The coronary slow flow phenomenon: characteristics, mechanisms and implications
Introduction
The coronary slow flow phenomenon (CSFP) is an angiographic clinical entity, characterized by delayed distal vessel opacification in the absence of significant epicardial coronary stenosis. Although it is well-known to interventional cardiologists for approximately four decades, the pathogenic mechanisms are incompletely understood. Rather than representing a simple angiographic curiosity, CSFP has direct clinical implications, as it has been linked to clinical manifestations of myocardial ischemia, life-threatening arrhythmias, sudden cardiac death, and recurrent acute coronary syndromes (1-4). However, current clinical practice tends to underestimate the impact of CSFP due to the yet unknown mechanisms, its relative rarity, and the subsequent difficulties in conducting randomized trials to evaluate different treatment options. In this article, we will summarize the characteristics, possible mechanisms, and clinical implications of this entity to provide further insight into its clinical significance and management strategies.
Definition
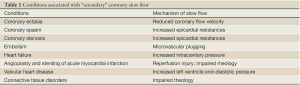
Although a number of formal definitions have been proposed, the CSFP essentially consists of a delay in the progression of the contrast injected into the coronary arteries during coronary angiography. Representative angiographic images of CSFP and normal subjects are shown in Movie 1,2. This condition, which may affect one or all coronaries, was originally described by Tambe et al. in 1972 (5). Since then it has been accepted as an independent clinical entity, which is called ‘CSFP’, ‘coronary slow flow syndrome’ ‘syndrome Y’, or “primary” coronary slow flow (6-9). Importantly, ‘primary’ CSFP should be distinguished from the delay in the contrast progression in the context of coronary reperfusion therapy such as angioplasty or stenting for acute myocardial infarction, or other “secondary” causes of coronary slow flow. These include coronary artery ectasia, coronary artery spasm, valvular heart disease, or connective tissue disorders (8-10) (Table 1).
Full table
Clinical manifestation
CSFP is a frequent angiographic observation, with a reported incidence of 1%-7% in patients undergoing diagnostic angiography because of clinical suspicion of cardiovascular disease (6,11). Clinically, this phenomenon occurs most commonly in young men and smokers, and patient admitted with acute coronary syndrome (12). The clinical course is complicated, with over 80% of patients experiencing recurrent chest pain, most occurring at rest, necessitating readmission to the coronary care unit in almost 20% of affected patients (12). Most importantly, coronary slow flow has been described to be associated with life-threatening arrhythmias and sudden cardiac death (3, 4), probably due to increased QTc dispersion in these patients (13).
Further, Yilmaz et al. (14) recently delineated the clinical and laboratory features of CSFP compared to the control subjects without CSFP. Metabolic syndrome was more frequent in CSFP in the presence of higher total cholesterol, low-density lipoprotein-cholesterol, fasting glucose and body mass index levels. These data are in line with the observations that insulin resistant states (15) and impaired glucose tolerance (16) correlate with CSFP occurrence. These data suggest that a common underlying pathophysiologic mechanism of the metabolic syndrome and CSFP may be endothelial dysfunction.
Diagnosis and evaluation
CSFP in coronary angiographic studies was initially described subjectively by visual judgement (5). A semi-quantitative assessment of coronary blood flow is the thrombolysis in myocardial infarction (TIMI) flow grade classification, which reflects the speed and completeness of the passage of the injected contrast through the coronary tree (17). Although this widely used method of grading coronary flow has been a valuable tool for comparison of flow data in clinical trials, variability in the visual assessment may limit the broad clinical applicability. In contrast, as an objective, quantitative index of coronary flow, corrected TIMI frame count (CTFC) facilitates the standardization of TIMI flow grades and flow assessment. It represents the number of cine-frames required for contrast to first reach standard distal coronary landmarks (18).
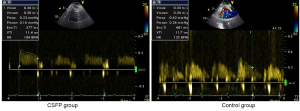
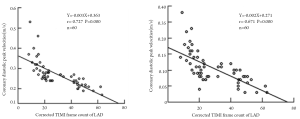
Currently, by using CTFC as a quantitative index of coronary flow, coronary angiography is the only tool for the diagnosis and assessment of CSFP. Yet, owing to its invasiveness, this method does not permit long-term clinical follow-up and dynamic treatment evaluation. Recent advances of transthoracic Doppler echocardiography (TTDE) have enabled the non-invasive demonstration of coronary flow patterns in the left anterior descending (LAD) coronary artery (19-24). In this context, we measured coronary flow in the distal LAD using TTDE technique with a high success rate (92.3%) (Figure 1). Patients with CSFP exhibited lower coronary diastolic velocities of LAD, which was negatively correlated with CTFC (Figure 2). TTDE may provide a useful tool for the monitoring of treatment effect and long-term follow-up for CSFP. However, there is a lack of confirmation in clinical trials, and there is need for further evaluation of TTDE in the diagnosis of CSFP.
Pathogenic mechanisms
Small vessel disease
The coronary circulation is traditionally considered as a two-compartment model. The first compartment consists of epicardial vessels, which are also referred to as “conductance vessels”, because they do not pose any resistance to blood flow. The second compartment consists of “small vessels” of <400 µm (“resistive vessels”), which primarily regulate myocardial blood flow in the absence of any significant obstructive epicardial stenosis (25,26). Small vessel dysfunction has been typically involved in the pathogenesis of CSFP since its first description (5). Confirming this hypothesis, investigators reported fibromuscular hyperplasia, medial hypertrophy, myointimal proliferation, as well as endothelial edema, thickening and degeneration in the coronary microvessels (27). In parellel to these data, Mangieri et al. (11) found thickening of vessel walls with luminal size reduction, mitochondrial abnormalities, and glycogen content reduction in left ventricular endomyocardial biopsies. Subsequently, Beltrame et al. (28) indicated that CSFP was associated with a chronically elevated resting coronary microvascular tone characterized by low coronary sinus oxygen saturation as well as blunted responses to endothelial stimuli such as cold pressor or acetylcholine testing. Based on these data, it can be suggested that a combination of structural and functional abnormalities coexists in the coronary microcirculation.
Endothelial dysfunction
A growing body of evidence has suggested that the endothelium plays an integral role in the regulation of vascular tone, platelet activity, leukocyte adhesion, vascular smooth muscle proliferation, and is intimately involved in the development of atherosclerosis. It has been reported that reduced endothelium dependent flow-mediated dilatation (FMD) of the brachial artery was detected in patients with CSFP, suggesting that endothelial dysfunction is implicated in etiology of CSFP (29). Noteworthy is the recent finding demonstrating that baseline and peak exercise endothelin-1 plasma concentrations were higher and nitric oxide plasma concentrations were lower in slow coronary flow patients (30,31). In addition, patients with slow coronary flow had raised level of plasma homocysteine (32,33) and asymmetric dimethylarginine (34), a nitric oxide synthase inhibitor, both of which have a detrimental effect on endothelial function. More recently, decreased adiponectin concentrations (35) and paraoxonase activity (36), two significant markers of endothelial dysfunction have also been shown to be responsible for the etiopathogenesis of CSFP.
Subclinical atherosclerosis
Utilizing IVUS technique and flow rate measurements, Cin et al. (37) demonstrated that patients with CSFP have diffuse intimal thickening, widespread calcification along the coronary vessel wall, and non-obstructive atheromatous coronary changes. In line with these results, Pekdemir et al. (38) showed that most patients with CSFP have longitudinally extended massive calcification throughout the epicardial coronary arteries. These data are evidence that CSFP may reflect diffuse, non-obstructive atherosclerotic disease of epicardial vessels together with microvascular disease. These findings are supported by previous IVUS study indicating that diffuse atherosclerosis is often present in angiographically normal coronary arteries.
Inflammation
Inflammation is a contributing factor to several cardiovascular conditions and inflammatory mechanisms have also been observed in the context of CSFP. Li et al. (39) showed that the plasma concentration of high-sensitivity C-reactive protein and interleukin-6 was increased in CSFP patients. Similarly, coronary slow flow was associated with higher levels of plasma soluble adhesion molecules, including intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin (40). Other inflammatory markers, such as red cell distribution width and serum uric acid levels, were also shown to be correlated with CSFP occurrence (41,42). Collectively, abnormalities in inflammatory parameters might be an indicator of endothelial dysfunction, both of which contribute to coronary slow flow.
Anatomic factors
Blood flow patterns in epicardial coronary arteries depend on the geometry and motion of these vessels (43). Disturbed laminar blood flow occurs in arterial segments with geometric irregularities such as curvatures, branches, and bifurcations (44). It is in these complex regions that low blood velocity rates tend to occur. Confirming this theory, recent observation with multidetector CT coronary angiography demonstrated that in patients with CSFP, the angulations of the main coronary arteries from the aorta were smaller determined (45). Based on this theory, we recently performed a case-control study to explore the correlation between anatomic properties of coronary arteries and CSFP occurrence. The results showed that the presence of CSFP was associated with higher tortuosity and more distal branches in coronary arteries. Accordingly, it is reasonable to assume that certain anatomic properties of coronary arteries could be predisposing to disturbed coronary flow and endothelial damage, ultimately leading to CSFP occurrence.
CSFP: a local or systemic condition?
Previous studies have suggested that a combination of morphological and functional abnormalities in small vessels and epicardial coronary arteries accounts for the etiology of CSFP. Another feature of CSFP, probably requiring special consideration is its frequent occurrence in association with more widespread vascular abnormalities.
Karakaya et al. (46) found that cerebral blood flow velocity is significantly lower in patients with CSFP. Endothelial abnormality appears to be a generalized process affecting both coronary and peripheral vasculature. Furthermore, in intravascular ultrasound investigation, Camsari et al. (47) found that there was a significant correlation between coronary intima-media thickness (IMT) and carotid IMT. In contrast, aortic distensibility and aortic strain, another predictor of subclinical atherosclerosis, were lower in patients with CSFP (48). It is reasonable to assume that early atherosclerosis is not restricted to the coronary circulation but also extends to large peripheral conduit arteries in patients with CSFP. Based on these data, our group hypothesized that CSFP is not an isolated local observation but may be part of generalized vascular disturbance (49). Taken together, CSFP may be caused by the interplay between local features coronary arteries and systemic pathophysiologic factors.
Therapy
Despite good prognosis of CSFP patients, the subsequent progress is frequently characterized by remitting, relapsing anginal episodes resulting in considerable impairment in quality of life. Unfortunately, currently available anti-anginal agents are of limited clinical value. To date, no large trial testing pharmacological approaches has been conducted, and the evidence available derives from small studies with inhomogeneous inclusion criteria. It was shown that dipyridamole and mibefradil, which both influence functional obstruction in arteries <200 µm, normalized CTFC but nitroglycerine, which dilates arteries with diameters >200 µm, did not (50,51). Importantly, statins appear beneficial for patients with CSFP, likely in part due to their anti-inflammatory properties (52-54). More recently, several studies demonstrated that nebivolol can both improve endothelial function and markedly ameliorate symptoms, thereby improving quality of life in patients with CSFP (55-57). Besides its beta-receptor blocking activity, nebivolol can cause endothelium-dependent vasodilatation through increased nitric oxide release (55).
Conclusions
CSFP is an important, angiographic finding typically observed in patients presenting with acute coronary syndrome, in particular unstable angina. This phenomenon should be considered a separate clinical entity with peculiar characteristics, pathogenic mechanisms, and defined diagnostic criteria. Previous studies have shown that small vessel disease, endothelial dysfunction, subclinical atherosclerosis, inflammation, and anatomic properties of coronary arteries are related to the occurrence of CSFP. Current findings support the hypothesis that CSFP may be part of systemic vascular disturbance.
Our current understanding is incomplete, but clinicians should be aware of this condition and its clinical significance. Further experimental investigations are needed to reveal the pathogenesis involved in CSFP. In addition, large-scale clinical studies are warranted to better characterize these phenomenon, and most importantly, investigate potential therapeutic approaches.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cutri N, Zeitz C, Kucia AM, et al. ST/T wave changes during acute coronary syndrome presentation in patients with the coronary slow flow phenomenon. Int J Cardiol 2011;146:457-8. [PubMed]
- Horjeti B, Goda A. Acute ischemia manifestation in a patient with coronary slow flow phenomenon. J Electrocardiol 2012;45:277-9. [PubMed]
- Wozakowska-Kapłon B, Niedziela J, Krzyzak P, et al. Clinical manifestations of slow coronary flow from acute coronary syndrome to serious arrhythmias. Cardiol J 2009;16:462-8. [PubMed]
- Saya S, Hennebry TA, Lozano P, et al. Coronary slow flow phenomenon and risk for sudden cardiac death due to ventricular arrhythmias: a case report and review of literature. Clin Cardiol 2008;31:352-5. [PubMed]
- Tambe AA, Demany MA, Zimmerman HA, et al. Angina pectoris and slow flow velocity of dye in coronary arteries--a new angiographic finding. Am Heart J 1972;84:66-71. [PubMed]
- Goel PK, Gupta SK, Agarwal A, et al. Slow coronary flow: a distinct angiographic subgroup in syndrome X. Angiology 2001;52:507-14. [PubMed]
- Li JJ, Wu YJ, Qin XW. Should slow coronary flow be considered as a coronary syndrome? Med Hypotheses 2006;66:953-6. [PubMed]
- Leone MC, Gori T, Fineschi M. The coronary slow flow phenomenon: a new cardiac "Y" syndrome? Clin Hemorheol Microcirc 2008;39:185-90. [PubMed]
- Fineschi M, Gori T. Coronary slow-flow phenomenon or syndrome Y: a microvascular angina awaiting recognition. J Am Coll Cardiol 2010;56:239-40. [PubMed]
- Gori T, Fineschi M. Two Coronary "Orphan" Diseases in Search of Clinical Consideration: Coronary Syndromes X and Y.Cardiovasc Ther 2011.
- Mangieri E, Macchiarelli G, Ciavolella M, et al. Slow coronary flow: clinical and histopathological features in patients with otherwise normal epicardial coronary arteries. Cathet Cardiovasc Diagn 1996;37:375-81. [PubMed]
- Beltrame JF, Limaye SB, Horowitz JD. The coronary slow flow phenomenon--a new coronary microvascular disorder. Cardiology 2002;97:197-202. [PubMed]
- Atak R, Turhan H, Sezgin AT, et al. Effects of slow coronary artery flow on QT interval duration and dispersion. Ann Noninvasive Electrocardiol 2003;8:107-11. [PubMed]
- Yilmaz H, Demir I, Uyar Z. Clinical and coronary angiographic characteristics of patients with coronary slow flow. Acta Cardiol 2008;63:579-84. [PubMed]
- Ozcan T, Gen R, Akbay E, et al. The correlation of thrombolysis in myocardial infarction frame count with insulin resistance in patients with slow coronary flow. Coron Artery Dis 2008;19:591-5. [PubMed]
- Binak E, Gunduz H, Sahin M, et al. The relation between impaired glucose tolerance and slow coronary flow. Int J Cardiol 2006;111:142-6. [PubMed]
- Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987;76:142-54. [PubMed]
- Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996;93:879-88. [PubMed]
- Tani T, Tanabe K, Kitai T, et al. Detection of severe stenosis and total occlusion in the left anterior descending coronary artery with transthoracic Doppler echocardiography in the emergency room. Echocardiography 2009;26:15-20. [PubMed]
- Citro R, Voci P, Pizzuto F, et al. Clinical value of echocardiographic assessment of coronary flow reserve after left anterior descending coronary artery stenting in an unselected population. J Cardiovasc Med (Hagerstown) 2008;9:1254-9. [PubMed]
- Anjaneyulu A, Raghu K, Chandramukhi S, et al. Evaluation of left main coronary artery stenosis by transthoracic echocardiography. J Am Soc Echocardiogr 2008;21:855-60. [PubMed]
- Pizzuto F, Voci P, Mariano E, et al. Assessment of flow velocity reserve by transthoracic Doppler echocardiography and venous adenosine infusion before and after left anterior descending coronary artery stenting. J Am Coll Cardiol 2001;38:155-62. [PubMed]
- Daimon M, Watanabe H, Yamagishi H, et al. Physiologic assessment of coronary artery stenosis by coronary flow reserve measurements with transthoracic Doppler echocardiography: comparison with exercise thallium-201 single piston emission computed tomography. J Am Coll Cardiol 2001;37:1310-5. [PubMed]
- Hozumi T, Yoshida K, Ogata Y. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation 1998;97:1557-62. [PubMed]
- De Bruyne B, Hersbach F, Pijls NH, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but "Normal" coronary angiography. Circulation 2001;104:2401-6. [PubMed]
- Maseri A, Crea F, Kaski JC, et al. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardio 1991;17:499-506.
- Mosseri M, Yarom R, Gotsman MS, et al. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation 1986;74:964-72. [PubMed]
- Beltrame JF, Limaye SB, Wuttke RD, et al. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am Heart J 2003;146:84-90. [PubMed]
- Sezgin AT, Sigirci A, Barutcu I, et al. Vascular endothelial function in patients with slow coronary flow. Coron Artery Dis 2003;14:155-61. [PubMed]
- Camsarl A, Pekdemir H, Cicek D, et al. Endothelin-1 and nitric oxide concentrations and their response to exercise in patients with slow coronary flow. Circ J 2003;67:1022-8. [PubMed]
- Sezgin N, Barutcu I, Sezgin AT, et al. Plasma nitric oxide level and its role in slow coronary flow phenomenon. Int Heart J 2005;46:373-82. [PubMed]
- Riza Erbay A, Turhan H, Yasar AS, et al. Elevated level of plasma homocysteine in patients with slow coronary flow. Int J Cardiol 2005;102:419-23. [PubMed]
- Barutcu I, Sezgin AT, Sezgin N, et al. Elevated plasma homocysteine level in slow coronary flow. Int J Cardiol 2005;101:143-5. [PubMed]
- Selcuk MT, Selcuk H, Temizhan A, et al. Asymmetric dimethylarginine plasma concentrations and L-arginine/asymmetric dimethylarginine ratio in patients with slow coronary flow. Coron Artery Dis 2007;18:545-51. [PubMed]
- Selcuk H, Selcuk MT, Temizhan A, et al. Decreased plasma concentrations of adiponectin in patients with slow coronary flow. Heart Vessels 2009;24:1-7. [PubMed]
- Yildiz A, Gur M, Yilmaz R, et al. Association of paraoxonase activity and coronary blood flow. Atherosclerosis 2008;197:257-63. [PubMed]
- Cin VG, Pekdemir H, Camsar A, et al. Diffuse intimal thickening of coronary arteries in slow coronary flow. Jpn Heart J 2003;44:907-19. [PubMed]
- Pekdemir H, Polat G, Cin VG, et al. Elevated plasma endothelin-1 levels in coronary sinus during rapid right atrial pacing in patients with slow coronary flow. Int J Cardiol 2004;97:35-41. [PubMed]
- Li JJ, Qin XW, Li ZC, et al. Increased plasma C-reactive protein and interleukin-6 concentrations in patients with slow coronary flow. Clin Chim Acta 2007;385:43-7. [PubMed]
- Turhan H, Saydam GS, Erbay AR, et al. Increased plasma soluble adhesion molecules; ICAM-1, VCAM-1, and E-selectin levels in patients with slow coronary flow. Int J Cardiol 2006;108:224-30. [PubMed]
- Kalay N, Aytekin M, Kaya MG, et al. The relationship between inflammation and slow coronary flow: increased red cell distribution width and serum uric acid levels. Turk Kardiyol Dern Ars 2011;39:463-8. [PubMed]
- Yildiz A, Yilmaz R, Demirbag R, et al. Association of serum uric acid level and coronary blood flow. Coron Artery Dis 2007;18:607-13. [PubMed]
- Ramaswamy SD, Vigmostad SC, Wahle A, et al. Fluid dynamic analysis in a human left anterior descending coronary artery with arterial motion. Ann Biomed Eng 2004;32:1628-41. [PubMed]
- Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007;49:2379-93. [PubMed]
- Kantarci M, Gündogdu F, Doganay S, et al. Arterial bending angle and wall morphology correlate with slow coronary flow: determination with multidetector CT coronary angiography. Eur J Radiol 2011;77:111-7. [PubMed]
- Karakaya O, Koçer A, Esen AM, et al. Impaired cerebral circulation in patients with slow coronary flow. Tohoku J Exp Med 2011;225:13-6. [PubMed]
- Camsari A, Ozcan T, Ozer C, et al. Carotid artery intima-media thickness correlates with intravascular ultrasound parameters in patients with slow coronary flow. Atherosclerosis 2008;200:310-4. [PubMed]
- Arat N, Altay H, Sabah I. Elastic properties of aorta are impaired in patients with slow coronary flow phenomenon. Indian Heart J 2008;60:119-24. [PubMed]
- Wang X, Geng LL, Nie SP. Coronary slow flow phenomenon: a local or systemic disease? Med Hypotheses 2010;75:334-7. [PubMed]
- Kurtoglu N, Akcay A, Dindar I. Usefulness of oral dipyridamole therapy for angiographic slow coronary artery flow. Am J Cardiol 2001;87:777-9. [PubMed]
- Beltrame JF, Turner SP, Leslie SL, et al. The angiographic and clinical benefits of mibefradil in the coronary slow flow phenomenon. J Am Coll Cardiol 2004;44:57-62. [PubMed]
- Cakmak M, Tanriverdi H, Cakmak N, et al. Simvastatin may improve myocardial perfusion abnormality in slow coronary flow. Cardiology 2008;110:39-44. [PubMed]
- Li JJ, Zheng X, Li J. Statins may be beneficial for patients with slow coronary flow syndrome due to its anti-inflammatory property. Med Hypotheses 2007;69:333-7. [PubMed]
- Caliskan M, Erdogan D, Gullu H, et al. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol 2007;30:475-9. [PubMed]
- Gunes Y, Tuncer M, Guntekin U, et al. Regional functions of the left ventricle in patients with coronary slow flow and the effects of nebivolol. Ther Adv Cardiovasc Dis 2009;3:441-6. [PubMed]
- Fragasso G. Nebivolol in patients with coronary slow flow: the right drug for the right case? Anadolu Kardiyol Derg 2009;9:296-7. [PubMed]
- Albayrak S, Ordu S, Yuksel H, et al. Efficacy of nebivolol on flow-mediated dilation in patients with slow coronary flow. Int Heart J 2009;50:545-53. [PubMed]