CC, Kearney KE, et al. Temporal Trends in Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting: Insights From the Washington Cardiac Care Outcomes Assessment Program. J Am Heart Assoc 2020;9:e015317. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Szerlip M, Anwaruddin S, Aronow HD, et al. Considerations for cardiac catheterization laboratory procedures during the COVID-19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) Members and Graduates. Catheter Cardiovasc Interv 2020;96:586-97. [Crossref] [PubMed]
- Atri D, Siddiqi HK, Lang J, et al. COVID-19 for the Cardiologist: A Current Review of the Virology, Clinical Epidemiology, Cardiac and Other Clinical Manifestations and Potential Therapeutic Strategies. JACC Basic Transl Sci 2020;5:518-36. [Crossref] [PubMed]
- Weisz G, Metzger DC, Caputo RP, et al. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J Am Coll Cardiol 2013;61:1596-600. [Crossref] [PubMed]
- Weisz G, Smilowitz NR, Metzger DC, et al. The association between experience and proficiency with robotic-enhanced coronary intervention-insights from the PRECISE multi-center study. Acute Card Care 2014;16:37-40. [Crossref] [PubMed]
- Lo N, Gutierrez JA, Swaminathan RV. Robotic-Assisted Percutaneous Coronary Intervention. Curr Treat Options Cardiovasc Med 2018;20:14. [Crossref] [PubMed]
- Mahmud E, Naghi J, Ang L, et al. Demonstration of the Safety and Feasibility of Robotically Assisted Percutaneous Coronary Intervention in Complex Coronary Lesions: Results of the CORA-PCI Study (Complex Robotically Assisted Percutaneous Coronary Intervention). JACC Cardiovasc Interv 2017;10:1320-7. [Crossref] [PubMed]
- Mahmud E, Schmid F, Kalmar P, et al. Feasibility and Safety of Robotic Peripheral Vascular Interventions: Results of the RAPID Trial. JACC Cardiovasc Interv 2016;9:2058-64. [Crossref] [PubMed]
- Mangels DR, Giri J, Hirshfeld J, et al. Robotic-assisted percutaneous coronary intervention. Catheter Cardiovasc Interv 2017;90:948-55. [Crossref] [PubMed]
- Smilowitz NR, Moses JW, Sosa FA, et al. Robotic-Enhanced PCI Compared to the Traditional Manual Approach. J Invasive Cardiol 2014;26:318-21. [PubMed]
- Smitson CC, Ang L, Pourdjabbar A, et al. Safety and Feasibility of a Novel, Second-Generation Robotic-Assisted System for Percutaneous Coronary Intervention: First-in-Human Report. J Invasive Cardiol 2018;30:152-6. [PubMed]
- Swaminathan RV, Rao SV. Robotic-assisted transradial diagnostic coronary angiography. Catheter Cardiovasc Interv 2018;92:54-7. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Welt FGP, Shah PB, Aronow HD, et al. Catheterization Laboratory Considerations During the Coronavirus (COVID-19) Pandemic: From ACC's Interventional Council and SCAI. J Am Coll Cardiol 2020;75:2372-5. [Crossref] [PubMed]
- Baldi E, Sechi GM, Mare C, et al. Out-of-Hospital Cardiac Arrest during the Covid-19 Outbreak in Italy. N Engl J Med 2020;383:496-8. [Crossref] [PubMed]


