
Catheter-based angiography versus CT angiography for the diagnosis of extracoronary fibromuscular dysplasia in patients with spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) describes a non-atherosclerotic, non-traumatic and non-iatrogenic separation of coronary arterial wall (1). Fibromuscular dysplasia (FMD) is commonly associated with SCAD, reported in ~50–86% (1). FMD commonly affects renal, carotid and vertebral arteries, and can lead to stenosis, aneurysm or dissection (2). The true prevalence of FMD is unknown, however, it was reported in 3.8–6.6% of renal donors on angiography (3). The gold-standard for diagnosing FMD remains catheter-based angiography (CA) due to its superior spatial resolution, but non-invasive modalities are more frequently used, especially computed tomography angiography (CTA) and magnetic resonance angiography (MRA) (2). However, comparison between CA and CTA on extracoronary FMD diagnosis have not been performed in SCAD patients.
We retrospectively reviewed non-atherosclerotic SCAD patients prospectively followed at Vancouver General Hospital who underwent both CA and CTA for FMD diagnosis in the renal and/or iliac arteries. Patients were consented in SCAD registries approved by the University of British Columbia Research Ethics Board. SCAD diagnosis/classification was confirmed by 2 experienced angiographers. FMD diagnosis was made from invasive or non-invasive angiography based on the American Heart Association definition of multifocal disease (2). SCAD patients underwent FMD imaging with either CA or CTA per physician discretion. Non-selective or selective CA of the renal and iliac arteries were typically performed during coronary angiography. Patients investigated with MRA or ultrasound were excluded as the spatial resolution are lower than CTA. FMD diagnosis on CA was made by 2 experienced angiographers, and CTA diagnosis by the interpreting radiologists. CTAs were acquired on a second or third generation dual source scanner (Siemens Healthineers, Forkheim, Germany), with spiral axial 128 slice by 0.6 collimation, or axial 192 slice by 0.6 collimation, respectively, with 40% overlap and 1mm slice thickness. Iterative reconstruction was utilized to reduce noise. Multiplanar reformat oblique images were reviewed; and coronal and sagittal maximal intensity projections were obtained with 40% overlap and 3 mm slice thickness.
Baseline continuous variables were expressed as mean +/− standard deviation and discrete variables as frequencies and percentages. Comparisons between categorical data were made with the Chi-square or Fisher’s exact tests. Continuous data were compared using the Student t-test. Statistical analysis was performed with SPSS (IBM SPSS Version 23, Armonk, New York, USA).
We identified 30 SCAD patients who underwent CA and CTA for renal and/or iliac arteries FMD screening. Mean age was 53.7±7.6 yr, 93.3% were women, 36.7% had hypertension, 40.0% had ST-elevation myocardial infarction (STEMI), and 60.0% had non-STEMI. Of these, 29 had both CA and CTA performed for renal arteries, and 24 had both performed for iliac arteries. The median imaging time between CA and CTA was 41 days (interquartile range 1–172.8 days). Overall, FMD was diagnosed in 27/30 (90.0%) on CA (Figure 1), but CTA identified only 6/27 with FMD when compared to CA, with sensitivity of 22.2%, specificity 100%, negative predictive value 12.5%, and positive predictive value 100%. All patients who had FMD identified on CTA also had FMD on CA. For the renal arteries, FMD was diagnosed in 20/29 (69.0%) on CA, but only 5/30 (16.7%) on CTA (P<0.001). For the iliac arteries, FMD was diagnosed in 14/26 (53.8%) on CA, but only 1/24 (4.2%) on CTA (P=0.004).
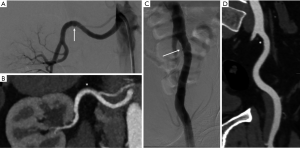
Several non-invasive imaging modalities can be utilized to diagnose FMD, including duplex ultrasonography, CTA and MRA, but CA remains the gold-standard given its superior spatial resolution (2). However, CA is invasive and is clinically relegated as a second-line imaging for FMD given logistical practicality and risks. Importantly, this shift towards less invasive imaging may compromise the diagnosis of FMD in clinical practice as shown by our study. Indeed, CA particularly with digital subtraction, was shown to have superior spatial resolution compared to CTA for multiple vascular beds, including cerebrovasculature for submillimetre arteries (4). MRA has even lower spatial resolution than CTA, and is more prone to missing FMD or falsely diagnosing FMD due to artifact or motion (5). Therefore, when screening for FMD, clinicians must take these factors into consideration. The different imaging modalities used could explain the marked variability in concomitant FMD prevalence in SCAD studies; the highest reported 86% co-prevalence was reported in the study where CA was primarily used (in ~80% of patients) (3). Of note, for very mild or subtle angiographic FMD cases, intravascular ultrasound may further improve diagnosis by visualizing endoluminal webs, ridges, membranes or folds (6).
In contrast to our study, Sabharwal reported 100% sensitivity with CTA for FMD diagnosis compared to CA (6). However, their study had several differences in methodology, such as patient demographics (~10 years older than our population) and inclusion criteria (all had confirmed FMD on CA prior to CTA). Furthermore, only 36.7% of our patients had hypertension, and many patients in our large Vancouver SCAD series had only angiographically mild and clinically silent extracoronary FMD, which makes it more challenging for CTA to diagnosis FMD compared to CA. Indeed, the cases where CTA missed the FMD diagnosis in our series had mild changes on CA, indicating that the lower spatial resolution was a challenge with diagnosing mild FMD with CTA. This should be taken into consideration when selecting the FMD imaging modality of choice in SCAD patients. In addition, if selective angiography of renal/iliac arteries were performed on CA, a complete systemic screen of the remainder vasculature (e.g., cerebrovascular, abdominal) that were not visualized on CA should be performed with CTA/MRA.
Study limitations: our study is small, single-center, retrospective and thus subject to bias. Diagnosis of FMD on CTA were not interpreted by core laboratory, instead reflecting standard clinical interpretation by practicing radiologists.
In conclusion, our study showed CA to be significantly more sensitive than CTA in diagnosing renal/iliac FMD. When choosing FMD imaging modality, we encourage clinicians to consider the diagnostic accuracies of each test, especially in the population of SCAD patients where extracoronary FMD changes are often mild and silent. Although invasive, we support the use of CA over CTA in diagnosing renal/iliac FMD during the index coronary angiography.
Acknowledgments
Funding: Dr. Saw has received unrestricted research grant supports (from the Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, National Institutes of Health, Michael Smith Foundation of Health Research, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, St Jude Medical, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, St Jude Medical, Boston Scientific, and Sunovion), consultancy and advisory board honoraria (AstraZeneca, St Jude Medical, and Abbott Vascular), and proctorship honoraria (St Jude Medical and Boston Scientific).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/cdt-20-71). Dr. Saw reports grants from CIHR, grants from Heart & Stroke Foundation of Canada, grants from Michael Smith Foundation, grants from AstraZeneca, grants, personal fees and other from Boston Scientific, personal fees and other from Abbott Vascular, personal fees from Gore, personal fees from Baylis, outside the submitted work. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saw J, Mancini GBJ, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2016;68:297-312. [Crossref] [PubMed]
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014;129:1048-78. [Crossref] [PubMed]
- Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44-52. [Crossref] [PubMed]
- Villablanca JP, Rodriguez FJ, Stockman T, et al. MDCT angiography for detection and quantification of small intracranial arteries: comparison with conventional catheter angiography. AJR Am J Roentgenol 2007;188:593-602. [Crossref] [PubMed]
- Olin JW, Kadian-Dodov D. Fibromuscular Dysplasia: Looking Beyond the "String of Beads". JACC Cardiovasc Imaging 2017;10:562-4. [Crossref] [PubMed]
- Gowda MS, Loeb AL, Crouse LJ, et al. Complementary roles of color-flow duplex imaging and intravascular ultrasound in the diagnosis of renal artery fibromuscular dysplasia: should renal arteriography serve as the "gold standard"? J Am Coll Cardiol 2003;41:1305-11. [Crossref] [PubMed]


