
Coronary artery calcium scoring, what is answered and what questions remain
Introduction
Atherosclerotic cardiovascular disease is number one cause of death in the world, accounting for nearly one-third of all deaths worldwide. There is a clinical dilemma, in that almost half of acute coronary events occur in previously asymptomatic patients (1), and nearly 70% of acute coronary events result from coronary lesions that are not flow-limiting before the event (2). Multiple scoring systems have been developed to predict the risk of coronary events in patients who do not have a history of cardiovascular diseases. The Framingham risk score (FRS) is probably the most extensively adopted (3). The main limitation of FRS, is that it does not incorporate family history and many components of metabolic syndrome. Hence, it was important to look for other imaging modalities as calcium artery calcium (CAC) score to properly assess the cardiovascular risk. In this paper we will review some of the established technical facts and clinical applications of CAC Scoring together with some of the controversial issues and limitations that might need better understanding and further studies to be better clarified.
Calcium scoring; imaging modalities and scoring techniques
Two modes of cardiac CT are used for CAC quantification. Formerly, electron beam computed tomography (EBCT) and more recently multidetector computed tomography (MDCT) have been used for this evaluation. Electron beam computed tomography allowed faster imaging with higher temporal resolution. However, MDCT have the advantage of higher spatial resolution and image quality especially with recent scanner generations, but optimally should be done with heart rate control to limit motion artifacts from high heart rates.
CAC is defined as a hyper-attenuating lesion >130 Hounsfield units with an area of ≥3 pixels. Baseline CAC has been quantified by several methods. The Agatston score is calculated by multiplying the lesion area (mm2) by a density factor (between 1 and 4) (4). Because of the stepwise nature of the density factor, changes in the Agatston score might not accurately capture changes in coronary calcium. In contrast to the Agatston score, the calcium volume score (CVS) represents an actual volume of CAC and reduces variability between scans (5) opposed to the increase in Agatston score which might just represent an increase in plaque attenuation rather than size over time. The mass score has also been advocated, with less inter-scanner variability, however limited outcome data is available with this measure, so it is rarely used. CAC is typically scanned in a prospectively ECG-triggered mode with 2.5-3.0 mm thick axial images. The radiation dose is low, with a typical effective dose of <1.5 mSv (6).
CAC score had been validated in an original histopathological study by Rumberger et al. which showed a high correlation between CAC area by EBCT and plaque area in 13 autopsy hearts (7). EBCT CAC Score was also directly compared to coronary angiography in 213 consecutive patients and a cut-point Agatston score of 371 was found to be associated with coronary arterial luminal stenosis >70% in at least one coronary artery with high sensitivity and specificity (8). Specific patterns of calcifications, namely; shell like and diffuse were shown to be more associated with severe stenosis rather than a nodular pattern (9).
Main Clinical applications of CAC
Cardiovascular risk prediction and relation to Framingham risk score (FRS)
CAC score has been extensively validated as a marker for cardiovascular risk and currently regarded as a feasible surrogate marker for screening of coronary artery disease. In the 2010 ACCF/AHA practice guidelines, measurement of CAC was considered reasonable for assessment of cardiovascular risk in asymptomatic adults at intermediate risk (10-20% 10-year) [Class IIa, level of evidence B], with similar recommendations for persons with diabetes, and a IIb recommendation for those persons at low-intermediate risk 6-10% 10 year risk (10). Similarly, in the 2010 appropriateness criteria, CAC scoring was judged as appropriate for intermediate CHD risk patients, and for the specific subset of low-risk patients in whom a family history of premature CHD was present. However, for those patients with low cardiovascular risk (<6%), CAC Score was considered of no benefit and inappropriate for cardiovascular risk assessment.
CAC score was studied in association with other traditional well established risk factor scoring systems, like the Framingham risk score (FRS) showing the following advantages: (I) Independent incremental value in prediction of all cause mortality in asymptomatic cohort group; Pooled data from 6 studies of 27,622 asymptomatic patients examined predictors of CAD deaths or MIs (10). The 11,815 subjects who had CAC Score of 0 had a low rate of events over the subsequent 3 to 5 years (0.4%). Compared with a CAC Score of 0, a CAC Score (100-400) indicated a relative risk (RR) of 4.3 (95% CI, 3.5-5.2; P<0.0001), a score of (400-1,000) indicated a RR of 7.2 (95% CI, 5.2-9.9; P<0.0001), and a score >1,000 indicated a RR of 10.8 (95% CI, 4.2-27.7; P<0.0001). The summary relative risk ratios in Figure 1 reveal an incremental relationship where higher CAC scores are associated with higher event rates and higher relative risk ratios. (II) Superior value over FRS in predicting the proximal stenosis burden: Brown et al. (11) demonstrated that the CAC Scores had a significant positive correlation with the proximal stenosis burden measured by invasive coronary angiography (The Spearman correlation coefficients, R=0.62 for Agatston score), as well as superior predictive value over other traditional Framingham risk factors on value multivariate regression analysis. (III) Reclassification of CAD risk categories: 60% of coronary atherosclerotic events occur in patients in low or intermediate-Framingham risk categories (12). Intermediate-risk patients with CACS >300 had a 2.8% annual rate of cardiac death or MI (roughly equivalent to a 10-year rate of 28%) that would put them in a high risk category (13). In the Rotterdam study (14), cut-off values of 615 and 50 were suggested to reclassify the elderly intermediate risk group population to either high or low risk groups respectively. A recent advanced analysis based on the Rotterdam study studied the change in the c-statistic and the overall net reclassification index (NRI) when each newer risk marker is added to the Framingham base model. [The c-statistic is a measure of discrimination (the ability to distinguish between 2 persons, 1 with and 1 without a CHD event), and NRI specifies the amount of correct reclassification of estimated events and nonevents to 10 years]. The maximum change in the c-statistic was observed for CAC score [increase, 0.05 (CI, 0.02-0.06)], followed by NT-proBNP level [increase, 0.02 (CI, 0.01-0.04)]. The highest overall net percentage of persons correctly reclassified was also observed for CAC score [NRI, 19.3% (CI, 12.5-26.2%)], with a smaller NRI for NT-proBNP [7.6% (CI, 2.8-12.5%)]. High Sensitivity C reactive protein did not lead to net reclassification or improve the C statistic. Changes in c-statistics and overall NRIs in total population were otherwise negligible or absent for every other newer marker (15).

Role of CAC in patients presenting with chest pain
The Appropriateness criteria published in 2010 considered CTA as appropriate indication in symptomatic patients with prior CAC score ≤400 while inappropriate for higher scores (CAC score >400). Some studies showed that CAC score can be regarded as the gate keeper to CTA in diagnosis of significant CAD in patients with chest pain.
The recently published guidelines from the National Institute of Health and Clinical excellence (NICE) in UK have two separate diagnostic pathways (16). The first is for patients with acute chest pain, and the second for those with intermittent stable chest pain. Patients classified as low likelihood of CAD by modified Diamond-Forrester (DF) score (10-30%) are offered CAC score scan as a first line test that was used to confidently rule out cardiac pain in those with zero calcium score indicating no need for further imaging and to refer those with higher scores to invasive or non invasive coronary angiogram to obtain additional information on stenosis severity See Figure 2. some controversy in respect to the diagnostic accuracy of CAC score will be discussed later.
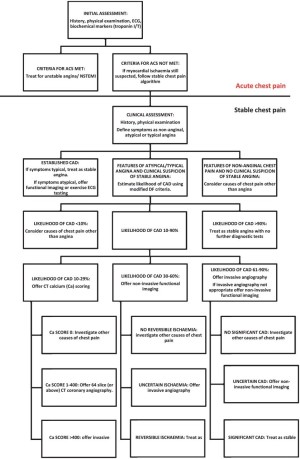
Other potential applications for CAC scoring have been identified are shown in Table 1 (22).
Table 1
| Authors | Year | Cohorts | Application of CAC score | Conclusion |
|---|---|---|---|---|
| Chen et al. (17) | 2001 | 93 subjects with stable angina | Differentiation between syndrome X and CAD | Calcium coverage score determined by EBCT could differentiate between syndrome X and CAD |
| Raggi et al. (18) | 2004 | 10,377 subjects with and without diabetes | Prognosis prediction of diabetic patients | All-cause mortality increased in asymptomatic diabetics in proportion to the CAC Score |
| Tong et al.(19) | 2004 | 159 young to middle-aged African-Americans | Reflection of the severity of hypertensive disease | CACS correlated increased LV mass independent from other risk factors |
| Wong et al. (20) | 2004 | 1,291 subjects with subclinical atherosclerosis | Monitoring the effects of lipid control | Greater progression of CAC may occur in those in whom HDL-cholesterol is not controlled |
| Sirineni et al. (14) | 2008 | 6,814 asymptomatic multiethnic subjects | Coronary age calculation | The coronary age of men and women of diverse ethnicities could be estimated based on CACS |
| Colletti et al. (21) | 2010 | 386 subjects | Prediction of future cardiac function | Subclinical atherosclerosis assessed by using CAC Score was associated with an increased future likelihood of RWMA |
CAC: coronary artery calcium, RWMA: regional wall motion abnormality, CAD: coronary artery disease, EBCT: electron beam computed tomography
Role of calcium score in specific patient groups
(I) Women
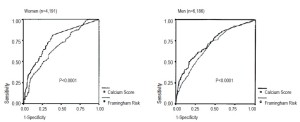
Coronary calcium scanning was shown to have a significant contribution in accurate detection of Coronary heart disease (CHD) on top of traditional cardiovascular risk factors in asymptomatic women. The MESA study screened 2,600 asymptomatic women, mean age 61.5 years, the median Agatston score was 0 (interquartile range, 0-26). CHD occurred in 53 (2%) subjects (17). The area under the curve (AUC) for CHD increased significantly from 0.805 for the base model to 0.835 with the addition of CAC scanning in women. Similar findings were observed in a study by Raggi et al. supporting the role of the Agatston score as a risk stratification tool for women (Figure 3) (18).
(II) Diabetes
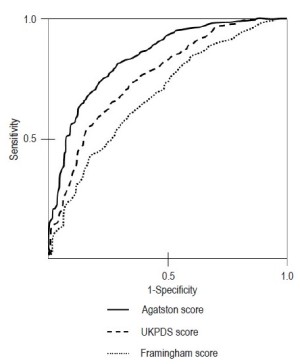
Asymptomatic persons with diabetes were shown to have significantly higher calcium score than non diabetics even after controlling for other risk factors (19-21). When compared to other traditional risk scoring systems, calcium scores have been shown to be significantly superior in predicting cardiac events having the largest area under the curve AUC (0.92) compared to United Kingdom Prospective Diabetes Study Risk Score, UKPDS (AUC: 0.74) and FRS (AUC: 0.6) see Figure 4 (23).
In an 8-year follow up study of 716 asymptomatic diabetics it was shown that those who had higher CAC score (>400) had significantly higher prevalence of annualized cardiac events (namely, myocardial infarction and cardiac death) compared to those with lower scores (5.6% versus 0.7%, P<0.01) (24). Also, it was shown that as the CAC score increases the cardiac events proportionally increases going from 0% to 18% as the calcium score of goes from <100 to >1,000.
However, on the other side, knowing that diabetics constitute a higher risk group, studies showed that having a zero calcium score can be helpful to re-stratify them into a lower risk category with low cardiac events and excellent survival rates. In a 5-year follow up study for 903 asymptomatic diabetics, the prevalence of zero calcium score in non diabetics was almost twice as that in diabetics with zero calcium score, yet, it was shown that there was no difference in the survival between the two groups. (98.8% and 99.4%, respectively, P=0.5) (19).
Similarly, another recent follow up study for about 300 patients with a mean follow up of 20 months showed that the event rate was 0% in both diabetics and non-diabetics with zero calcium (25).
Accordingly, persons with diabetes who are ≥40 years, it is recommended by the 2010 ACCF/AHA practice guidelines to use the CAC scan as a tool for cardiovascular risk assessment (Class IIA, level of evidence B) (10).
(III) Kidney transplant recipients (KTR)
KTR constitutes another known higher risk group patients and in a recent study where repeated Calcium score scans were available for 197 KTRs after 4.40±0.28 years, it was found that CAC scores increased significantly by a median of 11% during follow up. Higher baseline CAC score, history of cardiovascular event, use of a statin, and lower 25-hydroxyvitamin D3 level were independent determinants of CAC progression (26).
Controversies and limitations
Retesting in patients with zero CAC score, how often?
CAC score retesting in asymptomatic subjects and the “warranty period” of a zero score are subjects of debate. Obviously, if the patient becomes symptomatic, re-evaluation would be considered regardless of a previous zero CS. However, in those who remain symptom free, evidence suggests that the CS should not be repeated frequently (10,27,28). In a recent study by Min et al., the cumulative rate of “conversion” from a zero CAC score to ≥1 CAC score was 15% in the first 4 years and 25% in the fifth year (28) concluding that 4 years might be the ideal “warranty period” for a zero CAC score which is considerably longer that the warranty period that is offered by normal functional imaging tests (such as nuclear perfusion scans) which is considered to be around 1.5 to 2 years (29). Although looks reasonable, their suggested period might be underestimated because cardiac events -not just the conversion to a positive study - should be regarded as the end point especially that most patients’ scores remained <100 which is still considered a low risk category.
Clinical significance of zero calcium score. Does it rule out obstructive CAD in symptomatic patients confidently?
The clinical implication of a zero CAC score in patients with chest pain syndrome has been under debate. In a recently published study, 1,114 patients presented with chest pain and zero calcium score, with mean follow-up period of 2.8 years, early revascularization was done in 25 patients (2.2%) and there were 14 major adverse cardiac events (1.3%) (30). Other studies listed in Table 2 have used a positive calcium score as a positive test for identifying obstructive CAD with high sensitivity values. On the other hand, a zero calcium score was used as a negative test to identify negative (ie, no obstructive lesions) cases, virtually all studies demonstrate very high sensitivity (generally >95%) and even higher negative predictive power (achieving 99% in most studies), highlighting the ability of a zero CS to rule out obstructive CAD. An exception for this was for the CORE64 substudy (Coronary Evaluation Using Multi-Detector Spiral Computed Tomography Angiography Using 64 Detectors) (31), which took zero CAC score as a positive test to rule out obstructive CAD, thus simply inverting the sensitivity and specificity values.
One reason that zero CAC score might not be a good test to rule out obstructive CAD is the low prevalence of zero CAC score among symptomatic patients. As shown in Table 2, most of the studies showed a low prevalence of zero CAC score ranging from 12-28% thus, performing a test that only yields useful data in 20% of the cases is clearly not ideal, unless lower probability patients are enrolled. This algorithm using CAC as a first line test in low risk patients is implemented in the NICE guidelines (16). In these national guidelines, patients with a 1-29% pre-test probability of obstructive disease are screened using CAC as a first line test, progressing to CTA for scores of 1-400 and invasive angiography for CAC scores >400.
Table 2
| Study | Total patients, N | Patients with zero CAC score, n [%] | Sensitivity % | Specificity % | Observations |
|---|---|---|---|---|---|
| Fallavollita et al. (33) | 106 | 30 [28] | 85 | 45 | Only patients <50 years old |
| Budoff et al. (34) | 710 | 147 [21] | 95 | 44 | |
| Baumgart et al. (35) | 57 | 7 [12] | 97 | 21 | Findings corroborated by IVUS |
| John et al. (36) | 368 | 71 [19] | 96 | 31 | Retrospective analysis, multicenter study |
| Bielak et al. (37) | 213 | 40 [19] | 99 | 39 | |
| Knez et al. (38) | 2115 | 326 [15] | 99 | 29 | Retrospective analysis |
| Haberl et al. (39) | 133 | 25 [19] | 85 | 24 | |
| Lau et al. (40) | 50 | 6 [12] | 97 | 25 | |
| Becker et al. (41) | 1347 | 259 [19] | 99 | 31 | Predictive accuracy of 64%. Same center and similar population as the study by Knez et al. (39) |
| Drosch et al. (42) | 500 | 61 [12] | N/A | N/A | Retrospective analysis |
| Gottlieb et al. (31) | 291 | 72 [25] | 45 | 91 | Multicenter study. Zero CS was considered “positive,” thus inverting the sensitivity and specificity values compared with the other trials |
Role of CAC score in patients’ compliance to treatment
Several studies have shown CAC score to positively enhance patients’ adherence to both pharmacological and non pharmacological treatments, such as dietary changes and exercise. Generally, it was shown that patients with any coronary calcification had a better initiation and compliance to various therapeutic interventions compared to those with baseline zero calcium score. Similarly, higher adherence rate was observed in patients with higher calcium score compared to lower scores.
In The prospective Multi-Ethnic Study of Atherosclerosis (MESA) study, 6,814 subjects free of clinical cardiovascular disease underwent baseline CAC testing with follow up scans after 1.6 and 3.2 years. The risk ratios for medication continuation after a follow up of 3.2 years among those with CAC >400 versus CACS=0 were 1.10 (95% CI, 1.01-1.20) for lipid lowering medication, 1.05 (95% CI, 1.02-1.08) for Blood pressure lowering medication, and 1.14 (95% CI, 1.04 -1.25) for ASA initiation, respectively (32).
In the Prospective Army Coronary Calcium (PACC) study, among 1,640 healthy men followed up for 6 years, those with CAC were 3 times more likely to receive a statin (48.5% vs. 15.5%, P<0.001) and aspirin (53.0% vs. 32.3%, P<0.001) than those without CAC. In multivariable models controlling for National Cholesterol Education Program (NCEP) risk variables and baseline medication use, CAC was strongly and independently associated with use of either statin [odds ratio (OR) 3.53; 95% confidence interval (CI), 2.66 to 4.69], aspirin (OR 3.05; 95% CI, 2.30-4.05) or both (OR 6.97; 95% CI, 4.81-10.10) (33).
In another study of 505 asymptomatic patients, Kalia et al. showed that 3.6 years after visualizing their CAC scan, 90% of patients with CAC>400 complied with their statin therapy, compared to 75% for CAC 100-399, and only 44% for 0 CAC scores (34). Similarly, in a study of 980 asymptomatic patients, Orakzai et al. showed that ASA initiation, dietary changes, and exercise were much better in CAC>400 (61%, 67%, 56%) than in patients with 0 CAC (29%, 34%, 44%) (35).
Most importantly, the EISNER study (IMPACT OF CORONARY ARTERY CALCIUM SCANNING ON CORONARY RISK FACTORS AND DOWNSTREAM TESTING: A PROSPECTIVE RANDOMIZED TRIAL) was a randomized trial evaluating the potential benefit of undergoing CAC testing versus standard of care. Compared to the no-scan group, the scan group showed a net favorable change in systolic blood pressure (P=0.02), LDL-cholesterol (P=0.04), waist circumference for those with increased abdominal girth (P=0.01), and tendency to weight loss among overweight subjects (P=0.07). While mean FRS rose in the no-scan group, it remained static in the scan group [(0.7+5.1) versus (0.002+4.9), P=0.003). Within the scan group, increasing baseline CAC score was associated with a dose-response improvement in systolic and diastolic blood pressure (P<0.001), total cholesterol (P<0.001), LDL-cholesterol (P<0.001), triglycerides (P<0.001), weight (P<0.001) and FRS (P=0.003).
The missing piece of the puzzle in regards to scanning and compliance is whether undergoing a CAC scan results in better outcomes, which is a very difficult undertaking (as it relies on the downstream treatment to affect a change, not solely based on the performance of the scan itself), with sample size estimates in the 50,000-100,000 range.
Assessment of coronary artery calcium progression
It would be highly desirable to have a reliable measure of the progression of coronary atherosclerosis, to allow physicians to understand non-invasively which patients are, and are not, responding well to therapies without waiting for hard end points. Typically, Coronary calcium progresses at 10-20% of the baseline value per year, although data suggest that a progression rate of >15% per year is associated with a 17-fold increased risk for incident CAD events (36). Median inter-scan variability is about 7%, and mean interscan variability is 15% with MDCT, allowing serial follow up at no closer than 1 year intervals.
Assessment of CAC progression has been regarded as a dynamic measurement that might provide insight into ongoing current disease activity and more efficiently predicts future cardiac events, by its association with increased total plaque burden, rather than static traditional clinical parameters and baseline CACS. However we acknowledge some limitations; In earlier MSCT studies, there has been a tendency to overestimate the CAC score progression in patients with high baseline scores when measuring the absolute change, due to known increased interscan variability. However with the more recent MSCT generations, better image quality significantly contributed to improve reproducibility (37). Similarly, caution should be taken in patients with low baseline scores when using the percentage relative change as this measurement tends to magnify small increases in the baseline calcium score, comparison between published data on CAC progression would be cumbersome because of heterogeneous variables used in each study yielding different results for example, type of scanner used, calcium scoring method and the method used for assessment of progression (absolute vs. relative change). Formal guidelines need to be developed to standardize these factors.
Numerous studies tried to relate CAC progression to other traditional or novel cardiac risk factors as C-reactive protein (CRP), this relation was not always consistent but this may be related to different cohorts studied and different methodology used for measuring the progression rates. Berry et al. (38) showed that higher annual CAC progression (22% vs. 15% in men; 9% vs. 5% in women) was successful in defining a group of patients with high life-time risk though they were originally classified as low risk for cardiac events on FRS. Baseline calcium score was shown to be the most powerful predictor for CAC progression, a 5-year follow-up study by Gopal et al. (27) showed that only 2% of patients with baseline zero calcium score had CAC progression >50 suggesting that individuals with no detectable CAC on an initial scan, a repeat scan can be recommended no sooner than 5 years.
In most observational studies, higher CAC progression rates were shown to be significantly associated with more cardiac events and myocardial infarction when compared to lower progression rates. An observational study of 817 asymptomatic patients showed that the yearly percentage of calcium volume changes in those who developed MI were significantly higher at 47%±50% versus 26%±32% in those who did not (39). In this paper by Raggi et al. (39), the yearly mean absolute CVS change in the 45 patients who had a myocardial infarction was 147, compared with 63 in those patients without events (P<0.001).
It was demonstrated by Shemesh et al. (40) that the 180% increase in the annual progression rate in the group that suffered CV events was significantly higher than either the 124% in the asymptomatic and the 118% in the stable CAD groups (P<0.05).
In the largest study to date, Budoff et al. (41) followed 4,609 asymptomatic individuals undergoing two scans for 3.1 years for all-cause mortality. Progression of CAC was significantly associated with mortality regardless of the method used to assess progression (P<0.0001), and retained significance after adjusting for baseline score, age, sex, risk factors and time between scans. In those with CAC =0, progression was very limited and did not predict mortality. Serial assessment may have clinical value in assessing plaque progression and future cardiovascular risk.
Statins and its relation to coronary artery calcium progression
Statins reduce clinical cardiac end points across a spectrum of patient populations (42). It seemed intuitive to early researchers that statin should reduce the progression of CAC. However, later studies in this field did not yield significance in randomized controlled trials (RCTs).
Callister et al. produced the first report of CACS being used to assess the effects of statin therapy (43). They conducted a retrospective study of 149 patients with no history of CAD. Repeat scanning was carried out 12-15 months later. 105 patient received statin and a net reduction in CAC score was found in the treated group who had final LDL-cholesterol levels <3.10 mmol/L (–7±23%; P<0.001). Treated patients who had an average LDL-cholesterol of ≥3.10 mmol/L had an increase in CAC score (25±22%; P<0.001), further increases was noticed in untreated patients who had an average LDL-cholesterol of ≥3.10 mmol/L (52±36%; P<0.0001).
In a prospective study, 66 patients who were not on treatment and whose LDL-cholesterol was >3.4 mmol/L, had a repeat EBCT and treatment with Cerivastatin 0.3 mg/day was initiated after a mean interval of 14 months (44). A third examination was carried out after 12 months of treatment; the median annual relative increase in CAC score was 25% during the untreated period versus 8.8% in the treatment period (P<0.0001).
On the other hand, other studies could not prove similar significant results. St Francis Heart Study is a double blinded, placebo controlled, randomized clinical trial where 1,005 asymptomatic men and women aged 50-70 years with CAC score ≥80th percentile for age and gender were randomized to receive either Atorvastatin 20 mg daily and vitamin E (α-Tocopherol) 1,000 units daily or placebo (45) with mean duration of treatment of 4.3 years. Treatment reduced total cholesterol by 26.5-30.4%, LDL-cholesterol by 39-43% and triglycerides by 11.2-17%, but had no effect on progression of CAC score. More importantly, the treatment with statins did reduce CV events by 42% (P<0.05) in those persons taking statins as compared to the placebo group, demonstrating definitively that persons with CAC benefit by treatment with statins. Interestingly, in the overall study cohort of 4,609 patients, change in calcium score was a robust predictor of subsequent coronary disease events. Median score increased by 4 [0, 38] from baseline to the year two scan in subjects who did not sustain a coronary event at any time during the study. In contrast, median (interquartile range) increased by 247 [40, 471] U between the baseline and two-year examinations in subjects who first experienced a CAD event after the year two scan (P<0.0001). In multiple logistic regression, only age (P=0.03), male gender (P=0.04), LDL cholesterol (P=0.01), HDL cholesterol (P=0.04), and two-year change in calcium score (P=0.0001) were significantly associated with subsequent CAD events. Thus, progression of CAC was the strongest predictor of future CV events. It was nevertheless disappointing to find no effect of LDL-cholesterol lowering on CAC score progression.
In another multicenter, randomized double-blind trial (46), 471 patients had ≥2 cardiovascular risk factors and a CAC score of ≥30 with no history of CAD or evidence of significant coronary stenoses. Patients were randomized to receive either low (10 mg) or high dose (80 mg) Atorvastatin over 1 year. LDL-cholesterol was reduced in the 80 mg/day group from 2.7 to 2.2 mmol/L, whereas in the 10 mg/day group it remained at 2.8 mmol/L. The mean progression in 366 patients corrected for baseline score was 27% in the 80 mg/day group compared with 25% in the 10 mg/day. Thus, no difference was found in the progression between the two groups and no relationship was found between on-treatment LDL-cholesterol and progression. Accordingly, several factors might explain this conflicting data; (I) first CAC score might not be the best tool to monitor the treatment response, (II) longer follow up period are needed or (III) statins might be improving the cardiovascular outcome through other pleiotropic effects rather than mere lowering of the LDL-cholesterol or decreasing the CAC scores.
Cost effectiveness and impact on downstream testing
Dewey M et al. (47) compared the cost effectiveness of CTA, CAC score and stress magnetic resonance imaging with traditional diagnostic modalities (Invasive coronary angiography ICA, Exercise treadmill test, Echocardiography) for the diagnosis of CAD using a decision tree model. CAC score was more cost effective for a pretest likelihood of 30% to 40% than any of the traditional diagnostic modalities. However, the main findings showed that patients with a 10%-50% pretest likelihood of CAD, CTA was the most cost effective approach, while ICA was most cost effective for a pretest likelihood of at least 70%.
In The EISNER study, Rozanski et al. (48) randomized participants to CAC scanning and compared 4-years changes in coronary artery disease risk factors, Framingham risk score and differences in downstream medical resource utilization with conventional risk stratification strategy before risk factor counseling in a prospective randomized trial. Downstream medical testing and costs in the scan group were comparable to those of the no-scan group (overall cost 4,053 vs. 3,649 in scan vs. no scan group, P=0.09) balanced by lower and higher resource utilization for subjects with normal CAC scans and CAC score >400 respectively. Clearly, more studies related to cost effectiveness and downstream utilization after CAC are warranted.
Conclusions and future directions
The field of preventive cardiology and atherosclerosis imaging developed with the hope that early detection of subclinical disease and aggressive risk factor modification could significantly reduce the incidence of acute cardiovascular events. The field has witnessed enormous advancements in just a few years and CAC imaging has played a substantial role. CAC has been used to assess the cardiovascular risk and predicting future events in association with other traditional risk factors. More benefit will be gained if more evidence-based long term outcome data would be available.
The role of zero calcium score in the management of patients with chest pain together with the treatment strategies on CAC progression rates are still controversial. Further studies are needed to elucidate these data aiming at improving the primary and secondary preventive measures for CAD. It is important to educate the public and physicians as to the advantages and limitations for the most proper use of this powerful imaging tool. In the meantime, the screening of asymptomatic intermediate-risk patients for refinement of risk stratification and imaging in stable patients with chest discomfort to exclude obstructive CAD may constitute the best current indications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Murabito JM, Evans JC, Larson MG, et al. Prognosis after the onset of coronary heart disease. An investigation of differences in outcome between the sexes according to initial coronary disease presentation. Circulation 1993;88:2548-55. [PubMed]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657-71. [PubMed]
- Kannel WB, D’Agostino RB, Sullivan L, et al. Concept and usefulness of cardiovascular risk profiles. Am Heart J 2004;148:16-26. [PubMed]
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32. [PubMed]
- Callister TQ, Cooil B, Raya SP, et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998;208:807-14. [PubMed]
- Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006;114:1761-91. [PubMed]
- Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92:2157-62. [PubMed]
- Rumberger JA, Sheedy PF, Breen JF, et al. Electron beam computed tomographic coronary calcium score cutpoints and severity of associated angiographic lumen stenosis. J Am Coll Cardiol 1997;29:1542-8. [PubMed]
- Thilo C, Gebregziabher M, Mayer FB, et al. Correlation of regional distribution and morphological pattern of calcification at CT coronary artery calcium scoring with non-calcified plaque formation and stenosis. Eur Radiol 2010;20:855-61. [PubMed]
- Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2007;49:378-402. [PubMed]
- Brown BG, Morse J, Zhao XQ, et al. Electron-beam tomography coronary calcium scores are superior to Framingham risk variables for predicting the measured proximal stenosis burden. Am J Cardiol 2001;88:23E-6E. [PubMed]
- Lauer MS. Primary prevention of atherosclerotic cardiovascular disease: the high public burden of low individual risk. JAMA 2007;297:1376-8. [PubMed]
- Nasir K, Budoff MJ, Post WS, et al. Electron beam CT versus helical CT scans for assessing coronary calcification: current utility and future directions. Am Heart J 2003;146:969-77. [PubMed]
- Sirineni GK, Raggi P, Shaw LJ, et al. Calculation of coronary age using calcium scores in multiple ethnicities. Int J Cardiovasc Imaging 2008;24:107-11. [PubMed]
- Kavousi M, Elias-Smale S, Rutten JH, et al. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med 2012;156:438-44. [PubMed]
- Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin, NICE Clinical Guideline 95. 2010. Accessed 2011. Available online: http://www.nice.org.uk/nicemedia/live/12947/47938/47938.pdf
- Jain A, McClelland RL, Polak JF, et al. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging 2011;4:8-15. [PubMed]
- Raggi P, Shaw LJ, Berman DS, et al. Gender-based differences in the prognostic value of coronary calcification. J Womens Health (Larchmt) 2004;13:273-83. [PubMed]
- Raggi P, Shaw LJ, Berman DS, et al. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43:1663-9. [PubMed]
- Hoff JA, Quinn L, Sevrukov A, et al. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am Coll Cardiol 2003;41:1008-12. [PubMed]
- Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care 2001;24:335-8. [PubMed]
- Lee J. Coronary artery calcium scoring and its impact on the clinical practice in the era of multidetector CT. Int J Cardiovasc Imaging 2011;27:9-25. [PubMed]
- Anand DV, Lim E, Hopkins D, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J 2006;27:713-21. [PubMed]
- Becker A, Leber AW, Becker C, et al. Predictive value of coronary calcifications for future cardiac events in asymptomatic patients with diabetes mellitus: a prospective study in 716 patients over 8 years. BMC Cardiovasc Disord 2008;8:27. [PubMed]
- Van Werkhoven JM, Cademartiri F, Seitun S, et al. Diabetes: prognostic value of CT coronary angiography--comparison with a nondiabetic population. Radiology 2010;256:83-92. [PubMed]
- Maréchal C, Coche E, Goffin E, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis 2012;59:258-69. [PubMed]
- Gopal A, Nasir K, Liu ST, et al. Coronary calcium progression rates with a zero initial score by electron beam tomography. Int J Cardiol 2007;117:227-31. [PubMed]
- Min JK, Lin FY, Gidseg DS, et al. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: what is the “warranty period” for remaining normal? J Am Coll Cardiol 2010;55:1110-7. [PubMed]
- Hachamovitch R, Hayes S, Friedman JD, et al. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: what is the warranty period of a normal scan? J Am Coll Cardiol 2003;41:1329-40. [PubMed]
- Kim YJ, Hur J, Lee HJ, et al. Meaning of zero coronary calcium score in symptomatic patients referred for coronary computed tomographic angiography. Eur Heart J Cardiovasc Imaging 2012. [Epub ahead of print].
- Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 2010;55:627-34. [PubMed]
- Nasir K, McClelland RL, Blumenthal RS, et al. Coronary artery calcium in relation to initiation and continuation of cardiovascular preventive medications: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Qual Outcomes 2010;3:228-35. [PubMed]
- Taylor AJ, Bindeman J, Feuerstein I, et al. Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. J Am Coll Cardiol 2008;51:1337-41. [PubMed]
- Kalia NK, Miller LG, Nasir K, et al. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis 2006;185:394-9. [PubMed]
- Orakzai RH, Nasir K, Orakzai SH, et al. Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. Am J Cardiol 2008;101:999-1002. [PubMed]
- Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol 2004;24:1272-7. [PubMed]
- Budoff MJ, McClelland RL, Chung H, et al. Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: the Multi-Ethnic Study of Atherosclerosis. AJR Am J Roentgenol 2009;192:613-7. [PubMed]
- Berry JD, Liu K, Folsom AR, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation 2009;119:382-9. [PubMed]
- Raggi P, Cooil B, Shaw LJ, et al. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol 2003;92:827-9. [PubMed]
- Shemesh J, Apter S, Stolero D, et al. Annual progression of coronary artery calcium by spiral computed tomography in hypertensive patients without myocardial ischemia but with prominent atherosclerotic risk factors, in patients with previous angina pectoris or healed acute myocardial infarction, and in patients with coronary events during follow-up. Am J Cardiol 2001;87:1395-7. [PubMed]
- Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229-36. [PubMed]
- Sutton-Tyrrell K, Kuller LH, Edmundowicz D, et al. Usefulness of electron beam tomography to detect progression of coronary and aortic calcium in middle-aged women. Am J Cardiol 2001;87:560-4. [PubMed]
- Callister TQ, Raggi P, Cooil B, et al. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med 1998;339:1972-8. [PubMed]
- Achenbach S, Ropers D, Pohle K, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation 2002;106:1077-82. [PubMed]
- Arad Y, Spadaro LA, Roth M, et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005;46:166-72. [PubMed]
- Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 2006;113:427-37. [PubMed]
- Dewey M, Hamm B. Cost effectiveness of coronary angiography and calcium scoring using CT and stress MRI for diagnosis of coronary artery disease. Eur Radiol 2007;17:1301-9. [PubMed]
- Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011;57:1622-32. [PubMed]


