
Treatment of adults with Eisenmenger syndrome—state of the art in the 21st century: a short overview
Despite the increased availability of early reparative surgery and interventions—avoiding long-term complications such as severe pulmonary arterial hypertension—Eisenmenger syndrome remains a challenging diagnosis in a subset of adult congenital heart disease (ACHD) patients (1). It represents a cyanotic shunt condition with a pulmonary arterial pressure at the systemic (post-tricuspid shunts) or even at the suprasystemic level (pre-tricuspid shunt) which results in a bidirectional or reversed shunt and a plethora of multiple end-organ complications (2,3). Patients are almost always symptomatic with reduced exercise capacity and life expectancy is limited in this condition (2-6). The first description of the syndrome dates to the 19th century, with a case report by the Viennese physician Victor Eisenmenger (7). The initial description was labeled as Eisenmenger complex in a 32-year-old man with a non-restrictive VSD, but it was Paul Wood in his landmark paper who described the anatomical and pathophysiological phenotypes in a very large population and introduced the term Eisenmenger syndrome as it doesn’t matter where the large shunt happens to be (8). Since then, our understanding of the condition has evolved but only within the last one and a half decades medical therapeutic options became available improving symptoms and potentially survival in this vulnerable cohort.
The prevalence of Eisenmenger syndrome is not well defined but traditional estimates suggest that around 5% of ACHD patients under follow-up at large supra-regional centers have Eisenmenger syndrome (2,3). The prevalence of the condition is most likely much higher in low and middle income countries while in high resource countries it is likely to decrease further due to better diagnostic and surgical/interventional therapeutic options early in life; in fact, we shouldn’t see any Eisenmenger syndrome patients born in these countries anymore (9). In patients presenting late with established Eisenmenger syndrome, shunt closure is contraindicated as this will worsen prognosis in this setting (1). This short article aims to give an overview over the pathophysiology and especially the relevant medical aspects of care for Eisenmenger patients. It therefore represents a focused overview over selected aspects of the condition. It should be noted that recent reviews have also addressed this condition potentially covering some additional relevant aspects.
Pathophysiology
Eisenmenger syndrome represents a clinical diagnosis based on physiologic factors. The underlying anatomic diagnosis can therefore be variable. Most commonly, isolated large, unrestrictive, unrepaired shunt lesions at the ventricular or arterial level as well as complex univentricular conditions represent the underlying morphological lesions. Occasionally also large atrial septal defects (ASD) may be associated with this condition. Due to the prevailing distribution of vascular resistance between pulmonary and systemic circulation, patients initially develop left-to-right shunting with volume overload of the pulmonary circulation, often accompanied by increased pressure due to post-tricuspid shunt lesions. In this situation, over time, pulmonary vascular disease develops and progresses to the point where shunt reversal occurs. This is labelled as Eisenmenger reaction which manifests itself first during exercise and later also at rest. Patients therefore develop cyanosis, often accompanied by the typical stigmata of cyanotic disease such as clubbing and visible cyanosis. It should be noted, however, that not all patients are desaturated at rest and arterial desaturation may become apparent only during exercise in some patients (10). Important additional features of the condition are typical laboratory findings mainly secondary erythrocytosis, low platelet count, iron deficiency, and hyperuricemia. In addition, overt Eisenmenger syndrome is a multisystem disorder characterized by hepatic, renal, immunologic, neurological and orthopedic complications (2,3,11).
Clinical presentation and outcome
The clinical presentation of Eisenmenger patients is variable. Depending on the underlying heart defect and associated end-organ complications, patients may be oligosymptomatic, while the majority has dyspnea (NYHA class II or III). Occasionally, patients also present in NYHA class IV (6,12). Main symptoms include shortness of breath, fatigue, exercise intolerance and palpitations. Patients with Eisenmenger syndrome, by and large, represent the most symptomatic subgroup of ACHD patients and the group with the lowest average exercise capacity (13). Hemoptysis, an external manifestation of an intrapulmonary hemorrhage and previously reported as a common complication, is, however, nowadays rarely encountered (3,14). Due to their predisposition for bacterial infections, patients may present with pneumonia or bacterial endocarditis. Cerebral abscess should be suspected in patients presenting with neurological symptoms, pronounced cephalgia and signs of infection. Arrhythmias are not uncommon in this setting and may be associated with malignant arrhythmias and sudden cardiac death in selected patients. Furthermore, hyperbilirubinemia may lead to gallstones and associated symptoms. Hematological abnormalities include secondary erythrocytosis with hyperviscosity syndromes in selected patients (relatively rare) and bleeding complications due to thrombocytopenia and abnormal coagulation parameters. Due to the possibility of paradoxical embolism and air embolism, ischemic neurological complications may occur, and neurological symptoms should be taken seriously in Eisenmenger patients.
Survival prospects of Eisenmenger patients are variable and predicting individual prognosis remains challenging (15). Given the degree of pulmonary arterial hypertension, survival prospects of Eisenmenger patients are relatively preserved compared to patients with idiopathic pulmonary hypertension. On the other hand, there is a misconception and misperception of highly benign outcome of the Eisenmenger population. Recent data suggests, however, that untreated Eisenmenger patients have significantly confined life expectancy especially in the presence of complex underlying heart defects (5). The reason for the superior survival of Eisenmenger patients compared to idiopathic pulmonary arterial hypertension patients is likely due to a better adaptation of the right ventricle to increased afterload given the slower disease progression and potentially the higher plasticity of cardiac myocytes earlier in life (3,16,17). This has led to the postulate that ‘the integrity of right ventricular function, rather than the degree of vascular injury, … is the major determinant of symptoms and survival in pulmonary arterial hypertension’ (18). As such, progressive dilatation or worsening of right ventricular function in Eisenmenger syndrome should be considered an ominous sign as they may herald failure of the balanced but fragile physiology. Risk factors of mortality in Eisenmenger patients include age, lower oxygen saturation, complexity of the underlying heart defect, signs and symptoms of heart failure, levels of brain natriuretic peptides, low six minute walk test distance, NYHA functional class, presence of Down syndrome, absence of sinus rhythm or arrhythmias, the presence of a pre-tricuspid shunt, worse right ventricular function [especially lower tricuspid annular plane systolic excursion (TAPSE)], low albumin and increased C-reactive protein (2,12,15,19). Assessing prognosis is paramount as it may help to guide specific pulmonary arterial hypertension therapy in this setting. There has been a shift in the cause of death during the last decades: the leading causes of death include heart failure, infections, sudden cardiac death, thromboembolism, hemorrhage and peri-procedural complications in the current era (14).
Management strategies
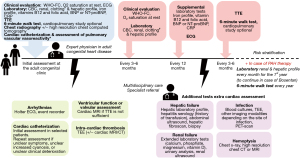
The key principle in the clinical management of Eisenmenger syndrome patients remains to cause no harm to the fragile balanced pathophysiological state (2). As a consequence, Eisenmenger patients require life-long expert assessment and therapy at specialized centers. Preventing complications and avoiding management errors should be the primary goal of regular outpatient assessment (1). Table 1 and Figure 1 summarize the key elements of diagnostic, preventive and therapeutic approaches (2).

Full table
Secondary erythrocytosis—a physiologic response: do not phlebotomize
Given the obvious central cyanosis, often accompanied by pronounced secondary erythrocytosis, non-congenital heart specialists may be tempted to offer regular, routine phlebotomies to the patients in a well-meant intention to ‘normalize’ blood rheology. However, this idea is based on a flawed physiologic concept, derived from patients with polycythemia vera (a completely different population), and has been shown to be associated with worse outcome, iron deficiency and increased risk of stroke in Eisenmenger patients (20,21). In fact, the elevated hemoglobin levels represent a physiologic adaptation to reduced oxygenation, intending to maintain tissue oxygen delivery by increasing the oxygen carrying capacity of blood. As such iatrogenic interventions disabling this adaptive mechanism have delirious consequences and should be avoided. Hyperviscosity symptoms are driving the indication for phlebotomy; i.e., selected patients with true hyperviscosity syndrome may benefit from occasional phlebotomies with isovolumic substitution in expert hands. As perceived hyperviscosity symptoms may indeed represent signs of iron deficiency or dehydration, meticulous clinical assessment is required prior to intervention and iron deficient anemia or dehydration should be excluded. When iron deficiency is identified, iron replacement should be initiated. Usually, a low dose of ferrous sulfate is administered orally to this end to avoid hyperreactive erythrocytosis responses. Parenteral iron therapy is also possible in selected patients, intolerant to oral iron substitution (22,23).
Relative anemia
Relative iron-deficient anemia is frequently missed or even ignored in patients with Eisenmenger syndrome because the hemoglobin level may be perceived as normal like in an acyanotic patient, but in fact, it is far too low for a patient with cyanotic congenital heart disease. The hemoglobin level must always be interpreted in relation to the oxygen saturation in each individual patient. Eisenmenger patients may need early iron replacement therapy or even red blood cell transfusion to achieve an appropriate hemoglobin level to improve the amount of oxygen carriers and to facilitate oxygen delivery to the tissue.
Oxygen therapy
Chronic oxygen therapy is not indicated as it does not substantially increase oxygenation of tissue, does not improve outcome and may lead to drying of the mucous pharyngeal tissue, potentially predisposing patients to epistaxis (2). Additionally, the need to carry oxygen tanks represents a burden for patients, further reducing mobility in this group of patients with severely curtailed exercise capacity.
Pregnancy
Pregnancy is contraindicated in women with Eisenmenger syndrome as it carries a maternal mortality risk of 30–50% associated with generally low prospects of successful pregnancy especially if maternal arterial oxygen saturation is below 85% (24).
Neurological complications
Neurologic complications are common in Eisenmenger patients and require immediate attention. These include both ischemic events (paradoxical embolism or air embolism) and infections complications (cerebral abscess). Given the risk of paradoxical/air embolism, Eisenmenger patients require the use of air-eliminating filters whenever intravenous lines are established.
Thrombotic and embolic complications
Pulmonary thrombosis has been reported in a substantial number of Eisenmenger patients and may represent in-situ thrombosis as well as the consequence of thromboembolism. As a consequence, routine anticoagulation of Eisenmenger patients has been suggested but has to be balanced against the generally increased risk of bleeding due to co-existing hematological abnormalities with in intrinsic, increased risk of bleeding. No study has shown any survival patients of Eisenmenger patients on oral anticoagulants (6,25). Oral anticoagulation is generally required in patients with supraventricular arrhythmias (especially atrial fibrillation/flutter) and in those with a history of thromboembolic events. Mechanical heart valves are another indication, but this clinical scenario is very rare in Eisenmenger patients.
Given the scarce data on safety and efficacy of new oral anticoagulants as well as suggestions of a potential harmful effect (26), these drugs should be used very restrictively (if at all) in this population (26,27).
Prevention of infectious complications
It is recommended to meticulously encourage patients to obtain yearly influenza immunization as well as immunization against pneumococcal disease every 5 years. Furthermore, respiratory infections should be pro-actively diagnosed and treated accordingly to avoid pulmonary or cardiac complications. As per current guidelines, Eisenmenger patients should be offered appropriate antibiotic endocarditis prophylaxis before dental procedures (1). This of course, does not obviate the need for good dental hygiene and regular visits to the dentist.
General anesthesia carries a high risk
If general anaesthesia is required for any non-cardiac or cardiac diagnostic or therapeutic procedure, this should be supervised by an experienced Anaesthesist, ideally at a tertiary center with experience in ACHD. The anesthetist needs to be familiar with fragile pathophysiology, response to narcotics and to fluid shift/volume loss.
Early referral to transplant center
In case of progressive deterioration discussion of heart lung transplantation should be initiated early and patients be offered referral to a transplantation service experienced in congenital heart disease. Due to surgical/technical difficulties as well as the multiorgan nature of end-stage Eisenmenger syndrome the prospects of obtaining a transplantation are often limited (albeit depending on local practice and geography).
Specific disease-targeting therapies
Since the advent of targeted therapies for pulmonary arterial hypertension approximately 20 years ago and subsequent positive results in Eisenmenger patients, these patients can be offered a therapeutic option that improves symptoms, exercise capacity and potentially outcome (6,28-30). Therapy is based on various pathophysiologic pathways. These include the nitric oxide, the endothelin and the prostacyclin pathway. The majority (as well as the most robust) data exist on the effect of endothelin receptor antagonists in patients with Eisenmenger syndrome. The landmark BREATHE-5 trial was the first to convincingly demonstrate that (I) Eisenmenger patients do not suffer from ‘fixed’ pulmonary hypertension and reduction in pulmonary vascular resistance can be achieved with appropriate therapy, (II) symptoms and exercise capacity can be improved by endothelin receptor antagonists (Bosentan in this case) and (III) this therapy is safe in this population (29). This has sparked interest in various treatment approaches using also phosphodiesterase-5 inhibitors (mainly Sildenafil) in this setting.
While the data is less robust for this substance, positive effects have also been observed with Sildenafil therapy (2,31). At most centers, endothelin receptor antagonist therapy remains the first choice for symptomatic Eisenmenger patients (especially those in NYHA class III and those deteriorating over time). If unsatisfactory results are achieved with monotherapy, Sildenafil is generally added. Therapy initiation or escalation is also useful in patients with low oxygen saturation and symptoms interfering with daily activities (32). We and others have had positive experience with Selexipag (an oral prostacyclin receptor agonist) especially in patients with persistent symptoms and those deemed at high risk of complications or mortality despite dual oral therapy. In addition, patients in NYHA class IV and those with ominous clinical signs should be considered for parenteral prostacyclin therapy (33). Where available, especially subcutaneous preparation appear a reasonable choice in this setting.
Macicentan a novel oral endothelin receptor antagonist with favorable pharmacodynamic characteristics appears to be a reasonable alternative to other endothelin receptor antagonists, especially in patients not tolerating Bosentan or women on oral contraceptives (30). While a recent study was neutral on the primary endpoint (improvement of 6-minute walk distance), a significant reduction in NT-pro BNP levels in the main study cohort and pulmonary vascular resistance in the hemodynamic substudy cohort was observed with this drug (30). While not confirmed yet by prospective randomized trials, available data consistently suggest that specific disease targeted drug therapy may improve prognosis in Eisenmenger patients. This observation is based on various large observational studies and patients should be informed about these data (6,28). All Eisenmenger syndrome patients need to be referred to an ACHD expert center with access to pulmonary hypertension specialists so that this fragile population is early evaluated for specific disease targeted therapy (1).
Summary
Eisenmenger syndrome represents a relatively rare population with a balanced, but very fragile pathophysiology and severe multiorgan disorder associated with significant symptoms, morbidity and high mortality. This population is best served in a tertiary care center with expertise in adult congenital heart disease. A pro-active approach, avoiding disturbing the fragile physiological balance, complications and early initiation of specific disease target therapies is warranted in this setting. Limited data exists on risk stratification and the approach to combination therapy in this setting.
Acknowledgments
Dr. Erwin Oechslin currently holds the Bitove Family Professorship of Adult Congenital Heart Disease.
Funding: Research at Dr. Dillers Institution is supported by the EMAH Stiftung Karla Voellm.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Pediatric Pulmonary Hypertension”. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/cdt-21-135). The series “Pediatric Pulmonary Hypertension” was commissioned by the editorial office without any funding or sponsorship. Drs. Lammers served as the unpaid Guest Editor of the series. Drs. Diller and Lammers both report support from Actelion Germany/Global and Johnson and Johnson. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baumgartner H, De Backer J. The ESC Clinical Practice Guidelines for the Management of Adult Congenital Heart Disease 2020. Eur Heart J 2020;41:4153-4. [Crossref] [PubMed]
- Chaix MA, Gatzoulis MA, Diller GP, et al. Eisenmenger Syndrome: A Multisystem Disorder-Do Not Destabilize the Balanced but Fragile Physiology. Can J Cardiol 2019;35:1664-74. [Crossref] [PubMed]
- Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007;115:1039-50. [Crossref] [PubMed]
- Diller GP, Kempny A, Alonso-Gonzalez R, et al. Survival Prospects and Circumstances of Death in Contemporary Adult Congenital Heart Disease Patients Under Follow-Up at a Large Tertiary Centre. Circulation 2015;132:2118-25. [Crossref] [PubMed]
- Diller GP, Kempny A, Inuzuka R, et al. Survival prospects of treatment naïve patients with Eisenmenger: a systematic review of the literature and report of own experience. Heart 2014;100:1366-72. [Crossref] [PubMed]
- Diller GP, Körten MA, Bauer UM, et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects. Eur Heart J 2016;37:1449-55. [Crossref] [PubMed]
- Eisenmenger V. Die angeborenen Defekte der Kammerscheidewand. Z Klin Med 1897;32:1-28.
- . WOOD P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. Br Med J 1958;2:755-62. [PubMed]
- Hasan B, Hansmann G, Budts W, et al. Challenges and Special Aspects of Pulmonary Hypertension in Middle- to Low-Income Regions: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:2463-77. [Crossref] [PubMed]
- Somerville J. How to manage the Eisenmenger syndrome. Int J Cardiol 1998;63:1-8. [Crossref] [PubMed]
- Brida M, Gatzoulis MA. Pulmonary arterial hypertension in adult congenital heart disease. Heart 2018;104:1568-74. [Crossref] [PubMed]
- Diller GP, Dimopoulos K, Broberg CS, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 2006;27:1737-42. [Crossref] [PubMed]
- Kempny A, Dimopoulos K, Uebing A, et al. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life--single centre experience and review of published data. Eur Heart J 2012;33:1386-96. [Crossref] [PubMed]
- Hjortshøj CMS, Kempny A, Jensen AS, et al. Past and current cause-specific mortality in Eisenmenger syndrome. Eur Heart J 2017;38:2060-7. [Crossref] [PubMed]
- Kempny A, Hjortshøj CS, Gu H, et al. Predictors of Death in Contemporary Adult Patients With Eisenmenger Syndrome: A Multicenter Study. Circulation 2017;135:1432-40. [Crossref] [PubMed]
- Hopkins WE. The remarkable right ventricle of patients with Eisenmenger syndrome. Coron Artery Dis 2005;16:19-25. [Crossref] [PubMed]
- Hopkins WE, Ochoa LL, Richardson GW, et al. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant 1996;15:100-5. [PubMed]
- Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis 2005;16:13-8. [Crossref] [PubMed]
- Moceri P, Dimopoulos K, Liodakis E, et al. Echocardiographic predictors of outcome in eisenmenger syndrome. Circulation 2012;126:1461-8. [Crossref] [PubMed]
- Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation 1993;87:1954-9. [Crossref] [PubMed]
- Ammash N, Warnes CA. Cerebrovascular events in adult patients with cyanotic congenital heart disease. J Am Coll Cardiol 1996;28:768-72. [Crossref] [PubMed]
- Blanche C, Alonso-Gonzalez R, Uribarri A, et al. Use of intravenous iron in cyanotic patients with congenital heart disease and/or pulmonary hypertension. Int J Cardiol 2018;267:79-83. [Crossref] [PubMed]
- Tay EL, Peset A, Papaphylactou M, et al. Replacement therapy for iron deficiency improves exercise capacity and quality of life in patients with cyanotic congenital heart disease and/or the Eisenmenger syndrome. Int J Cardiol 2011;151:307-12. [Crossref] [PubMed]
- Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009;30:256-65. [Crossref] [PubMed]
- Sandoval J, Santos LE, Córdova J, et al. Does anticoagulation in Eisenmenger syndrome impact long-term survival? Congenit Heart Dis 2012;7:268-76. [Crossref] [PubMed]
- Freisinger E, Gerß J, Makowski L, et al. Current use and safety of novel oral anticoagulants in adults with congenital heart disease: results of a nationwide analysis including more than 44 000 patients. Eur Heart J 2020;41:4168-77. [Crossref] [PubMed]
- Mongeon FP, Macle L, Beauchesne LM, et al. Non-Vitamin K Antagonist Oral Anticoagulants in Adult Congenital Heart Disease. Can J Cardiol 2019;35:1686-97. [Crossref] [PubMed]
- Dimopoulos K, Inuzuka R, Goletto S, et al. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation 2010;121:20-5. [Crossref] [PubMed]
- Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48-54. [Crossref] [PubMed]
- Gatzoulis MA, Landzberg M, Beghetti M, et al. Evaluation of Macitentan in Patients With Eisenmenger Syndrome. Circulation 2019;139:51-63. [Crossref] [PubMed]
- Arvanitaki A, Giannakoulas G, Baumgartner H, et al. Eisenmenger syndrome: diagnosis, prognosis and clinical management. Heart 2020;106:1638-45. [Crossref] [PubMed]
- Grünig E, Ohnesorge J, Benjamin N, et al. Plasma Drug Concentrations in Patients with Pulmonary Arterial Hypertension on Combination Treatment. Respiration 2017;94:26-37. [Crossref] [PubMed]
- Skoro-Sajer N, Gerges C, Balint OH, et al. Subcutaneous treprostinil in congenital heart disease-related pulmonary arterial hypertension. Heart 2018;104:1195-9. [Crossref] [PubMed]


