
Guide to functional mitral regurgitation: a contemporary review
Introduction
Mitral regurgitaion is categorized as functional mitral regurgitation (FMR) in the setting of normal mitral leaflet morphology, but dilated mitral annulus due to left ventricular dilation/dysfunction, typically secondary to ischemic or non-ischemic cardimoyopathic myocardial disease (1,2). The prevalence of FMR continues to rise, and 4million people in the USA are expected to be diagnosed with FMR by 2030 (3). Prior studies have shown that significant FMR developed in ~50% of patients after myocardial infarction (MI) (4-7) and up to 50% of patients with heart failure (HF) (8). Moreover, FMR portends poor prognosis, with mortality rates ranging from 15–40% at 1 year (9-12). Therefore, there has been much interest and effort to develop optimized methods for quantifying and classifying the severity of FMR, as well as developing effective therapeutic interventions to improve outcomes in patients with significant FMR.
Pathophysiology of FMR
FMR is characterized by normal MV leaflets which are apically displaced in the left ventricle and occur in 20–25% of patients with adverse prognosis after revascularization or MI (11). The MV consists of anterior and posterior leaflets, which attach to the mitral annulus. The mitral subvalvular apparatus, comprises of 2 main papillary muscles (PM) (anterolateral and posteromedial) arising from LV myocardium, and chordae tendineae, which support the mitral leaflets (1). Carpentier’s classification provides important categorization of mitral regurgitation, based on the etiology/mechanism of MV disease: type I represents normal valve movement, such as annular dilation or leaflet perforation; type II represents excessive movement; type III represents restrictive movement with IIIa defined as diastolic restriction such as rheumatic disease, and IIIb systolic restriction as in functional disease (13). FMR results from a complex relationship of LV dilation/dysfunction, abnormal valvular/subvalvular apparatus geometry, displacement of one or both PM, resultant MV leaflet tethering and incomplete systolic MV closure in both ischemic cardiomyopathy (ICM) and nonischemic cardiomyopathy (NICM) FMR. In FMR, there is a complex interplay of annular dilatation, PM displacement with increased systolic leaflet tethering and regional or global LV remodeling results in incomplete mitral leaflet closure in the setting of normal mitral leaflets (14). Global LV enlargement or remodeling/scarring can affect PMs, causing posteriorly directed or central MR (15). In addition, normal saddle-shape of annulus is important to maintain normal leaflet stress, and the loss of this shape/annular flattening can result in increased leaflet stress with chronic MR leading to increased mitral leaflet area, insufficient leaflet remodeling all which contribute to severe MR (1,16).
Ischemic MR (IMR)
IMR is the most frequent etiology of regurgitation in FMR. LV remodeling after MI results in PM displacement causing systolic tenting of the MV and this can occur with normal left ventricular ejection fraction (LVEF) as ventricular remodeling with regional wall motion abnormalities can cause MV tethering causing reduced closing forces causing regurgitation (17). The PM contributes nonextensible chordae to both anterior and posterior mitral leaflets. LV remodeling can lead the PM to displace apically, which results in a more apical position of the leaflets and their coaptation point causing a deformity of anterior leaflet described as ‘seagull sign’ (18). In addition, reduced closing forces can cause reduction in synchronicity between the two PMs, alter systolic annular contraction and decrease LV contractility which leads to a self-perpetuating physiology where resultant MR leads to further ventricular dilatation, leading to further PM displacement, annular enlargement and further regurgitation (18-20). Type IIIb Carpentier classification is the most common form of IMR, with restricted motions of margins of the leaflets in systole. IMR is defined by (I) prior history of MI, (II) tethering of posterior-medial scallop of posterior leaflet (most common), and (III) type IIIb Carpentier’s dysfunction with restricted leaflet motion in systole (13). IMR can be further classified based on echocardiogram parameters into asymmetric or symmetric tethering patterns. Asymmetric tethering is associated with inferolateral MI with inferolateral remodeling, and increased tenting areas with akinesia or dyskinesia at the base of the LV causing a MR jet that is posterior directed. Symmetric outflow tract tethering pattern is seen with large anterior or multiple infarcts leading to greater eccentric remodeling, bi-leaflet apical tethering, larger tenting areas and a MR jet that is centrally directed (20). In addition, FMR, including IMR, is dynamic in nature with flow mainly occurring in early and late systole and decreased regurgitation in mid-systole since LV maximally exerts force to close the leaflets in mid-systole, reducing the orifice area (14).
Nonischemic causes of FMR
Non-ischemic causes of FMR include idiopathic dilated cardiomyopathy and atrial fibrillation. Typically, NICM FMR is characterized by LV/LA dilation, mitral annular dilation, loss of mitral annulus contraction, and inadequate MV leaflet length (Carpentier type I MR) (21), with resultant MV mal-coaptation. While the pattern of mitral annular dilation is typically symmetric in NICM FMR due to global LV dysfunction, the mitral annular dilation is typically greatest in the septal-lateral direction, and correlates with the severity of LV dysfunction (22). Functional atrial mitral regurgitation has recently been recognized as an important cause of FMR due to atrial fibrillation. Atrial fibrillation frequently results in significant atrial dilation and remodeling, resulting in mitral annular enlargement and reduced leaflet coaptation causing MR even without LV systolic dysfunction. In addition, the dilated left atrium (LA) has been shown to cause anterior mitral leaflet flattening along the mitral annular plane and postural mitral leaflet bent toward the LV cavity, restricting its movement (23).
Prognostic implications
FMR is associated with higher cardiovascular mortality in patients with HF with reduced EF (HFrEF) (24). Studies have shown that patients with HFrEF and significant FMR have impaired left atrial function, higher LV filling and pulmonary pressures, higher incidence of right ventricular dysfunction and overall worse clinical status (25). In patients with non-ST segment elevation MI (NSTEMI), presence and degree of MR was associated with worse long-term prognosis, especially after first acute coronary syndrome event (9). FMR has been shown as an independent predictor of death and heart transplantation in patients with less severe symptoms (not advanced HF) of HF, indicating that FMR plays a major role in early phase of HF (26). When comparing MR five days post-MI, at one month and 20 months post MI, the severity of MR at baseline was associated with larger LV end-diastolic and end-systolic volumes, increased sphericity index and reduced EF and over worse LV function indicating increased likelihood of adverse outcomes (27). Studies have shown that significant functional MR developed in ~50% of patients after an MI (4,6) and is associated with more deaths and complications than the combination of all other consequences of MI (28).Moreover, FMR was associated with more deaths and complications than the combination of all other consequences of MI (28), as well as more hospitalizations and worse long-term prognosis than patients with chronic HF, but without significant FMR (12,29,30).
Imaging evaluation of functional MR
Echocardiogram
Echocardiogram is typically the primary diagnostic method of assessment. Echocardiography provides comprehensive assessment of LV systolic and diastolic function, MV and mitral annular morphology, left atrial size, and right ventricular function. Severity of MR is evaluated by integrating both qualitative and quantitative assessments. American Society of Echocardiography guidelines on MR grading is provided in Figure 1 (see Figure 1). Qualitative assessments include MV morphology, color flow imaging and continuous wave Doppler assessment of the MR jet. Functional MR jets are typically central if there is more symmetric annular dilation or posteriorly directed when there is posterior mitral leaflet restriction.

Flow convergence analysis is the most widely used method for quantifying mitral regurgitation, where color-flow imaging proximal to the regurgitant orifice is used to measure proximal iso-velocity surface area (PISA). This method allows the measurement of effective regurgitant orifice area (EROA) and regurgitant volume (RVol) (2). The PISA method is the most quantitative method, and it applies principle that blood approaching a circular orifice will form concentric, hemispheric shell of increasing velocity and decreasing surface area; however limitation of this method is assumption that EROA is circular in shape. This assumption is problematic in the setting of FMR, where the orifice tends to be ovoid in shape. In addition, it is essential that color wave doppler signal is well aligned with the regurgitant jet to measure EORA and poor alignment with an eccentric jet will lead to underestimated velocity and overestimation of EROA by PISA (31,32).
Quantification of FMR, using the EROA method, has been a topic of much controversy. In 2014, the ACC/AHA released guidelines, which decreased the threshold for severe FMR to EROA ≥0.2 cm or regurgitant volume ≥30 mL, based on studies demonstrating an increase risk in mortality with EROA ≥0.2 cm2 (12,33-35). This change in EROA threshold resulted from the fact that total LV forward stroke volume (SV) may be reduced in the setting of cardiomyopathy. Thus resultant MR volume (MRV) would likely be lower than in primary MR in most cases. Additionally, because the ERO in FMR is frequently elliptical and dynamic, and because the 2D PISA method assumes a round and static orifice, both EROA and vena contracta width measurements may be significantly underestimated (36,37). However, much debate ensued regarding the validity of the change in criteria and whether the quantification criteria for FMR should be based on prognosis alone (36-39). Thus, the ACC/AHA released an update to the Valve Guidelines in 2017, stating that the threshold criteria for FMR should now be the same as that for primary MR (EROA ≥0.4 cm2, RVol ≥60 mL, regurgitant fraction ≥50%) (40). Interestingly, the European guidelines have maintained the secondary MR threshold EROA ≥0.2 cm2, and a recent study suggested that the optimal cut off might be EROA ≥0.3 cm2 (22,41).
Alternatively, the vena contract method can also be used to quantify FMR. Vena contracta refers to the width of regurgitant jet as it escapes regurgitant orifice, reflecting the regurgitant orifice area. Vena contracta in IMR is elongated along the mitral coaptation line, and a mean vena contracta width is obtained from 4 chamber and 2 chamber views with vena contracta <0.3 cm considered mild MR and >0.7 cm considered as severe MR (20). With FMR, the major limitation to vena contracta assessment is that the orifice is usually slit-like or elliptical. Therefore, the vena contract method tends to underestimate MR severity.
3D echocardiography provides more comprehensive assessment of the MV apparatus compared to 2D echocardiography, and better delineates the spatial relationship between MV and LV and bimodal/saddle shape of the mitral annulus. It can be used to measure coaptation depth, tenting area and the angle subtended by posterior MV leaflet (15). These details can allow the clinician to elucidate the etiology of FMR, for example in IMR, typically the posteriomedial PM is displaced leading to asymmetric tethering and restricted closure of medial portion of the posterior leaflet and nonischemic MR usually involves displacement of both PMs leading to central MR and even tethering lengths demonstrated on 3D imaging (42) 3D echo cam overcomes the assumptions of a circular regurgitant orifice area, by permitting direct planimetry of the vena contracta regardless of orifice shape or number of jets (43).
Cardiac MRI
Cardiac MRI (CMR) has demonstrated to be the gold standard in quantification of ventricular size, function, as well as ventricular remodeling (44). Mitral regurgitant can also be quantified by CMR by deriving the mitral regurgitant volume and mitral regurgitation fraction as the calculated difference between the LV SV (determined by endocardial segmentation of cine images) and forward aortic flow volume using breath-held phase-contrast imaging (see Figure 2). The mitral regurgitant fraction is calculated using the equation: MRV/LV SV) × 100%. This indirect method has been shown by several prior publications to have excellent reproducibility (lower variability compared to echocardiography) (45).
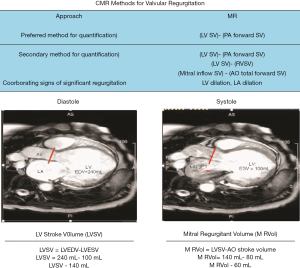
CMR also provide superior assessment of the subvalvular mitral apparatus and also provides tissue characterization of the myocardium, which can be an essential component of understanding the etiology of the underlying cardiomyopathy and the overall health of the myocardium. The presence of global wall motion abnormalities with equal PM displacement can cause IMR to appear similar to non-ischemic functional MR and location (46) and severity of LV myocardial fibrosis with ICM can impact the progression of ischemic functional MR (47,48). CMR can be used to accurately quantify MR and predict outcomes in patients undergoing surgical MV intervention for IMR. For example, viability assessment with CMR imaging has demonstrated that patients with IMR and large scar burden are at highest risk for mortality after surgical intervention (49). Furthermore, a recent study demonstrated a novel interaction between CMR quantification of IMR and myocardial size, in which the hazard ratio of patients with significant IMR (mitral regurgitant fraction ≥35%) and small myocardial infarct size was 1.51 (0.57, 3.98), while for patients with significant IMR and large MIS (≥30%) was 5.41 (2.34, 12.7) (50). Thus, this study demonstrated that risk associated with IMR is more comprehensively described as an interaction between IMR severity and myocardial infarct size, quantified by CMR. Myocardial fibrosis, as assessed by CMR, has also been shown as a significant predictor of LV remodeling after cardio-resynchronization therapy (CRT) and MR severity is associated with increased diffuse myocardial fibrosis, independent of the presence of late gadolinium enhancement (LGE) quantification (21). While CMR provides comprehensive assessment of FM, limitations of CMR include suboptimal image quality in patients who have cardiac arrhythmias, pacemakers and implantable cardioverter-defibrillators, inability perform adequate breath holds, and claustrophobia.
Cardiac CT
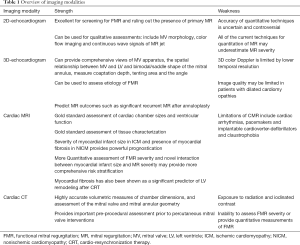
Cardiac CT can also be used in evaluating MR and by providing accurate volumetric measures of chamber dimensions, and assessment of the MV and mitral annular geometry. Cine cardiac CT can be performed and can provide assessment of LV function, though the temporal resolution is inferior to that of echocardiography and CMR and comes at the cost of significant increased radiation exposure. Cardiac CT provides an important role for pre-procedural planning for percutaneous MV replacement interventions, and is routinely acquired as part of the pre-procedural evaluation for percutaneous MV replacement, which are currently in development and are being deployed as part of several research trials. In regards to transcatheter percutaneous MV replacement, recent studies have demonstrated the importance of pre-procedural planning with cardiac CT to predict the risk of left ventricular outflow tract (LVOT) obstruction, which has been associated with increased procedural mortality (51). Using cardiac CT imaging, a neo-LVOT can be reconstructed with dedicated post-processing software, and an area of less than 2 cm2 is currently thought to increase the risk of obstruction (52). Summary of various imaging modalities in diagnosing and prognosticating function mitral regurgitation can be seen in Table 1 (see Table 1).
Full table
Therapeutic considerations of FMR
Medical therapy
Medical therapy is mostly aimed at optimizing LV remodeling. Guideline directed medical therapy (GDMT) for HF is typically the first-line treatment for patients with FMR (53). Beta blockers and angiotensin-converting enzyme inhibitors (ACEi) are recommended for all patients with FMR with LV dysfunction to reduce or reverse LV remodeling and thereby reduce severity of MR (54,55). Sacubitril/Valsartan has been shown to significantly improve LV systolic remodeling and less significant MR (56). Among patients with secondary functional MR, sacubitril/valsartan reduced MR to a greater extent as exhibited by decrease in ERO an regurgitant volume than valsartan alone (57). Diuretics can reduce symptoms of disease however no recent studies have reported their effects on preventing progression of the underlying disease. Few small cohort studies have shown beneficial effects of pharmacologic vasodilators such as nitroprusside, nitrates and hydralazine in treatment of severe MR. Patients showed improved hemodynamics, improved symptoms, lower central venous pressure and overall improvement in cardiac output (58).
CRT
While GDMT for secondary MR is important in afterload reduction and treating HF symptoms, cardiac resynchronization therapy (CRT) has also shown to be effective in treatment of MR. CRT is a class I recommendation for patients in sinus rhythm with NYHA functional class II to IV symptoms on GDMT with LVEF ≤35%, left bundle branch block and QRS ≥150 ms (59). CRT can have beneficial effect on secondary MR through reversal of LV remodeling, improving LV systolic function, and restoration of synchronous ventricular contraction. Patients at high surgical risk with secondary MR may benefit from CRT as shown by van Bommel et al., where patients with severe FMR who received CRT had significant reduction in MR measured by vena contracta width, EROA, tenting area, left atrial volume and jet area with superior survival after CRT (60). However, benefit of CRT may be limited in patients with IMR, given these patients tend to have LV dilation and leaflet tethering or scar at the LV pacing lead tip which can impede resynchronization (61).
Surgery
MV annuloplasty is a surgical technique where primary target of treatment is aimed at mitral annular dilation. First introduced in 1968 by Alain Carpentier, this technique involves use of a rigid or semi-rigid ring to downsize the annulus diameter to its native geometry. This will bring the annulus and leaflets together and into alignment to achieve a central line of coaptation (62). Recurrent MR is frequent outcome after MV annuloplasty for secondary MR patients, with risk factors of recurrence include severe pre-operative MR, centrally directed or multiple jets, greater amount of LV dilation, symmetric anterior leaflet tethering, ≥11 mm coaptation height and presence of basal aneurysm/dyskinesis (63). MV repair is generally associated with lower perioperative mortality, while replacement provides better long-term correction with lower risk of recurrence.
Studies in IMR population showed that MV replacement is a suitable option for those with chronic ischemic mitral regurgitation with impaired LV function. However, no significant difference was found in LV reverse remodeling or survival in patients who underwent MV repair vs. those who underwent MV replacement (64). While there have been studies showing lower recurrence rates of MR after replacement compared to repair,, one meta-analysis suggested that mitral valve repair may be associated with improved short and long term survival (65). However, there is still no clear mortality benefit when comparing both surgical interventions in ischemic or non-ischemic secondary MR. ACC/AHA guidelines on valvular heart disease recommend MV surgery for chronic severe FMR who are undergoing CABG or aortic valve replacement, a chordal-sparing MV replacement instead of MV repair for severely symptomatic patients with chronic severe IMR despite GDMT as class IIA indication (40). When comparing MV repair vs. replacement, mitral regurgitation recurred more frequently in the repair group resulting in more HF symptoms and admissions (4).In addition, in patients that undergo repair, early hazard of increased neurologic events and supraventricular arrhythmias was noted (5).
Percutaneous therapy
Minimally invasive percutaneous approach to correct MR has gained a lot of popularity and momentum over the past several years. MitraClip device, inspired by end-to-end MV repair technique performed by suturing together the leading edges of the scallops of the mitral leaflets at the site of regurgitation creating a double orifice MV, is intended for patients who are at high surgical risk. This is an edge-to-edge leaflet repair system with a deliverable clip that grasps the A2/P2 leaflets at the site of regurgitation, creating the double orifice (66). EVEREST II trial was one of the first trials to compare MitraClip with surgical MV repair or replacement in patients with severe regurgitation; this data showed that MitraClip was not as effective as surgery for complete resolution of MR or LV remodeling, however it demonstrated a better safety profile at 30 days compared to surgery. However, 73% of patients in this study had primary MR and only 27% had secondary MR (67,68). The TRAMI (Transcather Mitral Valve Interventions) trial enrolled 1,064 patients who were subsequently treated with MitraClip, in which 71% had FMR with NYHA class III/IV HF symptoms. There were no procedural deaths and at 3-month follow up about 66% of patients remained in NYHA class I/II (69).
Recently, two major MitraClip randomized control trials evaluated the survival benefit in patients with FMR which demonstrated conflicting results and have resulted in much debate and controversy: percutaneous repair with the MitraClip Device for severe functional/secondary mitral regurgitation (MITRA-FR) trial and the Cardiovascular Outcomes Assessment of MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial. The MITRA-FR trial was a multicenter, randomized controlled trial that compared MitraClip to optimal medical therapy in patients with severe, chronic, secondary MR—defined as RV ≥30 mL/beat or EROA ≥22 mm2 and all patients were deemed inoperable. Outcomes of this study showed no significant difference in primary end point of composite death from any cause or unplanned HF hospitalization at 12 months. In addition, this study reported higher rates of stroke, renal failure, and hemorrhage in the intervention group. However, criticisms of this study include that the underlying cardiomyopathy, rather than the MR, was the true underlying driver of poorer outcomes in patients with severe MR. In addition, higher number of patients had residual MR after MitraClip procedure, had higher rate of device implantation failure, and high mortality and rehospitalization rates of the cohort raising concerns that the population enrolled were simply too sick to benefit from any intervention (70).
On the other hand, the COAPT trial, which was also a randomized, multicenter trial of MitraClip in symptomatic HF patients with moderate to severe or severe MR with NYHA II-IVa symptoms demonstrated contrasting results (71). The primary endpoint in this trial was HF hospitalizations within 24 months, and patients in the MitraClip arm demonstrated significantly lower rates of HF hospitalizations than patients in the GDMT arm, with better quality of life scores, functional capacity, degree of MR and LV remodeling in the intervention arm. The results of this study demonstrated an impressive reduction in recurrent hospitalizations and all-cause mortality after percutaneous MitraClip placement compared to medical therapy alone. Overall, the patients enrolled in the COPAT trial were healthier than MITRA-FR trial and had to be on maximally tolerated GDMT before enrollment, which may represent a more highly selective cohort being enrolled in the COAPT trial. The COAPT trial also enrolled patients with less adverse LV remodeling and patients demonstrated lower rates of residual MR in the intervention arm, which may partially explain the positive survival benefit in this trial, in contrast to the results from the MITRA-FR and EVEREST II trials. The results of the COPAT trial led to FDA approval for MitraClip for the treatment of FMR. However, given the conflicting results of the MITRA-FR and COAPT trials, many questions remain regarding optimal patient selection criteria, and it is unlikely that all patients with FMR will equally benefit from MV repair. Therefore, further studies are needed to determine optimal selection criteria for patients with significant FMR, who are being considered for MitraClip intervention.
Currently, edge to edge leaflet repair is the only guideline recommended transcatheter treatment for FMR; however, there are various new devices in development for percutaneous MV repair such as, Carillon mitral contour system, and Cardioband to name a few (72). Devices such as Neochord (NeoChord DS 1000) which are less invasive, are already in use worldwide for MV repair. Typically, MV repair is performed with the patient in cardioplegic arrest to allow exposure of MV. Transcatheter MV replacement devices for functional MR are in development; however, these devices are often bulky and limited to transapical delivery method. Lastly, transcatheter MV-in-valve and valve-in-valve ring using transcatheter aortic valve implantation devices are currently alternative strategies that can be pursued in patients with prior surgical MV intervention, who are considered to have high surgical risk with significant FMR (73-75).
Conclusions
FMR, regardless of underlying etiology, is strongly associated with poor prognosis in patients with HF. Echocardiography remains the primary imaging modality for the diagnosis of FMR; however, advanced imaging modalities are being increasingly utilized for improved assessment of degree of MR, as well as to delineate the etiology and tissue characterization of the underlying cardiomyopathy. GDMT is the initial treatment strategy, aimed at treating the HF symptoms. The role surgical intervention for FMR remains to be unclear and controversial. MitraClip intervention holds great promise for this high-risk disease. Ongoing studies and randomized control trials for emerging transcatheter MV interventions are essential to further guide optimal treatment strategies in this complicated patient population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Heart Valve Disease”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-19-277). The series “Heart Valve Disease” was commissioned by the editorial office without any funding or sponsorship. Kwon D served as the unpaid Guest Editors of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asgar AW, Mack MJ, Stone GW. Secondary Mitral Regurgitation in Heart Failure: Pathophysiology, Prognosis, and Therapeutic Considerations. J Am Coll Cardiol 2015;65:1231-48. [Crossref] [PubMed]
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932-41. [Crossref] [PubMed]
- Mihos CG, Xydas S, Yucel E, et al. Mitral valve repair and subvalvular intervention for secondary mitral regurgitation: a systematic review and meta-analysis of randomized controlled and propensity matched studies. J Thorac Dis 2017;9:S582-94. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Chello M, et al. Papillary muscle approximation in mitral valve repair for secondary MR. J Thorac Dis 2017;9:S635-9. [Crossref] [PubMed]
- Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation 2005;112:745-58. [Crossref] [PubMed]
- Perez de Isla L, Zamorano J, Quezada M, et al. Prognostic significance of functional mitral regurgitation after a first non-ST-segment elevation acute coronary syndrome. Eur Heart J 2006;27:2655-60. [Crossref] [PubMed]
- Hickey MS, Smith LR, Muhlbaier LH, et al. Current prognosis of ischemic mitral regurgitation. Implications for future management. Circulation 1988;78:I51-9. [PubMed]
- Lamas GA, Mitchell GF, Flaker GC, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation 1997;96:827-33. [Crossref] [PubMed]
- Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675-80. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [Crossref] [PubMed]
- Levine RA, Hung J, Otsuji Y, et al. Mechanistic insights into functional mitral regurgitation. Curr Cardiol Rep 2002;4:125-9. [Crossref] [PubMed]
- Ray S. The echocardiographic assessment of functional mitral regurgitation. Eur J Echocardiogr 2010;11:i11-7. [Crossref] [PubMed]
- Saito K, Okura H, Watanabe N, et al. Influence of chronic tethering of the mitral valve on mitral leaflet size and coaptation in functional mitral regurgitation. JACC Cardiovasc Imaging 2012;5:337-45. [Crossref] [PubMed]
- Kumanohoso T, Otsuji Y, Yoshifuku S, et al. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg 2003;125:135-43. [Crossref] [PubMed]
- Aklog L, Filsoufi F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation 2001;104:I68-75. [Crossref] [PubMed]
- McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004;128:916-24. [Crossref] [PubMed]
- Varma PK, Krishna N, Jose RL, et al. Ischemic mitral regurgitation. Ann Card Anaesth 2017;20:432-9. [Crossref] [PubMed]
- Jampates S, Koneru S, Popovic Z, et al. Mitral Regurgitation Severity Is Associated with Increased Diffuse Myocardial Fibrosis by Extracellular Volume Quantification in Non-ischemic Cardiomyopathy. J Am Coll Cardiol 2016;67:1828. [Crossref]
- Bartko PE, Arfsten H, Heitzinger G, et al. A Unifying Concept for the Quantitative Assessment of Secondary Mitral Regurgitation. J Am Coll Cardiol 2019;73:2506-17. [Crossref] [PubMed]
- Ito K, Abe Y, Takahashi Y, et al. Mechanism of atrial functional mitral regurgitation in patients with atrial fibrillation: A study using three-dimensional transesophageal echocardiography. J Cardiol 2017;70:584-90. [Crossref] [PubMed]
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48. [Crossref] [PubMed]
- Palmiero G, Melillo E, Ferro A, et al. Significant functional mitral regurgitation affects left atrial function in heart failure patients: haemodynamic correlations and prognostic implications. Eur Heart J Cardiovasc Imaging 2019;20:1012-9. [Crossref] [PubMed]
- Bursi F, Barbieri A, Grigioni F, et al. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail 2010;12:382-8. [Crossref] [PubMed]
- Amigoni M, Meris A, Thune JJ, et al. Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: prognostic significance and relation to ventricular size and function. Eur Heart J 2007;28:326-33. [Crossref] [PubMed]
- Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295-301. [Crossref] [PubMed]
- Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759-64. [Crossref] [PubMed]
- Nickenig G, Schueler R, Dager A, et al. Treatment of Chronic Functional Mitral Valve Regurgitation With a Percutaneous Annuloplasty System. J Am Coll Cardiol 2016;67:2927-36. [Crossref] [PubMed]
- Grayburn PA, Weissman NJ, Zamorano JL. Quantitation of mitral regurgitation. Circulation 2012;126:2005-17. [Crossref] [PubMed]
- Bargiggia GS, Tronconi L, Sahn DJ, et al. A new method for quantitation of mitral regurgitation based on color flow Doppler imaging of flow convergence proximal to regurgitant orifice. Circulation 1991;84:1481-9. [Crossref] [PubMed]
- Dalton JE, Kattan MW. Recent advances in evaluating the prognostic value of a marker. Scand J Clin Lab Invest Suppl 2010;242:59-62. [Crossref] [PubMed]
- Lancellotti P, Troisfontaines P, Toussaint AC, et al. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation 2003;108:1713-7. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [Crossref] [PubMed]
- Buck T, Plicht B, Kahlert P, et al. Effect of dynamic flow rate and orifice area on mitral regurgitant stroke volume quantification using the proximal isovelocity surface area method. J Am Coll Cardiol 2008;52:767-78. [Crossref] [PubMed]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-8. [Crossref] [PubMed]
- Antonelli P, Chiumello D, Cesana BM. Statistical methods for evidence-based medicine: the diagnostic test. Part II. Minerva Anestesiol 2008;74:481-8. [PubMed]
- Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol 2016;76:175-82. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal 2017;38:2739-91. [Crossref] [PubMed]
- Veronesi F, Corsi C, Sugeng L, et al. Quantification of mitral apparatus dynamics in functional and ischemic mitral regurgitation using real-time 3-dimensional echocardiography. J Am Soc Echocardiogr 2008;21:347-54. [Crossref] [PubMed]
- Thavendiranathan P, Liu S, Datta S, et al. Quantification of chronic functional mitral regurgitation by automated 3-dimensional peak and integrated proximal isovelocity surface area and stroke volume techniques using real-time 3-dimensional volume color Doppler echocardiography: in vitro and clinical validation. Circ Cardiovasc Imaging 2013;6:125-33. [Crossref] [PubMed]
- Kwon DH, Hachamovitch R, Popovic ZB, et al. Survival in patients with severe ischemic cardiomyopathy undergoing revascularization versus medical therapy: association with end-systolic volume and viability. Circulation 2012;126:S3-8. [Crossref] [PubMed]
- Krieger EV, Lee J, Branch KR, et al. Quantitation of mitral regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart 2016;102:1864-70. [Crossref] [PubMed]
- Chinitz JS, Chen D, Goyal P, et al. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging 2013;6:220-34. [Crossref] [PubMed]
- Hagendorff A, Doenst T, Falk V. Echocardiographic assessment of functional mitral regurgitation: opening Pandora's box? ESC Heart Fail 2019;6:678-85. [Crossref] [PubMed]
- Agricola E, Oppizzi M, Maisano F, et al. Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr 2004;5:326-34. [Crossref] [PubMed]
- Kusunose K, Obuchowski NA, Gillinov M, et al. Predictors of Mortality in Patients With Severe Ischemic Cardiomyopathy Undergoing Surgical Mitral Valve Intervention. Journal of the American Heart Association 2017;6:e007163 [Crossref] [PubMed]
- Cavalcante JL, Kusunose K, Obuchowski NA, et al. Prognostic Impact of Ischemic Mitral Regurgitation Severity and Myocardial Infarct Quantification by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging 2020;13:1489-501. [Crossref] [PubMed]
- Yoon SH, Bleiziffer S, Latib A, et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv 2019;12:182-93. [Crossref] [PubMed]
- Kohli K, Wei ZA, Yoganathan AP, et al. Transcatheter Mitral Valve Planning and the Neo-LVOT: Utilization of Virtual Simulation Models and 3D Printing. Curr Treat Options Cardiovasc Med 2018;20:99. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-132. [Crossref] [PubMed]
- Levine AB, Muller C, Levine TB. Effects of high-dose lisinopril-isosorbide dinitrate on severe mitral regurgitation and heart failure remodeling. Am J Cardiol 1998;82:1299-301, a10.
- Comin-Colet J, Sanchez-Corral MA, Manito N, et al. Effect of carvedilol therapy on functional mitral regurgitation, ventricular remodeling, and contractility in patients with heart failure due to left ventricular systolic dysfunction. Transplant Proc 2002;34:177-8. [Crossref] [PubMed]
- Bayard G, Da Costa A, Pierrard R, et al. Impact of sacubitril/valsartan on echo parameters in heart failure patients with reduced ejection fraction a prospective evaluation. Int J Cardiol Heart Vasc 2019;25:100418 [Crossref] [PubMed]
- Kang DH, Park SJ, Shin SH, et al. Angiotensin Receptor Neprilysin Inhibitor for Functional Mitral Regurgitation. Circulation 2019;139:1354-65. [Crossref] [PubMed]
- Rosario LB, Stevenson LW, Solomon SD, et al. The mechanism of decrease in dynamic mitral regurgitation during heart failure treatment: importance of reduction in the regurgitant orifice size. J Am Coll Cardiol 1998;32:1819-24. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [Crossref] [PubMed]
- van Bommel RJ, Marsan NA, Delgado V, et al. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation 2011;124:912-9. [Crossref] [PubMed]
- Kang DH, Sun BJ, Kim DH, et al. Percutaneous versus surgical revascularization in patients with ischemic mitral regurgitation. Circulation 2011;124:S156-62. [Crossref] [PubMed]
- Nicolini F, Agostinelli A, Vezzani A, et al. Surgical treatment for functional ischemic mitral regurgitation: current options and future trends. Acta Biomed 2015;86:17-26. [PubMed]
- Bach DS, Bolling SF. Improvement Following Correction of Secondary Mitral Regurgitation in End-Stage Cardiomyopathy With Mitral Annuloplasty. The American Journal of Cardiology 1996;78:966-9. [Crossref] [PubMed]
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. [Crossref] [PubMed]
- Vassileva CM, Boley T, Markwell S, et al. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur J Cardiothorac Surg 2011;39:295-303. [Crossref] [PubMed]
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674-81. [Crossref] [PubMed]
- Feldman T, Kar S, Elmariah S, et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol 2015;66:2844-54. [Crossref] [PubMed]
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014;64:172-81. [Crossref] [PubMed]
- Schillinger W, Hunlich M, Baldus S, et al. Acute outcomes after MitraClip therapy in highly aged patients: results from the German TRAnscatheter Mitral valve Interventions (TRAMI) Registry. EuroIntervention 2013;9:84-90. [Crossref] [PubMed]
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med 2018;379:2297-306. [Crossref] [PubMed]
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med 2018;379:2307-18. [Crossref] [PubMed]
- Prendergast BD, Baumgartner H, Delgado V, et al. Transcatheter heart valve interventions: where are we? Where are we going? Eur Heart J 2019;40:422-40. [Crossref] [PubMed]
- Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous Transvenous Transseptal Transcatheter Valve Implantation in Failed Bioprosthetic Mitral Valves, Ring Annuloplasty, and Severe Mitral Annular Calcification. JACC Cardiovasc Interv 2016;9:1161-74. [Crossref] [PubMed]
- Frerker C, Schmidt T, Schluter M, et al. Transcatheter implantation of aortic valve prostheses into degenerated mitral valve bioprostheses and failed annuloplasty rings: outcomes according to access route and Mitral Valve Academic Research Consortium (MVARC) criteria. EuroIntervention 2016;12:1520-6. [Crossref] [PubMed]
- Wilbring M, Alexiou K, Tugtekin SM, et al. Pushing the limits-further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg 2014;147:210-9. [Crossref] [PubMed]


