
Determining the thresholds for abnormal left ventricular strains in healthy subjects by echocardiography: a meta-analysis
Introduction
Multiple studies have tested the prognostic utility of left ventricular global longitudinal (LVGLS), circumferential (LVGCS) and radial (LVGRS) strains in a wide range of clinical applications, including cardiomyopathies, coronary heart disease and valvular heart disease (1-5). Despite significant investigation, there is no consensus of what constitutes normal variability and what is abnormal strain in an otherwise healthy patient (6,7). Strain measurement variability may stem from biologic variability (e.g., impact of gender, age, body size) or measurement system variability (echocardiographic image quality, software speckle detection, strain calculation). Biologic variability can be resolved by appropriately large, multi-institutional samples of healthy individuals that take into account population diversity. Measurement system variability can be addressed by having a small sample with repeated echocardiographic exams obtained using different ultrasound systems or software. Yet it is close to impossible to perform a study in which both biologic and system variability are assessed.
One way to overcome this problem is to perform a meta-analysis with both biologic and measuring system variability addressed. While meta-analyses of strain measurements have been reported (8-10), they focused on the estimation of the pooled mean of the strain values with corresponding 95% confidence intervals (95% CI) for the mean alone. When determining normal ranges of systolic function parameters in cardiac imaging, such as ejection fraction, fractional area change, tricuspid annular plane systolic excursion and of course strain, the focus is on the threshold at which the value measured becomes abnormally low in magnitude to reflect impaired systolic function, otherwise known as the lower limit of normal (LLN) (7). It is important to note that the 95% CI of the pooled mean by meta-analysis does not accurately reflect the range of normal strain values and does not measure the LLN or its 95% CI, and therefore cannot be used in defining the cut-points for abnormal strain. Our meta-analysis aims to pool the LLNs and update the pooled mean data for two-dimensional (2D-) and three dimensional (3D-) LVGLS, LVGCS and LVGRS by speckle tracking echocardiography in healthy subjects, in order to redefine thresholds of abnormal strains, as well as analyzing baseline parameters that could be associated with left ventricular (LV) strain measurements. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was followed and presented in the conduct of this meta-analysis, without a separate review protocol.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-711).
Methods
Literature search and study selection
PubMed, Cochrane and Embase databases were searched for relevant studies with no restriction on start date until 31 December 2019. The search terms used were (left ventricle) AND (echocardiography) AND (strain OR speckle tracking), with filters adult (age) and human (subject) applied. To be included, studies needed to report: (I) original data of the mean ± standard deviation or standard error, or median (lower quartile, upper quartile); (II) at least one of left ventricular global longitudinal, circumferential and/or radial strain measured by speckle tracking; (III) in at least 50 healthy individuals; (IV) and either sex must make up at least one third of the healthy cohort. The largest study of healthy subjects were selected when there are multiple studies from the same authors. Healthy subjects are defined by absence of known cardiovascular disease, risk factors including hypertension, diabetes and obesity, chronic disease including malignancy and single or multi-organ failure and cardiac medications, with normal cardiac examination and investigations, both explicitly stated and confirmed by baseline characteristics reported. Reference lists of relevant articles were checked, while case reports, guidelines, editorials and letters were excluded.
Data extraction
We extracted the following parameters from eligible studies into spreadsheets: author surname, year, number of subjects, country, definition of group studied, age, sex, body mass index, systolic blood pressure, heart rate, left ventricular ejection fraction (LVEF), left ventricular end diastolic volume (LVEDV), vendor software, frame rate, and type of strain (2D- or 3D-, and views from which longitudinal strain is measured). The strain outcomes of interest extracted were left ventricular global longitudinal (LVGLS), circumferential (LVGCS) and radial (LVGRS) strains. If strain for endo, mid and epicardial were presented separately, mid-wall strain was recorded. One author (TKMW) screened studies for inclusion and extracted the data, and another author (ZP) confirmed all the appropriate studies for inclusion and data entered.
Statistical analyses
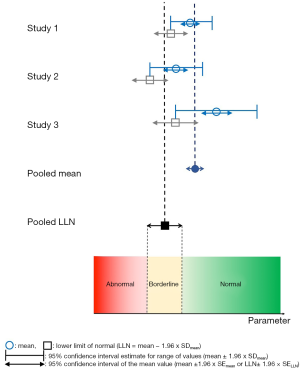
LVGLS, LVGCS and LVGRS by 2D and 3D were separately analyzed. By convention from contemporary echocardiography guidelines and studies, the LLN of strain derived from individual studies of healthy subjects is the boundary of the 95% CI with the lower magnitude of strain, which would be the less negative boundary for LVGLS and LVGCS, and the less positive boundary for LVGRS (7). The process of obtaining pooled mean, pooled lower limit of normal, and corresponding 95% CIs from individual studies using meta-analysis is illustrated in Figure 1. The first step is obtaining mean and standard deviation of individual samples (i.e., studies). If medians (med), lower (q1) and upper (q3) quartiles were reported for a study, we used the equations proposed by Wan et al. below to derive estimates of the sample mean and standard deviation (11):
[1]
[2]
The mean and SD of mean of individual studies can then be used in meta-analysis to obtain pooled mean and corresponding 95% CI. In an identical manner, we used LLN and SDLLN of individual samples (studies), to calculate the pooled LLN and corresponding 95% CI using meta-analysis. We defined LLN for longitudinal and circumferential strains as the upper boundary of the 95% CI for the sample mean strain calculated as mean plus 1.96 × standard error of the mean as this corresponds to a lower “absolute” (i.e., less negative) value for strain. LLN for radial strain were calculated in a standard manner. To calculate standard error of the lower limit of normal LLN (SELLN) of individual samples, we used the following formula (where SDmean is the standard deviation of the sample mean and n is number of patients in the sample) (12):
[3]
From this, we calculated as the parameter k as:
[4]
Where k is used in conjunction with LLN of individual samples in the same way SDmean is used in conjunction with sample means.
To perform meta-analysis, we pooled both the mean ± SDmean and LLN ± k across studies using the DerSimonian-Laird method and random effects models. We also analyzed the mean and LLN of LVGLS by baseline characteristics subgroups (either categorical or mean quantitative variables) if reported by at least two studies. Heterogeneity of studies were assessed using the Cochrane Q test (P value) and I2 (inconsistency) statistic. Study bias was not separately assessed as we were determining abnormal strain thresholds in healthy subjects. Univariable meta-regression was used to identify associations between baseline characteristics with LVGLS mean and LLN, using the reported means or medians for continuous variables or proportion for categorical variables for characteristics at study level. A positive beta coefficient indicates higher quantitative parameter or the presence of a categorical parameter correlates with a more positive (i.e., less negative or less absolute) strain. The reference group of countries and vendors/programs for comparison were Europe and GE Echopac as they were the most common subgroup for their classifications. Funnel plots were used to assess for publication bias. OpenMeta-Analyst software was used for all analysis (13), and P<0.05 was deemed to be statistically significant.
Results
A total of 773 studies were obtained from the literature search excluding duplicates, and after reviewing all abstracts, 102 studies passed initial screening for full-text review, and 44 studies were eligible for analysis (14-38), totaling 8,910 subjects (Figure 2) (39-57). In one study we were able to obtain unpublished means and standard deviations for LVGLS from the authors (15). Characteristics of the eligible studies are displayed in Table 1. Studies were published between 2008–2019, the number of healthy subjects varied from 50–2,008, mean age 28–67 years old, and male sex made up 39–65% (overall 50%). 2D-strain was reported in 38 studies and 3D-strain in 7.
Full table
Table 2 lists the pooled means and LLNs with heterogeneity testing of strain parameters on 2D and 3D. For 2D LV strain measurements, the pooled 2D-LVGLS mean and LLN (95% CI) were −20.1% (−20.7%, −19.6%) and −15.4% (−16.0%, −14.7%) respectively (Figure 3). The pooled 2D LVGCS mean and LLN (95% CI) were −21.9% (−23.4%, −20.3%) and −15.3% (−16.9%, −13.8%) respectively (Figure 4). The pooled 2D LVGRS mean and LLN (95% CI) were 48.4% (43.8%, 53.0%) and 25.5% (17.8%, 33.1%) respectively (Figure 5).

Full table
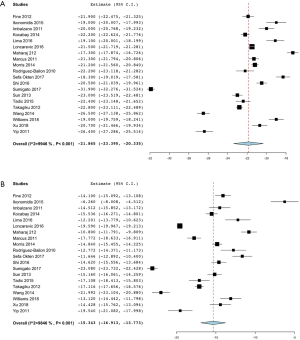
For 3D LV strain measurements, the pooled 3D LVGLS mean and LLN (95% CI) were −18.5% (−20.1%, −16.9%) and −14.5% (−16.8%, −12.3%) respectively (Table 2). The pooled 3D LVGCS mean and LLN were −25.0% (−30.8%, −19.3%) and −17.6% (−22.6%, −12.6%) respectively. The pooled 3D LVGRS mean and LLN were 49.4% (40.8%, 58.0%) and 27.9% (16.8%, 38.9%) respectively.
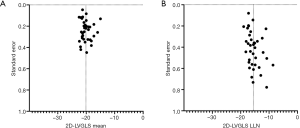
Significant heterogeneity was found in all pooled 2D and 3D LV global strain analyses by Cochrane Q test and I2 statistic. Funnel plots were symmetrical without revealing significant publication bias for all analyses, and the plots for 2D-LVGLS mean and LLN are shown in (Figure 6).
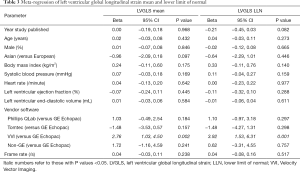
Results of the meta-regression analysis for 2D-LVGLS are shown in Table 3. The only factor associated with 2D-LVGLS means and LLNs was vendor software, specifically velocity vector imaging (VVI) having less negative values than GE Echopac. There was no significant interaction between LVGLS mean or LLN with year of publication, sex, region, body mass index, heart rate, systolic blood pressure, left ventricular ejection fraction or end-diastolic volume, other vendors and frame rate.
Full table
Discussion
Prior meta-analyses of LV strain assessment in healthy subjects focused on accurately defining the point estimate of the pooled average LV strain (8,9). A recent updated meta-analysis was able to use patient-level data in 16 studies and 2,396 subjects to determine the pooled means with 95% CIs and LLN of LVGLS only by vendor (10). The novelty of our findings is that we now also provide the estimate for the pooled LLN and the 95% CI of LLN using meta-analysis techniques for LVGLS, LVGCS and LVGRS, and for 2D- and 3D-strain, on top of being able to pool larger number of patients at the study level. This information is highly relevant as it can help define what should be considered an abnormal strain value in clinical practice. We also provide meta-regression results showing persistence of a small, but still present, between-vendor difference in strain despite recommendations for standardization of strain imaging with different LLNs (6). Finally, we did not detect any temporal trends in strain variability for both mean value and lower limit of normal. Notably, we focused on healthy participants with no cardiovascular disease or risk factors that could affect LV strain measurement so some large population studies were excluded (58,59), and also excluded studies that didn’t use speckle-tracking to measure LV strain such as tissue Doppler techniques (60).
Interpretation of the pooled means and LLNs for normal strain
The goal of meta-analysis is to combine results from multiple studies that address a similar question in order to increase power of point estimate of some clinically relevant parameter. Most commonly, we seek to obtain pooled parameters of a single important point estimate, such as relative risk or odds ratio. In that manner previous meta-analyses of LV strain in healthy subjects focused on a point estimate of pooled mean strain, with a corresponding 95% CI providing a measure of precision of this point estimate (8,9). However, as pooled 95% CI of the mean depend on sample size, they eventually become narrow with larger numbers, and do not reflect the general distribution of strain values in healthy subjects. These issues prompted us to develop a meta-analysis approach to define the LLN, with the specific purpose of detecting threshold beyond which strain measurements become “abnormal”. We addressed this task by emulating the approach of Bland who reported an elegant formula for estimating the standard error for the boundaries of the 95% CI of the mean, i.e., LLN (12). We can then combine these LLNs in a meta-analysis fashion to estimate the pooled LLN and its 95% CI for all strain parameters. With this approach it is important to note that, by default, the pooled standard error for the LLN is higher than that of the pooled standard error of the mean. Furthermore, if we used 99% confidence interval for our definition of the LLNs, the estimated LLN would be even further from the mean with its own, wider, confidence interval.
How should the pooled LLNs found in this meta-analysis be interpreted? Using the LLN of 2D-LVGLS as an example, the pooled estimate of −15.4% could be set as the cut-point for abnormal 2D-LVGLS. However, the 95% CI of LLN, in this case −16.0% to −14.7% presents further complexities to its interpretation, as this is the range for which the real LLN is thought to most likely (but not definitely) lie. The pragmatic approach to its use this would be if 2D-LVGLS was below (more negative than) −16.0% then it is normal, and if it is above (less negative) than −14.7%, then it is abnormal. The range of values between −16.0% to −14.7% would then be in the borderline grey zone. Our pooled LLN for LVGLS is similar to the −18% to −14% LLNs depending on vendor in the most recent updated meta-analysis (10).
The pooled means do have value in illustrating the average strain values for healthy subjects, subgroups and allow comparisons between studies. Our updated meta-analysis reported similar pooled means (95% CI) to the −19.7% (−20.4%, −18.9%), −23.3% (−24.6%, −22.1%) and 47.3% (43.6%, 51.0%) for 2D-LVGLS, LVGCS and LVGRS respectively reported in the earliest 2D-LV strain meta-analysis (8), and the more recent updated meta-analysis −21.0% (−19.2%, −22.7%) (10). We also found similar pooled means for 3D-LV strain to the −19.1% (−19.9%, −18.2%), −22.4% (−21.0%, −23.9%) and 47.5% (41.5−53.5%) reported in the previous 3D-LV strain meta-analysis (9), and in fact, 3D and 2D estimates were also similar. Still, there remains a significant heterogeneity between individual studies observed in previous meta-analyses and ours, even though the pooled mean LV strains for healthy subjects were consistent, reflecting the multitude of factors that influence these measurements (8,9).
Factors that influence strain by subgroups and regression
The only parameter we found on meta-regression for both the mean and LLN of 2D-LVGLS was vendor software. VVI had less negative 2D-LVGLS mean (by 2.8%) and LLN (by 3.9%) than GE Echopac whereas the Phillips QLab and Tomtech did not. The earliest 2D-LVGLS meta-analysis compared non-GE versus GE vendor software in its meta-regression, and this was not associated with mean 2D-LVGLS, although P value was 0.08 and VVI was not specifically analyzed (8). The more recent updated meta-analysis reported separate pooled means and LLNs by vendor software (GE −21.2% and −18.2%, Toshiba −19.9% and −15.8%, Philips −19.6% and −15.5%, and Siemens −16.9% and −14.0% respectively) showed similar pattern to our findings (10). Furthermore, vendor software was significantly associated with 3D-strain in the other previous meta-analysis, where Toshiba and 3DWM tracking software had less negative mean 3D-LVGLS than Echopac, GE and Phillips (9). Other studies including a wider distribution of normal and abnormal strains have also found VVI to produce less negative values to GE (61). We therefore recommend using a different LLN for VVI adjusted by the meta-regression beta-coefficient from the overall values, or using one of the alternative vendor software instead. Further large studies are required to establish this threshold for VVI as current data (two studies in this meta-analysis) is limited.
Limitations
This meta-analysis has some limitations. We assumed that all eligible studies had experienced readers following standardized methods and accurately measured LV strain which may not always be the case (6). Smaller studies and those with over-representation of male or female populations by more than two thirds were excluded to reduce bias, although this reduced the total number of subjects that couldn’t have been meta-analyzed with higher power. Heterogeneity was present for the range of normal LV strain reported across studies, as well as study design, populations and equipment, however this is expected and presented in all previous strain meta-analyses (8,9), and we also analyzed for parameters that may affect variations in LV strain measurements. Subgroup analysis particularly for the minority groups like the elderly age-group and non-GE vendors have wider 95% CI and may be underpowered. Notably, we defined LLN mathematically as the boundary of the 95% CI of the mean from individual studies and pooled this, which is by convention and arbitrary for echocardiographic chamber quantification (7) but also not based on prognostic significance. The meta-regression analysis has some biases and power in terms of missing baseline characteristics in some studies as well as the lack of patient-level data. Publication bias may be present as for any meta-analysis although we did not find significant evidence for this.
Conclusions
This meta-analysis study aimed to define the thresholds of abnormal LV strains in healthy subjects by determining the pooled LLN and the 95% confidence interval of LLN. Based on this, we can classify LV strain parameters as normal, borderline or abnormal. For example, for 2D-LVGLS the pooled LLN (95% CI) was −15.4% (−16.0%, −14.7%), therefore if 2D-LVGLS is less negative than −14.7% it would be abnormal, between −16.0% and −14.7% would be borderline, and more negative than −16.0% is normal. The pooled LLNs and updated pooled mean for 2D and 3D LVGLS, LVGCS and LVGRS parameters are provided. Meta-regression analysis revealed significant differences in LV strain by vendor software, but no differences by time, clinical or echocardiographic factors. These abnormal and borderline thresholds and methodology and methodology have significant clinical and academic applications for future practice and studies of LV strain.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-711
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-711). TKMW received a clinical and research fellowship grant from the National Heart Foundation of New Zealand (number 1775). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014;100:1673-80. [Crossref] [PubMed]
- Sengeløv M, Jorgensen PG, Jensen JS, et al. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure With Reduced Ejection Fraction. JACC Cardiovasc Imaging 2015;8:1351-9. [Crossref] [PubMed]
- Ersbøll M, Valeur N, Mogensen UM, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013;61:2365-73. [Crossref] [PubMed]
- Magne J, Cosyns B, Popescu BA, et al. Distribution and Prognostic Significance of Left Ventricular Global Longitudinal Strain in Asymptomatic Significant Aortic Stenosis: An Individual Participant Data Meta-Analysis. JACC Cardiovasc Imaging 2019;12:84-92. [Crossref] [PubMed]
- Alashi A, Khullar T, Mentias A, et al. Long-Term Outcomes After Aortic Valve Surgery in Patients With Asymptomatic Chronic Aortic Regurgitation and Preserved LVEF: Impact of Baseline and Follow-Up Global Longitudinal Strain. JACC Cardiovasc Imaging 2020;13:12-21. [Crossref] [PubMed]
- Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1-11. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Yingchoncharoen T, Agarwal S, Popovic ZB, et al. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 2013;26:185-91. [Crossref] [PubMed]
- Truong VT, Phan HT, Pham KNP, et al. Normal Ranges of Left Ventricular Strain by Three-Dimensional Speckle-Tracking Echocardiography in Adults: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr 2019;32:1586-1597.e5. [Crossref] [PubMed]
- D'Elia N, Caselli S, Kosmala W, et al. Normal Global Longitudinal Strain: An Individual Patient Meta-Analysis. JACC Cardiovasc Imaging 2020;13:167-9. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Bland M. An Introduction to Medical Statistics. 4th ed. Oxford: Oxford University Press, 2015.
- Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. Journal of Statistical Software 2012;49:1-15. [Crossref]
- Abou R, Leung M, Khidir MJH, et al. Influence of Aging on Level and Layer-Specific Left Ventricular Longitudinal Strain in Subjects Without Structural Heart Disease. Am J Cardiol 2017;120:2065-72. [Crossref] [PubMed]
- Asch FM, Miyoshi T, Addetia K, et al. Similarities and Differences in Left Ventricular Size and Function among Races and Nationalities: Results of the World Alliance Societies of Echocardiography Normal Values Study. J Am Soc Echocardiogr 2019;32:1396-1406.e2. [Crossref] [PubMed]
- Bernard A, Addetia K, Dulgheru R, et al. 3D echocardiographic reference ranges for normal left ventricular volumes and strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:475-83. [Crossref] [PubMed]
- Bogunovic N, van Buuren F, Esdorn H, et al. Physiological left ventricular segmental myocardial mechanics: Multiparametric polar mapping to determine intraventricular gradients of myocardial dynamics. Echocardiography 2018;35:1947-55. [Crossref] [PubMed]
- Cameli M, Mandoli GE, Nistor D, et al. Left heart longitudinal deformation analysis in mitral regurgitation. Int J Cardiovasc Imaging 2018;34:1741-51. [Crossref] [PubMed]
- Caselli S, Montesanti D, Autore C, et al. Patterns of left ventricular longitudinal strain and strain rate in Olympic athletes. J Am Soc Echocardiogr 2015;28:245-53. [Crossref] [PubMed]
- Değirmenci H, Demirelli S, Arisoy A, et al. Myocardial deformation and total atrial conduction time in the prediction of cardiac involvement in patients with pulmonary sarcoidosis. Clin Respir J 2017;11:68-77. [Crossref] [PubMed]
- Fine NM, Shah AA, Han IY, et al. Left and right ventricular strain and strain rate measurement in normal adults using velocity vector imaging: an assessment of reference values and intersystem agreement. Int J Cardiovasc Imaging 2013;29:571-80. [Crossref] [PubMed]
- Ikonomidis I, Tzortzis S, Triantafyllidi H, et al. Association of impaired left ventricular twisting-untwisting with vascular dysfunction, neurohumoral activation and impaired exercise capacity in hypertensive heart disease. Eur J Heart Fail 2015;17:1240-51. [Crossref] [PubMed]
- Imbalzano E, Zito C, Carerj S, et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography 2011;28:649-57. [Crossref] [PubMed]
- Inoue K, Okayama H, Nishimura K, et al. Right ventricular septal pacing preserves global left ventricular longitudinal function in comparison with apical pacing: analysis of speckle tracking echocardiography. Circ J 2011;75:1609-15. [Crossref] [PubMed]
- Jørgensen PG, Jensen MT, Mogelvang R, et al. Impact of type 2 diabetes and duration of type 2 diabetes on cardiac structure and function. Int J Cardiol 2016;221:114-21. [Crossref] [PubMed]
- Kaku K, Takeuchi M, Tsang W, et al. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 2014;27:55-64. [Crossref] [PubMed]
- Kleijn SA, Pandian NG, Thomas JD, et al. Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: results from a multicentre study. Eur Heart J Cardiovasc Imaging 2015;16:410-6. [Crossref] [PubMed]
- Kocabay G, Muraru D, Peluso D, et al. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed) 2014;67:651-8. [Crossref] [PubMed]
- Kotwica T, Relewicz J, Rojek A, et al. Role of galectin-3 in subclinical myocardial impairment in psoriasis. J Eur Acad Dermatol Venereol 2019;33:136-42. [Crossref] [PubMed]
- Kusunose K, Yamada H, Nishio S, et al. Validation of longitudinal peak systolic strain by speckle tracking echocardiography with visual assessment and myocardial perfusion SPECT in patients with regional asynergy. Circ J 2011;75:141-7. [Crossref] [PubMed]
- Larsen AH, Clemmensen TS, Wiggers H, et al. Left Ventricular Myocardial Contractile Reserve during Exercise Stress in Healthy Adults: A Two-Dimensional Speckle-Tracking Echocardiographic Study. J Am Soc Echocardiogr 2018;31:1116-1126.e1. [Crossref] [PubMed]
- Lima MS, Villarraga HR, Abduch MC, et al. Comprehensive left ventricular mechanics analysis by speckle tracking echocardiography in Chagas disease. Cardiovasc Ultrasound 2016;14:20. [Crossref] [PubMed]
- Loncarevic B, Trifunovic D, Soldatovic I, et al. Silent diabetic cardiomyopathy in everyday practice: a clinical and echocardiographic study. BMC Cardiovasc Disord 2016;16:242. [Crossref] [PubMed]
- Maharaj N, Peters F, Khandheria BK, et al. Left ventricular twist in a normal African adult population. Eur Heart J Cardiovasc Imaging 2013;14:526-33. [Crossref] [PubMed]
- Marcus KA, Mavinkurve-Groothuis AM, Barends M, et al. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J Am Soc Echocardiogr 2011;24:625-36. [Crossref] [PubMed]
- Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging 2009;2:80-4. [Crossref] [PubMed]
- Menting ME, McGhie JS, Koopman LP, et al. Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography 2016;33:1665-75. [Crossref] [PubMed]
- Mochizuki Y, Tanaka H, Matsumoto K, et al. Impaired Mechanics of Left Ventriculo-Atrial Coupling in Patients With Diabetic Nephropathy. Circ J 2016;80:1957-64. [Crossref] [PubMed]
- Morris DA, Otani K, Bekfani T, et al. Multidirectional global left ventricular systolic function in normal subjects and patients with hypertension: multicenter evaluation. J Am Soc Echocardiogr 2014;27:493-500. [Crossref] [PubMed]
- Muraru D, Cucchini U, Mihaila S, et al. Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects: reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr 2014;27:858-871.e1. [Crossref] [PubMed]
- Ng AC. Left ventricular longitudinal and radial synchrony and their determinants in healthy subjects. J Am Soc Echocardiogr 2008;21:1042-8. [Crossref] [PubMed]
- Reckefuss N, Butz T, Horstkotte D, et al. Evaluation of longitudinal and radial left ventricular function by two-dimensional speckle-tracking echocardiography in a large cohort of normal probands. Int J Cardiovasc Imaging 2011;27:515-26. [Crossref] [PubMed]
- Rodríguez-Bailón I, Jimenez-Navarro MF, Perez-Gonzalez R, et al. Left ventricular deformation and two-dimensional echocardiography: temporal and other parameter values in normal subjects. Rev Esp Cardiol 2010;63:1195-9. [PubMed]
- Sefa Okten M, Tuluce K, Yakar Tuluce S, et al. Screening first-degree relatives of patients with idiopathic dilated cardiomyopathy. Herz 2017;42:669-76. [Crossref] [PubMed]
- Shi J, Pan C, Kong D, et al. Left Ventricular Longitudinal and Circumferential Layer-Specific Myocardial Strains and Their Determinants in Healthy Subjects. Echocardiography 2016;33:510-8. [Crossref] [PubMed]
- Sugimoto T, Dulgheru R, Bernard A, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:833-40. [Crossref] [PubMed]
- Sullere V, Jain D, Sullere S, et al. Global longitudinal strain, ejection fraction, effort tolerance and normal echocardiography measurements in healthy Indians. Indian Heart J 2018;70:637-41. [Crossref] [PubMed]
- Sun JP, Lam YY, Wu CQ, et al. Effect of age and gender on left ventricular rotation and twist in a large group of normal adults--a multicenter study. Int J Cardiol 2013;167:2215-21. [Crossref] [PubMed]
- Tadic M, Ilic S, Cuspidi C, et al. Left Ventricular Mechanics in Untreated Normotensive Patients with Type 2 Diabetes Mellitus: A Two- and Three-dimensional Speckle Tracking Study. Echocardiography 2015;32:947-55. [Crossref] [PubMed]
- Takigiku K, Takeuchi M, Izumi C, et al. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J 2012;76:2623-32. [Crossref] [PubMed]
- Voilliot D, Huttin O, Hammache N, et al. Impact of Global and Segmental Hypertrophy on Two-Dimensional Strain Derived from Three-Dimensional Echocardiography in Hypertrophic Cardiomyopathy: Comparison with Healthy Subjects. J Am Soc Echocardiogr 2015;28:1093-102. [Crossref] [PubMed]
- Wang J, Fang F, Wai-Kwok Yip G, et al. Changes of ventricular and peripheral performance in patients with heart failure and normal ejection fraction: insights from ergometry stress echocardiography. Eur J Heart Fail 2014;16:888-97. [Crossref] [PubMed]
- Williams LK, Misurka J, Ho CY, et al. Multilayer Myocardial Mechanics in Genotype-Positive Left Ventricular Hypertrophy-Negative Patients With Hypertrophic Cardiomyopathy. Am J Cardiol 2018;122:1754-60. [Crossref] [PubMed]
- Xia JZ, Xia JY, Li G, Ma WY, Wang QQ. Left ventricular strain examination of different aged adults with 3D speckle tracking echocardiography. Echocardiography 2014;31:335-9. [Crossref] [PubMed]
- Xu L, Wang N, Chen X, et al. Quantitative evaluation of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography among young adults with essential hypertension in China. Medicine 2018;97:e12448. [Crossref] [PubMed]
- Yang WI, Kim JS, Kim SH, et al. An exaggerated blood pressure response to exercise is associated with subclinical myocardial dysfunction in normotensive individuals. J Hypertens 2014;32:1862-9. [Crossref] [PubMed]
- Yip GW, Zhang Q, Xie JM, et al. Resting global and regional left ventricular contractility in patients with heart failure and normal ejection fraction: insights from speckle-tracking echocardiography. Heart 2011;97:287-94. [Crossref] [PubMed]
- Biering-Sørensen T, Biering-Sorensen SR, Olsen FJ, et al. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging 2017;10:e005521. [Crossref] [PubMed]
- Biering-Sørensen T, Kabir M, Waks JW, et al. Global ECG Measures and Cardiac Structure and Function: The ARIC Study (Atherosclerosis Risk in Communities). Circulation. Circ Arrhythm Electrophysiol 2018;11:e005961. [Crossref] [PubMed]
- Dalen H, Thorstensen A, Aase SA, et al. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr 2010;11:176-83. [PubMed]
- Anwar S, Negishi K, Borowszki A, et al. Comparison of two-dimensional strain analysis using vendor-independent and vendor-specific software in adult and pediatric patients. JRSM Cardiovasc Dis 2017;6:2048004017712862. [Crossref] [PubMed]





