
Factors affecting the efficacy of SGLT2is on heart failure events: a meta-analysis based on cardiovascular outcome trials
IntroductionOther Section
In large cardiovascular outcome trials (CVOTs) (1-10) sodium-glucose transporter 2 inhibitors (SGLT2is) have been observed with the obvious efficacy of reducing heart failure events among the overall subjects of trials. However, these individual CVOTs were not designed with adequate statistical power to evaluate heart failure endpoints in various subgroups defined by clinically important factors, such as left ventricular ejection fraction (LVEF) level, New York Heart Association (NYHA) functional class, geographic region, and race. Moreover, different CVOTs reported the inconsistent results as for the identical subgroup. For instance, the SCORED trial (1) did not show sotagliflozin with a significant reduction in the risk of heart failure composite endpoint among patients with LVEF <40%, whereas three other trials (2,4,5) showed that. For another example, the EMPEROR-Reduced trial (4) showed that empagliflozin significantly reduced the risk of heart failure composite endpoint among Black patients, whereas five other trials (1,2,5,6,10) did not show that.
Furthermore, dose the efficacy of SGLT2is in reducing heart failure events vary in different underlying diseases? Dose the efficacy of SGLT2is in reducing heart failure events vary with specific SGLT2is? There have been not certain answers for the two questions until now. Thus, we carried out this meta-analysis based on CVOTs of SGLT2is, to evaluate the efficacy of SGLT2is on heart failure-associated endpoints in relevant subgroups defined by six clinically important factors. We present the following article in accordance with the PRISMA reporting checklist (available at: http://dx.doi.org/10.21037/cdt-20-984).
MethodsOther Section
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11) was used to guide the performance of this meta-analysis study (available at: http://dx.doi.org/10.21037/cdt-20-984). The study protocol for this meta-analysis has been published in the INPLASY website before the beginning of study selection and is available at https://inplasy.com/inplasy-2020-11-0094.
Search strategy and inclusion criteria
We searched literature in Embase, PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) using detailed retrieval strategies (they are presented in Table S1) from the start date of database to 21 November 2020. Original studies we included in this meta-analysis were CVOTs of SGLT2is, namely randomized controlled trials (RCTs) assessing the efficacy of any SGLT2i versus placebo or active drug on cardiovascular endpoints in patients with type 2 diabetes (T2D) or with congestive heart failure (CHF) or with chronic kidney disease (CKD). This meta-analysis did not consider any of the conference articles and grey articles. The outcome assessed in this study was heart failure composite outcome that was defined as a composite of cardiovascular death (CVD) or hospitalization for heart failure (HHF). If the composite outcome of CVD or HHF was not available in original studies, a composite of CVD or HHF or an urgent visit for heart failure would be used instead.
Study selection, data extraction and quality assessment
Two authors independently performed study selection and data extraction, and independently evaluated the quality of included RCTs using the Cochrane risk of bias assessment tool (12). The pre-specified data extracted from included studies contained study type, type of underlying diseases, type of interventions, type of control, study outcomes from various subgroups defined by each of the factors of interest. According to the Cochrane risk assessment tool (12) included RCTs were assessed with or without the following seven kinds of bias risks: risk of selection bias (concerning random sequence generation), risk of performance bias (concerning blinding of participants and personnel), risk of selection bias (concerning allocation concealment), risk of detection bias (concerning blinding of outcome assessment), risk of attrition bias (concerning incomplete outcome data), risk of reporting bias (concerning selective reporting), and risk of other bias. Any disagreements between them would be resolved by discussion with a third author.
Statistical analysis
We used the trial-level survival data, namely hazard ratios (HRs) and 95% confidence intervals (CIs) extracted from original articles, to perform fixed-effects meta-analysis. I2 statistic was calculated to measure statistical heterogeneity. I2>50% is considered as substantial heterogeneity. Subgroup meta-analysis was done according to each of the following 6 factors: type of underlying diseases (CHF, CKD, and T2D), type of SGLT2is (empagliflozin, canagliflozin, dapagliflozin, ertugliflozin, and sotagliflozin), LVEF level (<40%, 40% to <50%, and ≥50%), NYHA class (NYHA class II, and NYHA class III or IV), geographic region (North America, Latin America, Europe, and Asia), and race (White, Black, and Asian). Cochran’s Q test was performed to test for subgroup effects. P<0.05 means statistical significance. If substantial heterogeneity was observed, random-effects meta-analysis would be additionally conducted to assess the robustness of pooled results. All statistical analyses were completed in the Stata software (version 15.1).
ResultsOther Section
Characteristics of included trials
Figure S1 presents the complete process of study selection. After that, we included 13 articles (1-10,13-15) reporting a total of 10 CVOTs (1-10) for quantitative synthesis in this meta-analysis. All of the 10 CVOTs included in this meta-analysis study were placebo-controlled randomized trials, which consisted of two sotagliflozin trials [i.e., the SCORED trial (1) conducted in patients with T2D and CKD, and the SOLOIST-WHF trial (2) conducted in patients with T2D and CHF], one ertugliflozin trial [i.e., the VERTIS CV trial (6) conducted in patients with T2D], three dapagliflozin trials [i.e., the DAPA-CKD trial (3) conducted in patients with CKD, the DAPA-HF trial (5) conducted in patients with CHF, and the DECLARE-TIMI 58 trial (8) conducted in patients with T2D], two empagliflozin trials [i.e., the EMPEROR-Reduced trial (4) conducted in patients with CHF, and the EMPA-REG OUTCOME trial (10) conducted in patients with T2D], and two canagliflozin trials [i.e., the CREDENCE trial (7), and the CANVAS Program trial (9) conducted in patients with T2D]. The quality assessment result (Figure S2) showed that all the original studies included were with the low risk of bias. The original data used for pooled analysis in this study are given in https://cdn.amegroups.cn/static/public/cdt-20-984-1.xlsx.
Subgroup analysis according to NYHA class
Figure 1 shows the results of meta-analysis of the effect of SGLT2is on heart failure composite outcome in relevant subgroups defined by NYHA class. Compared with placebo, SGLT2is significantly reduced the risk of heart failure composite outcome in the subgroup of patients with NYHA class II (HR 0.66, 95% CI, 0.59–0.74; I2=0; P for drug effect <0.001), and in the subgroup of patients with NYHA class III or IV (HR 0.86, 95% CI, 0.75–0.99; I2=0; P for drug effect =0.032). SGLT2is provided greater benefits in patients with NYHA class II (reducing heart failure composite outcome by 34% according to the HR value) than in patients with NYHA class III or IV (reducing the composite outcome by only 14%), and the subgroup effect according to NYHA class was statistically significant (Psubgroup=0.004).

Subgroup analysis according to race
Figure 2 shows the results of meta-analysis of the effect of SGLT2is on heart failure composite outcome in relevant subgroups defined by race. Compared with placebo, SGLT2is significantly reduced the risk of heart failure composite outcome in the subgroup of White patients (HR 0.81, 95% CI, 0.76–0.86; I2=38.9%; P for drug effect <0.001), in the subgroup of Black patients (HR 0.63, 95% CI, 0.49–0.82; I2=0; P for drug effect=0.001), and in the subgroup of Asian patients (HR 0.64, 95% CI, 0.53–0.77; I2=0; P for drug effect <0.001). SGLT2is provided greater benefits in Black patients (reducing heart failure composite outcome by 37% according to the HR value) and Asian patients (reducing the composite outcome by 36%) than in White patients (reducing the composite outcome by only 19%), and the subgroup effect according to race was statistically significant (Psubgroup=0.016).
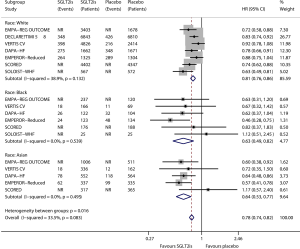
Subgroup analysis according to LVEF level
Figure 3 shows the results of meta-analysis of the effect of SGLT2is on heart failure composite outcome in relevant subgroups defined by LVEF level. Compared with placebo, SGLT2is significantly reduced the risk of heart failure composite outcome in the subgroup of patients with LVEF <40% (HR 0.76, 95% CI, 0.69–0.82; I2=0; P for drug effect <0.001), in the subgroup of patients with LVEF ≥40% and <50% (HR 0.67, 95% CI, 0.45–1.00; I2=0; P for drug effect =0.048), and in the subgroup of patients with LVEF ≥50% (HR 0.61, 95% CI, 0.43–0.86; I2=0; P for drug effect =0.004). The subgroup effect according to LVEF level was not statistically significant (Psubgroup=0.429).
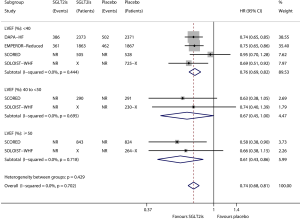
Subgroup analysis according to different underlying diseases
Figure S3 shows the results of meta-analysis of the effect of SGLT2is on heart failure composite outcome in relevant subgroups defined by underlying diseases. Compared with placebo, SGLT2is significantly reduced the risk of heart failure composite outcome in the subgroup of patients with T2D (HR 0.76, 95% CI, 0.72–0.80; I2=19.3%; P for drug effect <0.001), in the subgroup of patients with CHF (HR 0.74, 95% CI, 0.69–0.80; I2=0; P for drug effect <0.001), and in the subgroup of patients with CKD (HR 0.73, 95% CI, 0.68–0.78; I2=0; P for drug effect <0.001). The subgroup effect according to underlying diseases was not statistically significant (Psubgroup=0.673). In the overall patients, SGLT2is versus placebo reduced the composite outcome by 25% (HR 0.75, 95% CI, 0.72–0.78; I2=0; P for drug effect <0.001).
Subgroup analysis according to different SGLT2is
Figure S4 shows the results of meta-analysis of the effect of SGLT2is on heart failure composite outcome in relevant subgroups defined by type of SGLT2is. Compared with placebo, a significant reduction in the risk of heart failure composite outcome was observed with empagliflozin (HR 0.71, 95% CI, 0.64–0.80; I2=16.6%; P for drug effect <0.001), canagliflozin (HR 0.74, 95% CI, 0.66–0.84; I2=0; P for drug effect <0.001), dapagliflozin (HR 0.78, 95% CI, 0.71–0.85; I2=0; P for drug effect <0.001), and sotagliflozin (HR 0.72, 95% CI, 0.62–0.82; I2=0; P for drug effect <0.001; while a reduced trend in the risk of the composite outcome was observed with ertugliflozin (HR 0.88, 95% CI, 0.75–1.03; P for drug effect =0.114). The subgroup effect according to type of SGLT2is was not statistically significant (Psubgroup=0.244).
Subgroup analysis according to geographic region
Figure S5 shows the results of meta-analysis of the effect of SGLT2is on heart failure composite outcome in relevant subgroups defined by geographic region. Compared with placebo, SGLT2is significantly reduced the risk of heart failure composite outcome in the subgroup of patients in North America (HR 0.77, 95% CI, 0.69–0.87; I2=0; P for drug effect <0.001), in the subgroup of patients in Latin America (HR 0.73, 95% CI, 0.63–0.83; I2=0; P for drug effect <0.001), in the subgroup of patients in Europe (HR 0.84, 95% CI, 0.77–0.91; I2=3.3%; P for drug effect <0.001), and in the subgroup of patients in Asia (HR 0.70, 95% CI, 0.60–0.81; I2=0; P for drug effect <0.001). The subgroup effect according to geographic region was not statistically significant (Psubgroup=0.127).
DiscussionOther Section
This study assessed the effects of six clinically important factors (i.e., type of underlying diseases, type of SGLT2is, LVEF level, NYHA class, geographic region, and race) on the efficacy of SGLT2is on heart failure composite outcome by meta-analysis of 10 CVOTs of SGLT2is. Accordingly, this study produces the following two findings.
First, SGLT2is led to greater benefits in patients with NYHA class II (reducing heart failure composite outcome by 34%) than in patients with NYHA class III or IV (reducing the outcome by only 14%), while SGLT2is led to greater benefits in Black patients (reducing the outcome by 37%) and Asian patients (reducing the outcome by 36%) than in White patients (reducing the outcome by only 19%). Meanwhile, SGLT2is significantly reduced heart failure composite outcome independent of LVEF level (<40%, 40% to <50%, or ≥50%) and geographic region (North America, Latin America, Europe, or Asia).
Second, SGLT2is reduced heart failure composite outcome by 25% (HR 0.75, 95% CI, 0.72–0.78) independent of type of underlying diseases (CHF, CKD, or T2D), type of SGLT2is (empagliflozin, canagliflozin, dapagliflozin, ertugliflozin, or sotagliflozin).
A recent meta-analysis (16) based on the two trials of DAPA-HF (5) and EMPEROR-Reduced (4) assessing the effects of 10 factors (i.e., age, sex, type 2 diabetes status, angiotensin receptor neprilysin inhibitor treatment, history of HHF, body-mass index, estimated glomerular filtration rate level, NYHA functional class, geographic region, and race) on the efficacy of SGLT2is on heart failure composite outcome, produced the certain findings that the former 7 factors had no significant effects on the efficacy of SGLT2is, and meanwhile produced the uncertain findings that the later 3 factors had the possibility of affecting on the efficacy of SGLT2is. By incorporating more evidence our meta-analysis revealed that NYHA class and race but not geographic region had significant effects on the efficacy of SGLT2is. Moreover, that meta-analysis (16) failed to evaluate the efficacy of SGLT2is in relevant subgroups defined by LVEF level, whereas our meta-analysis explored this subgroup effect by incorporating the two latest trials of SCORED (1) and SOLOIST-WHF (2).
Another recent meta-analysis (17) including 8 CVOTs of SGLT2is assessing the efficacy of SGLT2is in the subgroups of patients with different underlying diseases (CHF, CKD, or T2D), showed that SGLT2is improved cardiovascular outcomes including both HHF and CVD independent of CHF, T2D, and/or CKD status. This finding from that meta-analysis (17) is consistent with the result of subgroup analysis according to type of underlying diseases conducted in our meta-analysis. However, that meta-analysis (17) failed to evaluate the subgroup effect according to specific SGLT2is whereas our meta-analysis revealed different SGLT2is with the similar efficacy in reducing heart failure composite outcome.
The mechanisms for SGLT2is in reducing heart failure events have not been completely clear so far. Early natriuresis, changes in tissue sodium handling, reductions in plasma volume, vascular resistance reduction, and blood pressure reduction might be the main mechanisms for that (18). Meanwhile, the benefits of SGLT2is for heart failure outcomes are paralleled by reverse cardiac remodeling (19,20) and improvement in quality of life (5,19,21). The reason why SGLT2is are more effective in patients with NYHA class II than in NYHA III-IV probably is that the effects of reverse cardiac remodeling and early natriuresis et al. which SGLT2is produce are more effective in early heart failure than in advanced heart Failure. The reason why SGLT2is are more effective in Black and Asian patients than in Caucasian patients probably is that Asians and Blacks are minorities and not treated with optimal medical therapy, and therefore they benefit the most of this new drug therapy.
This study has three main limitations. First, when we conducted subgroup analysis according to type of underlying diseases, we only considered one kind of disease; whereas we failed to do more specific subgroup analyses by simultaneously considering three kind of diseases (i.e., T2D, CHF, and CKD), such as the analysis in the subgroup of patients with CHF and CKD without T2D and the analysis in the subgroup of patients with CHF, CKD and T2D, since this is a trial-level meta-analysis but not a patient-level meta-analysis. Second, since only a few original studies were included in some subgroups, the corresponding subgroup analyses were with the lack of statistic power. Thus, relevant subgroup effects revealed by the present meta-analysis need to be validated by an updated meta-analysis with adequate studies included in relevant subgroups. Third, test of publication bias has the limited value when the number of included studies is not more than 10. Because in most of the subgroup analyses conducted in this meta-analysis the corresponding subgroups included a limited number of original studies, we did not perform test of publication bias.
In conclusion, SGLT2is reduce heart failure composite outcome by 25% independent of type of underlying diseases, type of SGLT2is, LVEF level, and geographic region. SGLT2is lead to greater reduction in the composite outcome in patients with NYHA class II than in patients with NYHA class III or IV, and in Black and Asian patients than in White patients.
AcknowledgmentsOther Section
Funding: This work is supported by the Shenzhen Key Medical Discipline Construction Fund (SZXK063).
FootnoteOther Section
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at: http://dx.doi.org/10.21037/cdt-20-984
Peer Review File: Available at: http://dx.doi.org/10.21037/cdt-20-984
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/cdt-20-984). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med 2021;384:129-39. [Crossref] [PubMed]
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 2021;384:117-28. [Crossref] [PubMed]
- Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2020;383:1436-46. [Crossref] [PubMed]
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020;383:1413-24. [Crossref] [PubMed]
- McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995-2008. [Crossref] [PubMed]
- Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med 2020;383:1425-35. [Crossref] [PubMed]
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019;380:2295-306. [Crossref] [PubMed]
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019;380:347-57. [Crossref] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644-57. [Crossref] [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus. Circulation 2018;138:458-68. [Crossref] [PubMed]
- Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J 2016;37:1526-34. [Crossref] [PubMed]
- Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and Clinical Outcomes in Patients With Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease. Circulation 2018;137:119-29. [Crossref] [PubMed]
- Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819-29. [Crossref] [PubMed]
- Salah HM, Al'Aref SJ, Khan MS, et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-Systematic review and meta-analysis of randomized placebo-controlled trials. Am Heart J 2021;232:10-22. [Crossref] [PubMed]
- Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020;17:761-72. [Crossref] [PubMed]
- Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol 2021;77:243-55. [Crossref] [PubMed]
- Lee MM, Brooksbank K, Wetherall K, et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021;143:516-25. [PubMed]
- Butler J, Anker SD, Filippatos G, et al. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J 2021;42:1203-12. [Crossref] [PubMed]


