Spontaneous coronary artery dissection—A review
Spontaneous coronary artery dissection (SCAD) is an infrequent and often missed diagnosis especially in women presenting with acute coronary syndrome (ACS). The incident case in a 42-year-old woman was diagnosed at autopsy in 1931 (1). SCAD can result in significant morbidities such as myocardial ischemia and infarction, ventricular arrhythmias and sudden cardiac death (2-4). Limited prognostic data is available due in part to under-recognition and lack of prospective studies. With the advent of new imaging modalities, particularly with intracoronary imaging, there has been improved recognition of SCAD. The aim of this paper is to review the epidemiology, etiology, presentation, diagnosis and management of SCAD.
Definition and epidemiology
SCAD is defined as a non-traumatic and non-iatrogenic separation of the coronary arterial walls, creating a false lumen (4). This separation can occur between the intima and media or between the media and adventitia, with intramural hematoma (IMH) formation within the arterial wall that compresses the arterial lumen, decreasing antegrade blood flow and subsequent myocardial ischemia or infarction (4,5).
It is difficult to ascertain the true prevalence of SCAD as it is often under-diagnosed and have varying presentations from mild chest pains to sudden cardiac death. Older literature encompassed mostly post-mortem reports (3), but with recent awareness of the disease and improved imaging, SCAD cases are increasingly reported antemortem. In patients presenting with ACS, SCAD is noted to occur in 3-4%, diagnosed with optical coherence tomography (OCT) (2,6-8). Among stable patients presenting for routine coronary angiography, SCAD was diagnosed in 0.3% (8), although we suspect these were delayed diagnoses following a previously undiagnosed ACS event. Among women presenting with ACS, the prevalence was reported to be higher at 8.7% among those <50 years old (9). In the subgroup of women presenting with ST elevation, prevalence of SCAD was even higher at 10.8% (9). At our center, upon thorough angiographic review, we found the prevalence of SCAD to be much higher at 24% in women <50 years of age who had a myocardial infarction (MI) (10). The majority of these cases were mis-diagnosed, highlighting the challenge and clear under-diagnosis of SCAD.
Pathogenesis and pathophysiology
There are two proposed mechanisms for the formation of IMH with SCAD. The first involves an intimal tear resulting in blood from the endoluminal space entering the intimal space, creating a false lumen filled with blood. The second mechanism of IMH formation is thought to be due to rupture of the vasa vasorum, which are small arterioles within the walls of arteries supplying blood to the walls. When such rupture occurs, blood can pool within the intramural space, creating a false lumen filled with hematoma. Figure 1 shows the distinctive features between the two mechanisms (4).
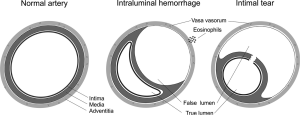
Clinically, the mechanism of the tear is probably unimportant, and coronary angiography is notoriously suboptimal to visualize intimal tears. Intracoronary imaging with intravascular ultrasound (IVUS), and in particular OCT, has increased the detection of intimal rupture substantially (11,12).
The usual pathogenesis of ACS involves atherosclerotic plaque rupture that is distinct from non-atherosclerotic forms of SCAD. Dissections due to atherosclerosis tend to be less extensive, as medial atrophy and scarring from atherosclerosis limits propagation of the dissection (4). Non-atherosclerotic SCAD is typically a culmination of disease pathways that predispose arterial beds to injury. These include fibromuscular dysplasia (FMD), multiple pregnancy, systemic inflammation (systemic lupus erythematosus, Crohn’s disease, polyarteritis nodosa and sarcoidosis), connective tissue disorder (Marfan’s syndrome, Ehler Danlos, cystic medial necrosis), hormonal therapy, and coronary artery spasm (Table 1). Previously, it was thought that a large proportion of SCAD was idiopathic; however, our group has shown that with proper screening for FMD and other potential arteriopathies, the proportion considered idiopathic was much lower (13).

Full table
SCAD has been observed in women who are peripartum or with multiple prior pregnancies, and thus a significant association with pregnancy has been postulated (14,15). Hormonal changes during pregnancy are thought to alter normal elastic fibres, impair collagen synthesis and mucopolysaccharide content, causing weakened media. Progesterone is thought to be the culprit hormone, and estrogen, on the other hand, creates a hypercoagulable state. The weakened arterial walls at risk of dissection with a prothrombotic state increases the risk of false lumen creation and thrombosis. Eosinophilic infiltrates in arterial adventitia have also been observed in autopsies of peripartum women. It is believed that these eosinophilic granules cause breakdown of the medial-adventitial layer via lytic substances, predisposing the artery to dissection. However, it is unclear if the eosinophilic granules cause SCAD or is resultant of SCAD. Interestingly, SCAD in pregnant women often occurs during late pregnancy or the post-partum period (5,13,14). That whether the different composition of hormones at that time causes increased risk is unknown. The hemodynamic changes during late pregnancy can also precipitate SCAD. The aorta undergoes microstructural changes secondary to increased shear stress from augmented cardiac output and increased circulatory volume during pregnancy. This could also extend to the coronary arteries (16). During labor, the increased intraabdominal pressures could also predispose more arterial stress leading to SCAD. Patients with peri-partum SCAD may also have underlying predisposing arteriopathies such as FMD (17).
Although early retrospective studies suggested that as high as 30% of SCAD cases were peripartum (13), more recent series showed much lower proportion of SCAD related to the peripartum state (6,13,14,18). In our series of 168 patients (13), pregnancy-related SCAD accounted for only 2.4% of cases. Older series may be biased by selective reporting of high morbidity and mortality cases. In fact, pregnancy-related SCAD do appear to have worse clinical outcomes (14) that prompt seeking of medical attention.
In patients with underlying predisposing arteriopathies, there can often be precipitating stressors such as intense exercise or emotional stress, which may trigger the SCAD event (13). Intense exercises, particularly an isometric type, can increase cardio-circulatory stresses and shear forces against the coronary arterial wall. Other aerobic type exercises, severe emotional stress, and activities that cause intense Valsalva-type straining have also been shown to precipitate SCAD, especially in patients with predisposing arteriopathy (13) (Table 1).
Related to arterial integrity, a recent study showed that coronary artery tortuosity is present at a higher proportion in patients with SCAD as compared to patient with normal coronary arteries (19). Repeat dissections were common within tortuous segments (80%). Whether arterial tortuosity predisposes to arterial fragility, or is merely a marker of SCAD (since FMD can cause arterial tortuosity) is unknown.
SCAD and FMD
Our group has discovered a strong association between SCAD and FMD (Figure 2), a condition that also predominantly affects women (20). FMD is a non-inflammatory, non-atherosclerotic disorder of the arterial vasculature that leads to arterial stenosis, occlusion, aneurysm or dissection. It can involve any small to medium-sized arterial beds, especially the renal and internal carotid arteries. The etiology of FMD is unknown, however, hormonal influences had been proposed and a small proportion may be genetically inherited. Since 90% of cases affect women (21), sex hormones were thought to influence development of FMD, with some similarities to SCAD.
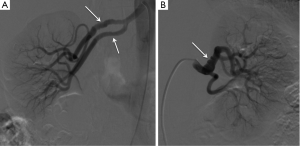
Three histopathological case reports have been previously published describing coronary FMD as the cause of SCAD in these patients (22-24). In our most recent series, we found that FMD is an important predisposing arteriopathy when it is screened for routinely, and was found in 72% of 168 patients with SCAD (13). Presumably, FMD accounted for a large proportion of previously erroneously labeled idiopathic SCAD. The retrospective series from the Mayo Clinic also described this interesting association; they found iliac FMD in 50% of femoral angiograms performed on SCAD patients (18).
Both FMD and SCAD are considered infrequent conditions. The high prevalence of concomitant FMD among SCAD patients suggests causal implications. Fibroplasia, hyperplasia and aneurysmal changes can occur in all three segments of the arterial wall, intima, media and adventitia with FMD, and can result in weakened arterial walls. These processes predispose segments of the artery to dissections, which may explain the strong association.
Presentation and patient characteristics
Patients presenting with SCAD have a spectrum of clinical presentation, and fortunately, the majority appear to present with ACS with good in-hospital prognosis (13). The first reported case of SCAD was a 42-year-old woman who suffered a sudden cardiac death after a day of severe retching and vomiting (1). She had described chest pain and nausea prior to her demise. Subsequent case reports also documented prior chest pain in post-mortem diagnosis of SCAD, with sudden cardiac arrest being the majority of the cause of death. In recent retrospective studies, chest pain was the presenting symptom in the majority of SCAD cases (2,15,20,25). Ventricular arrhythmia occurred in 8-14% of patients (2,8). In our recent series, all cases of SCAD presented with troponin-positive ACS, with 26% presenting with ST-elevation MI, and 3.6% with ventricular arrhythmia (13). Two older studies showed a higher proportion presenting with ST-elevation MI (80-84%), whereas non-S-T elevation MI were 8-16%, and 4% presented with unstable angina (2,21). Case series had also noted that patients may have severe left ventricular (LV) dysfunction which often has subsequent full recovery (26-28). However, LV recovery may occur with standard forms of MI, but whether there may be more marked LV recovery with SCAD as postulated by some, is yet unknown. There are no published studies that routinely evaluated LV function on presentation and subsequent follow-up for SCAD patients.
The conventional teaching is that SCAD occurred predominantly in young women. However, in our recent series, the average age was 52.1±9.2 years, and 58% were actually age 50 years or older. And in fact, 62.3% of the women with SCAD were post-menopausal. Thus, SCAD does not exclusively affect young women. Table 2 summarizes clinical features in patients presenting with ACS that would raise suspicion of an underlying diagnosis of SCAD.

Full table
Imaging
Angiography
The early case reports and series on SCAD had relied on post-mortem diagnosis. Current widespread availability of coronary angiography enabled earlier diagnosis of SCAD. We have characterized three distinct angiographic appearances and patterns of SCAD to aid diagnosis (29):
- Type 1 (evident arterial wall stain): this is the pathognomonic angiographic appearance of SCAD with contrast dye staining of the arterial wall with multiple radiolucent lumens (Figure 3).
- Type 2 (diffuse stenosis of varying severity): this angiographic appearance is not well appreciated and is often missed or misdiagnosed. SCAD commonly involves the mid to distal segments of coronary arteries, and can be so extensive that it reaches the distal tip. There is an appreciable (often subtle) abrupt change in arterial caliber, with demarcation from normal diameter to diffuse narrowing. This diffuse (typically >20 mm) and usually smooth narrowing can vary in severity from an inconspicuous mild stenosis to complete occlusion (Figure 4).
- Type 3 (mimic atherosclerosis): this appearance is the most challenging to differentiate from atherosclerosis (Figure 5) and most likely to be misdiagnosed. Angiographic features that favor SCAD are: (i) lack of atherosclerotic changes in other coronary arteries; (ii) long lesions (11-20 mm); (iii) hazy stenosis; and (iv) linear stenosis. Angiographer needs to have a high index of suspicion for SCAD (Table 2) and employ intracoronary imaging for such cases.
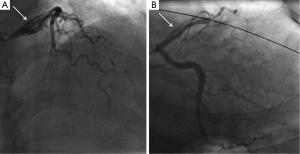
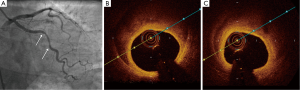
There are no apparent differences in clinical presentations with the three angiographic classifications of SCAD. Type 1 SCAD should be easily diagnosed with angiography. Type 2 SCAD can be distinctive enough for diagnosis once angiographers become familiar with the pattern. However, some type 2 cases (e.g., shorter lengths 20-30 mm) will require intracoronary imaging or repeat angiography to make the diagnosis. Type 3 SCAD is often indistinguishable from atheroma, and requires intracoronary imaging for diagnosis. Details on intracoronary imaging are discussed in the next session.
The natural history of SCAD appears to entail spontaneous healing in the vast majority of cases (13). Repeat angiogram in selected patients in previous studies showed variable healing at 1 month (2), <1 year (12) and full resolution at 40 months (15). In our series, 79 out of 79 patients treated conservatively had spontaneous angiographic healing at ≥4 weeks following their SCAD event (13). However, we also observed that luminal narrowing started improving before 4 weeks for patients with earlier repeat angiograms.
Intracoronary imaging
The current gold-standard coronary angiography is unfortunately an imperfect tool for the diagnosis of SCAD, because it is only a 2-dimensional luminogram. It is excellent to assess luminal narrowing; however, it is poor in assessing the arterial wall, where the key abnormalities occur with SCAD. The pathognomonic type 1 angiographic SCAD appearance of multiple radiolucent lumen and contrast dye stains in the arterial wall can be demonstrated well on angiography; however, this is not the most frequent angiographic manifestation of SCAD. In fact, the most common angiographic SCAD appearance is the type 2 long diffuse stenosis, which is seen in 2/3 of SCAD cases in our series (13). Type 3 angiographic appearance that mimics atherosclerosis was seen in <5% of our cases. Most angiographers are not familiar with the non-classic angiographic type 2 and 3 appearances of SCAD, contributing to under-diagnosis of SCAD.
IVUS and OCT are both tools that allow angiographers to better visualize the arterial wall structure and composition. Due to financial restraints, most centers only employ one of the two to supplement angiographic studies. Each modality, IVUS or OCT, has its pitfalls and benefits in detecting pathology in arterial walls. IVUS has a lower spatial resolution (150-200 µm), but has deeper penetration allowing for the full vessel and extent of the IMH to be visualized. IVUS can delineate true and false lumens and detect IMH, which appears as a homogenous collection behind the intimal-medial membrane. A small study that utilized IVUS to detect SCAD showed IMH in all five patients who had angiographically normal coronary arteries (11). Many other case reports noted IMH on either normal or smooth long narrowing on angiograms (30-34). These findings were noted to resolve by 3 months after the event (32).
OCT, on the other hand, is a much higher resolution (10-20 µm) modality and can visualize true lumen, false lumen, and even intimal tears exceedingly well. However, it has poorer penetration than IVUS, and may not visualize the full extent of the IMH (35). In the prospective OCT study by Alfonso et al., out of 17 patients with clinical and angiographic suspicion of SCAD, OCT ruled out SCAD in 6 patients and identified an intimal flap in the remaining 11 (only 3 of which showed the flap on normal angiogram). There were 64% with an intimal tear and 82% with IMH on OCT (12). Subacute IMH had also been visualized and documented 7 days post-event (36).
In comparative studies of IVUS and OCT (37,38), OCT was shown to be more sensitive and better at detecting more characteristics of SCAD than IVUS, especially for identifying intimal tears and flaps. Whereas, both OCT and IVUS were able to identify IMH equally.
Other imaging modalities
The majority of studies evaluating SCAD with computerized-tomography (CT) scans were done prior to the widespread use of cardiac CT angiography (CCTA) (34,39). Multi-detector CT scans were used in those cases to follow the course of SCAD and found the presence of IMH on CT 3 days post-event and near resolution on CT with only mild wall thickening at 10 days post-event. Despite improvements with CCTA technology, the spatial resolution is still much lower than conventional angiography. Smaller diameter arteries (<2.5 mm) are not well visualized. Therefore, CCTA is not recommended as first-line imaging to rule out SCAD. However, CCTA might be useful as a non-invasive technique to assess arterial healing after SCAD of larger proximal-mid coronary arteries. A case study on a 34-year-old woman presenting at one-month post-partum with neck pain and S-T elevation on ECG showed a false lumen on CCTA at initial presentation and full resolution at 2 months on repeat CCTA (40). Another case study noted full resolution at 23 days post-event (41).
Magnetic resonance imaging (MRI) has been used in detection of SCAD in one published case study (40). Hyperintensity was noted in the area seen to have an IMH on IVUS and CCTA. Full resolution of hyperintensity was noted at 23 days post-event. This finding is unlikely to be specific or sensitive to detecting SCAD. However, like CCTA, it can be a good non-invasive technique to assess for serial progression of arterial healing with minimal radiation exposure.
Diagnosis
Clinicians should have a high index of suspicion for SCAD especially in young women presenting with MI without traditional cardiovascular risk factors (Table 2). An early invasive coronary angiography for these patients is recommended. If type 1 SCAD appearance is evident, then there is little controversy and the diagnosis of SCAD can be made.
Angiographers should then assess for the presence of atherosclerotic changes in other coronary arteries, and utilize intracoronary imaging if there is uncertainty as to non-atherosclerotic SCAD. If type 1 SCAD appearance is not evident, SCAD diagnosis would be most objectively confirmed by OCT or IVUS. For diffuse (>20 mm) and smooth stenosis (type 2 SCAD), intracoronary nitroglycerin should be administered to relieve potential overlying spasm. If the lesion remains after nitroglycerin, then OCT or IVUS may be pursued. If there are concerns of compromising arterial flow with intracoronary imaging, then repeat angiogram >4 weeks later should be pursued instead to reassess the stenosis, as SCAD typically resolves spontaneously. If the appearance mimics atherosclerosis (type 3 SCAD) and there is a high clinical index of suspicion for SCAD (Table 2), then OCT or IVUS should be pursued. This proposed simple algorithm should help improve the diagnosis of SCAD (Figure 6) (29).
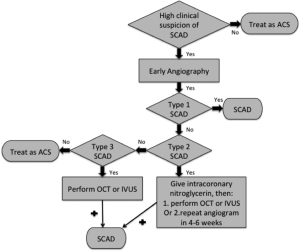
Of note, SCAD may involve any coronary artery, and can affect more than one coronary artery. In our series, multivessel SCAD involving >1 noncontiguous segment affected ~10% of patients (13).
Management
Early diagnosis is important for managing SCAD patients, because the use of unnecessary and potentially harmful pharmacologic therapies may be avoided. Unlike in the case of atherosclerotic coronary artery disease, there are no prospective randomized data that specifically address the management of SCAD.
Medical therapy
It is unclear if the standard ACS pharmaceutical agents are beneficial for SCAD. The role of antiplatelet therapy for SCAD patients not treated with stents is unclear. Considering the totality of evidence for aspirin in ACS and secondary prevention of CAD (42), along with low side-effect profile and bleeding risks, aspirin appears reasonable for acute and long-term SCAD treatment. The addition of clopidogrel for SCAD patients not treated with stents is also of uncertain benefit. Considering that a proportion of SCAD involves intimal tear, which is prothrombotic, this would empirically benefit from dual antiplatelet therapy. Reducing false lumen thrombus burden with antiplatelet agents could theoretically reduce true lumen compression (43). We routinely administer aspirin and clopidogrel for SCAD patients acutely; aspirin is then continued life-long and clopidogrel for up to 1 year. The role of new P2Y12 antagonists (prasugrel and ticagrelor) for SCAD is unclear. The role of GPIIb/IIIa inhibitors for acute SCAD management has also not been evaluated, but because of their greater potency, higher bleeding risk, and potential risk of extending dissection, they are not routinely used for SCAD.
The role of anticoagulation for SCAD is controversial with the risk of dissection extension balanced by the potential benefit of resolving overlying thrombus and improving true lumen patency. Heparin agents are typically administered for ACS patients on hospital presentation; however, we would discontinue heparin once SCAD is proven on angiography to avoid extension of IMH.
Thrombolytic therapy should be avoided in SCAD, as there have been reports of harm and clinical deterioration due to extension of dissection and IMH (44,45). In the retrospective review by Shamloo et al. (44), of 87 SCAD patients who received thrombolysis, 60% had clinical deterioration requiring rescue percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Thus, early coronary angiography is important if SCAD is suspected because medical management deviates from standard ACS therapy. Nevertheless, in remote centers without onsite or established transfer algorithms for primary PCI, thrombolysis should not be withheld for ST-elevation MI patients since the overall frequency of thrombotic occlusion is much higher than SCAD. There were anecdotal reports of successful thrombolysis with SCAD, thought related to lysis of false lumen thrombi (46). Nonetheless, these reports are limited and the bulk of data suggest harm with thrombolysis for SCAD.
Beta-blockers reduce arterial shear stress and are presumably beneficial in reducing coronary arterial wall stress, similar to the benefits in aortic dissection (47). Extrapolating the benefits from aortic dissection literature, we routinely administer beta-blockers acutely and long-term for SCAD. The use of nitroglycerin may be useful in alleviating ischemic symptoms from overlying vasospasm during acute SCAD presentation, but are not routinely used long-term. Angiotensin-converting enzyme inhibitors are only routinely administered when there is significant LV dysfunction after the MI (ejection fraction ≤40%; class 1 indication) (48). The use of statins for non-atherosclerotic SCAD has not been studied, and we would only administer statins in patients with preexisting dyslipidemia.
Revascularization
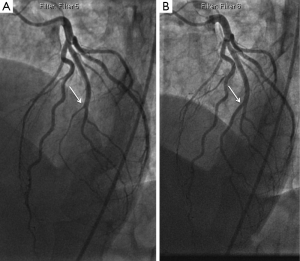
The decision to revascularize the dissected artery depends on the patient’s clinical status and affected coronary anatomy. In most cases, conservative treatment is preferred for stable patients without ongoing pain. Patients with ongoing chest pain, ischemia, ST elevation, or hemodynamic instability should undergo PCI, especially when the dissection affects major arteries with sizable myocardial jeopardy (13) (Figure 7). Patients with dissected left main or proximal segments of left anterior descending (LAD), circumflex or right coronary artery should be intervened percutaneously if feasible. Emergency CABG should be considered if the dissection involves the left main. When the dissected artery segment is distal, of small calibre, or when the dissection is extensive, stenting may not be practical. In our cohort, 80% were treated conservatively with good outcomes, with 4.8% recurrent MI in-hospital and 0% mortality (13). The success rate with PCI for SCAD is poor compared with atherosclerotic lesions, and long-term durability with PCI was only ~30% in our cohort (13). Likewise, in the study by Tweet et al., among 43 SCAD patients who underwent PCI, technical success was achieved in only 65% (18).
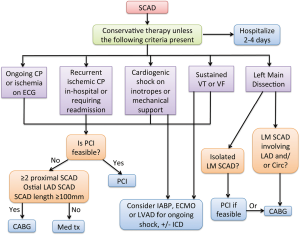
PCI of dissected coronary arteries can be notoriously challenging and often terminate with suboptimal results. To begin, it may be challenging to advance the coronary guidewire into the distal true lumen. The IMH of the dissected segment can also propagate antegradely or retrogradely with angioplasty, further compromising arterial blood flow and extending the dissection. The dissections often involve distal coronary arteries, which are too small to implant stents. Even if the dissected arteries are large, the dissections are often extensive requiring long stents and thus increasing the risks of restenosis. Furthermore, IMH resorb and heal over time, and may result in late strut malapposition, increasing the risk of very late stent thrombosis especially after cessation of dual anti-platelet therapy. Lastly, the natural history of the dissected segments is such that the vast majority heals spontaneously, and patients appear to have good long-term outcome if they survive their initial event. In our cohort, 79 out of 79 SCAD patients underwent repeat coronary angiography and all showed healing of the dissected segments (13). Thus, we recommend reserving PCI for patients with ongoing chest pain and ischemia when the lesion is amenable to stenting, and to consider CABG for extensive dissections involving the left main.
If PCI is attempted, there are strategies that may improve outcome. If the lesion is relatively focal, we recommend selecting longer stents that would provide adequate coverage for both edges of the lesion (at least 5-10 mm longer proximally and distally). This attempts to accommodate extension of the IMH proximally and distally when compressed by the stent. We recommend OCT or IVUS to ensure adequate stent coverage and wall apposition. For longer lesions, a multistep approach of stenting the distal edge, followed by the proximal edge, and then stenting the middle of the dissection, may be useful in preventing IMH propagation (49). The use of bioresorbable stents also has theoretical benefits of avoiding late stent malapposition following resorption of IMH.
There is no consensus as to repeat imaging after SCAD, irrespective of revascularization strategies. Because a significant proportion of patients have recurrent chest pains after their initial event, we find it useful to repeat coronary angiography several weeks later to investigate potential ischemic causes of pain, and to assess arterial healing.
Prognosis
Initial mortality rates appeared to be over-estimated due mainly to post-mortem reporting and little ante-mortem data. A collection of case reports from 1980-2000 showed mortality rates ranging from 0% to 7% (9). More recent studies reported lower in-hospital mortality rates ranging from 1% to 5% (2,9,15). In our recent study, there was no in-hospital mortality in our 168 patient-cohort (20). One-year mortality rates after discharge were similar, at 1% to 4% (9,15). One study reported 10-year mortality rate as high as 7.7% (15). However, when analyzed with matched ACS controls, long-term mortality was lower for SCAD patients than ACS controls in this retrospective study, but cardiac events were similar in the two groups. SCAD patients can also present with recurrence of repeat SCAD, MIs, repeat or subsequent revascularizations, congestive heart failure, and other cardiac events. Many also require subsequent hospital visits for recurrent chest pains, and repeat coronary angiograms. Event-free rates at 1 year range from 74% to 96% (2,9,13). In our series, the 2-year major adverse cardiac event-rate was 10.4% to 16.9%, with recurrent dissection rate of 13.1% (13). The retrospective Mayo Clinic study observed a recurrent dissection rate of 17% (18).
Female SCAD patients potentially have a poorer prognosis. An earlier study analyzing 222 patients from several published studies showed that the strongest predictors of death were female sex (odds ratio 4.27) and absence of early treatment (odds ratio 35.5) (50). In particular, the subgroup patients with postpartum SCAD appear to have the worst prognosis. A study by Ito et al. (51) looked at 7 of 23 SCAD patients who presented postpartum. All survived their initial hospitalization. However, postpartum patients were found to have larger infarcts and lower mean LV ejection fraction (34% vs. 49%; P<0.01). Postpartum women also tended to have proximal artery dissections (86% vs. 19%; P<0.004). We have previously reviewed pregnancy-related SCAD and provided some guidelines as to management of this challenging subset of SCAD patients (14).
Summary
SCAD is an infrequent condition that is under-diagnosed among patients presenting with ACS. Risk factors for SCAD are multifold, including young women, FMD, systemic inflammation, connective tissue disorders and pregnancy, and often compounded by precipitating stressors. The long-term outcome of patients who survived their initial SCAD presentation is good; however, recurrent events are frequent and these patients should be followed closely by cardiovascular specialists. Treatments typically entail conservative medical management for stable patients with ischemia resolution; however, revascularization with PCI or CABG may be necessary in a small proportion of patients. Ongoing prospective studies will hopefully clarify the long-term cardiovascular outcomes of SCAD patients.
Acknowledgements
Disclosure: Dr. Saw has received research grants for SCAD research from Canadian Institutes of Health Research, AstraZeneca, Abbott Vascular, St Jude Medical, and Servier.
References
- Pretty H. Dissecting aneurysm of coronary artery in a woman aged 42. BMJ 1931;1:667.
- Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv 2009;74:710-7. [PubMed]
- Bergen E, Huffer L, Peele M. Survival after spontaneous coronary artery dissection presenting with ventricular fibrillation arrest. J Invasive Cardiol 2005;17:E4-6. [PubMed]
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013;29:1027-33. [PubMed]
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010;96:801-8. [PubMed]
- Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart 2010;96:1119-25. [PubMed]
- Nishiguchi T, Tanaka A, Ozaki Y, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2013. [Epub ahead of print].
- Hering D, Piper C, Hohmann C, et al. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol 1998;87:961-70. [PubMed]
- Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250-4. [PubMed]
- Saw J, Aymong E, Mancini GB, et al. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol 2014;30:814-9. [PubMed]
- Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol 2002;89:466-8. [PubMed]
- Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol 2012;59:1073-9. [PubMed]
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645-55. [PubMed]
- Vijayaraghavan R, Verma S, Gupta N, et al. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014;130:1915-20. [PubMed]
- DeMaio SJ Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol 1989;64:471-4. [PubMed]
- Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol 1967;83:336-41. [PubMed]
- Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44-52. [PubMed]
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579-88. [PubMed]
- Eleid MF, Guddeti RR, Tweet MS, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656-62. [PubMed]
- Saw J, Poulter R, Fung A, et al. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012;5:134-7. [PubMed]
- Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation 2012;125:3182-90. [PubMed]
- Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Hum Pathol 1987;18:654-6. [PubMed]
- Mather PJ, Hansen CL, Goldman B, et al. Postpartum multivessel coronary dissection. J Heart Lung Transplant 1994;13:533-7. [PubMed]
- Brodsky SV, Ramaswamy G, Chander P, et al. Ruptured cerebral aneurysm and acute coronary artery dissection in the setting of multivascular fibromuscular dysplasia: a case report. Angiology 2007-2008;58:764-7. [PubMed]
- Tokura M, Taguchi I, Kageyama M, et al. Clinical features of spontaneous coronary artery dissection. J Cardiol 2014;63:119-22. [PubMed]
- Bergen E, Huffer L, Peele M. Survival after spontaneous coronary artery dissection presenting with ventricular fibrillation arrest. J Invasive Cardiol 2005;17:E4-6. [PubMed]
- Akyuz A, Alpsoy S, Akkoyun DC. Spontaneous coronary artery dissection and woven coronary artery: three cases and a review of the literature. Korean Circ J 2013;43:411-5. [PubMed]
- Lempereur M, Grewal J, Saw J. Spontaneous coronary artery dissection associated with β-HCG injections and fibromuscular dysplasia. Can J Cardiol 2014;30:464.e1-3.
- Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115-22. [PubMed]
- Choi SW, Nam CW, Bae HJ, et al. Spontaneous coronary artery dissection diagnosed by intravascular ultrasound and followed up by cardiac computed tomography. Korean J Intern Med 2013;28:370-3. [PubMed]
- Chung H, Lee SJ, Park JK, et al. Spontaneous coronary artery dissection mimicking coronary spasm diagnosed by intravascular ultrasonography. Korean Circ J 2013;43:491-6. [PubMed]
- Jang JH, Kim DH, Yang DH, et al. Spontaneous coronary artery dissection by intravascular ultrasound in a patient with myocardial infarction. Korean J Intern Med 2014;29:106-10. [PubMed]
- Jinnouchi H, Sakakura K, Matsuda J, et al. Recurrent spontaneous coronary artery dissection observed with multiple imaging modalities. Int Heart J 2013;54:181-3. [PubMed]
- Porto I, Aurigemma C, Pennestrì F, et al. Intravascular ultrasound-documented healing of spontaneous coronary artery dissection. Circ Cardiovasc Interv 2010;3:519-22. [PubMed]
- Alfonso F, Paulo M, Dutary J. Endovascular imaging of angiographically invisible spontaneous coronary artery dissection. JACC Cardiovasc Interv 2012;5:452-3. [PubMed]
- Das Neves BC, Núñez-Gil IJ, Alfonso F, et al. Evolutive recanalization of spontaneous coronary artery dissection: insights from a multimodality imaging approach. Circulation 2014;129:719-20. [PubMed]
- Paulo M, Sandoval J, Lennie V, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging 2013;6:830-2. [PubMed]
- Poon K, Bell B, Raffel OC, et al. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ Cardiovasc Interv 2011;4:e5-7. [PubMed]
- Koh JS, Jeong YH, Yoon SE, et al. A case of spontaneous coronary artery dissection healed by medical treatment: serial findings of coronary angiography, intravascular ultrasound and multi-detector computed tomography. Korean Circ J 2011;41:346-8. [PubMed]
- Russo V, Marrozzini C, Zompatori M. Spontaneous coronary artery dissection: role of coronary CT angiography. Heart 2013;99:672-3. [PubMed]
- Nakashima T, Noguchi T, Morita Y, et al. Detection of intramural hematoma and serial non-contrast T1-weighted magnetic resonance imaging findings in a female patient with spontaneous coronary artery dissection. Circ J 2013;77:2844-5. [PubMed]
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [PubMed]
- Choi JW, Davidson CJ. Spontaneous multivessel coronary artery dissection in a long-distance runner successfully treated with oral antiplatelet therapy. J Invasive Cardiol 2002;14:675-8. [PubMed]
- Shamloo BK, Chintala RS, Nasur A, et al. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol 2010;22:222-8. [PubMed]
- Zupan I, Noc M, Trinkaus D, et al. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter Cardiovasc Interv 2001;52:226-30. [PubMed]
- Leclercq F, Messner-Pellenc P, Carabasse D, et al. Successful thrombolysis treatment of a spontaneous left main coronary artery dissection without subsequent surgery. Eur Heart J 1996;17:320-1. [PubMed]
- Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J 2012;33:26-35b. [PubMed]
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1-e157. [PubMed]
- Walsh SJ, Jokhi PP, Saw J. Successful percutaneous management of coronary dissection and extensive intramural haematoma associated with ST elevation MI. Acute Card Care 2008;10:231-3. [PubMed]
- Thompson EA, Ferraris S, Gress T, et al. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J Invasive Cardiol 2005;17:59-61. [PubMed]
- Ito H, Taylor L, Bowman M, et al. Presentation and therapy of spontaneous coronary artery dissection and comparisons of postpartum versus nonpostpartum cases. Am J Cardiol 2011;107:1590-6. [PubMed]


