
Medical treatment of pulmonary hypertension in adults with congenital heart disease: updated and extended results from the International COMPERA-CHD Registry
Introduction
All patients with congenital heart disease (CHD) are considered as chronic heart disease patients, regardless of whether they have native disease or have been interventionally/surgically treated (1,2). Owing to medical advances in recent decades, in the industrialized world, up to 97% of patients with CHD now survive to adulthood (3). However, it is common for residua, sequelae, and cardiac or non-cardiac comorbidities to develop that adversely impact the course of the disease and increase morbidity and mortality (4). Pulmonary vascular disease (PVD) and pulmonary hypertension (PH) are the most important long-term complications, besides defect-specific residua, heart failure, and cardiac arrhythmias. The most severe form of PVD in CHD is Eisenmenger syndrome, which develops as a complication in a primary left-to-right shunt lesion (5).
For a long time, severe forms of PH were considered largely unresponsive to medical therapy, and lung transplantation combined with intracardiac repair or heart-lung transplantation were the final choice. However, the development of pulmonary vasoactive drugs has expanded the therapeutic armamentarium and favorably influenced the quality of life and survival of many affected patients (6). Despite the fact that several drugs are at present approved for the treatment of pulmonary arterial hypertension (PAH), there are only limited data on their use in CHD (7).
The aim of the current study was to evaluate and update data on targeted pharmacological management of patients with CHD and PVD from the COMPERA registry, which enrolls patients with all forms of PH (8).
Methods
This study was an extended and updated analysis of data from the international COMPERA registry (study identifier: Clinicaltrials.gov NCT01347216) on PH (8). At the time of the present analysis (September 01, 2020), 62 centers from 12 European countries (Austria, Belgium, Germany, Greece, Hungary, Italy, Latvia, Lithuania, the Netherlands, Slovakia, Switzerland, and the United Kingdom) participated in the sub-registry on CHD (COMPERA-CHD). Inclusion criteria for the present analysis were the diagnosis of PH in CHD, a minimum age of 18 years, any targeted PAH medication, including endothelin receptor antagonists (ERA), phosphodiesterase-5 inhibitors (PDE5i), the soluble guanylate cyclase (sGC) stimulator riociguat, or prostacyclin analogues (including selexipag as an oral prostacyclin agonist), and written informed consent. Exclusion criteria were lack of cognitive competence to consent to participate in the research project or refusal to consent.
The PAH associated with CHD (PAH-CHD) group comprised patients on targeted PAH medication for PVD with Eisenmenger’s syndrome, non-Eisenmenger patients with severe PAH based on a CHD (“Non-EM-PAH”), and patients who had undergone a modified Fontan operation. The “Non-EM-PAH” group included adults with CHD after previous reparative surgery or interventions for an intra- or extracardiac systemic-to-pulmonary shunt, as well as CHD associated with prevalent systemic-to-pulmonary shunts, or PAH with small/coincidental defects. In addition, patients with Eisenmenger syndrome without targeted PAH medication were also included.
Data collection and documentation
Patients were enrolled consecutively, and internet-based documentation included cardiac diagnoses, patient demographics, type of PAH according to Dana Point/Nice classification, surgical or interventional treatments, clinical status (including functional class, 6-min walk test, selected laboratory variables, and quality of life), cardiac and non-cardiac comorbidities, and detailed information on PAH medication and supportive medication. Data at entry were supplemented by data from routine readmissions or in the case of a predetermined clinical event (worsening functional class, PAH-related hospitalization, unanticipated changes in PAH therapy, transplantation, death, or other serious adverse events). Plausibility and completeness checks, queries, and/or on-site monitoring were undertaken to secure high data quality.
The registry has been approved by the institutional review boards of all contributing centers and written informed consent was obtained from all participating patients before the start of documentation (lead ethics committee at Technical University Dresden, Germany: approval EK 129052007 as of 22 May 2007). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Guidelines on good pharmacoepidemiological practice (GPP) and data protection guidelines were followed.
Statistical analysis
The study population was descriptively presented as percentage, median with interquartile range (IQR), or mean ± standard deviation. Categorical variables were compared using χ2-test or Fisher’s exact test. Group differences, after testing for normal distribution, were assessed by t-test. Otherwise, the 2-sided Mann-Whitney test was used. A P value of <0.05 was considered significant. Adjustment for multiple testing was omitted. Data analysis was performed using SPSS 24.0 (IBM Inc., Armonk, NY, USA).
The author(s) of this manuscript have certified that they comply with the Principles of Ethical Publishing in the American Journal of Cardiology (9).
Results
As of September 1, 2020, a total of 10,265 patients were enrolled in the COMPERA registry at 62 centers; 10,037 cases were adults and 228 were children (Table 1). Within the entire group, 769 patients had CHD (7.7%).
Table 1
| Parameter | December 1, 2018 | September 1, 2020 | Difference | % difference |
|---|---|---|---|---|
| Countries participating (n) | 11 | 12 | – | – |
| Centers participating (n) | 49 | 62 | 13 | +26.5 |
| Total PAH patients included (n) | 8,200 | 10,037 | 1,837 | +22.4 |
| Total PAH-CHD-patients included (n) | 680 | 769 | 91 | +13.4 |
| Eisenmenger syndrome (n) | 240 | 279 | 39 | +16.3 |
| Non-EM-PAH (n) | 167 | 212 | 45 | +26.9 |
| Fontan (n) | 7 | 9 | 2 | +28.6 |
| PAH-CHD—not assigned (n) | 186 | 190 | 4 | +2.2 |
| Eisenmenger Syndrome without PAH medication (n) | 80 | 79 | 1 | −1.3 |
| Sex, female (n) | 453 (66.6%) | 512 (66.6%) | 56 | 0 |
| Age (years; median ± SD) | 45.5±16.8 | 45.3±16.8 | 0.2 | – |
| Functional class (FC) | ||||
| FC I/II [n] | 26.7% [181] | 28.2% [217] | 1.5 | 5.6 |
| FC III [n] | 57.6% [392] | 56.0% [431] | 1.6 | −2.8 |
| FC IV [n] | 4.0% [27] | 3.8% [29] | 0.2 | −5.0 |
| FC unknown [n] | 11.8% [80] | 12.0% [92] | 0.2 | 1.7 |
| 6-minute-walk distance, meters [n] | 367±120 [454] | 369±121 [508] | 2 | 0.5 |
| Serum NT-pro-BNP, median (pg/mL) [n] | 543 [357] | 532 [416] | 11 | −2.0 |
EM, Eisenmenger syndrome; FC, functional class according to Perloff; Non-EM-PAH, patients with congenital heart disease-associated pulmonary arterial hypertension but without Eisenmenger syndrome; PAH, pulmonary arterial hypertension; PAH-CHD, congenital heart disease-associated pulmonary arterial hypertension; n, number.
A subgrouping was possible in 579 of these patients: 279 were on targeted PAH therapy because of Eisenmenger syndrome, 212 because of CHD and severe PVD which, however, did not meet the criteria for Eisenmenger syndrome (“Non-Eisenmenger-PAH”), and 9 because of PVD after Fontan surgery. In addition, 79 patients with Eisenmenger syndrome but without targeted PAH therapy were included (Table 1).
Thus, compared with the initial description of the COMPERA-CHD collective (as of December 1, 2018) (8), over a 21-month period in the PAH-CHD-group, the number of Eisenmenger patients rose by 39 (16.3%), and the number of “Non-Eisenmenger-PAH” patients increased by 45 (26.9%). The number of Fontan patients and untreated Eisenmenger patients changed only marginally (Table 1). At the same time, the number of included PAH patients in COMPERA increased by 1,837 (22.4%).
Type of CHD
According to the frequency of occurrence, CHD could be assigned to five main groups: post-tricuspid shunts; pre-tricuspid shunts; complex anomalies; congenital anomalies of the left heart, aortic valve, and aorta; and CHD that could not be further categorized (Table 2).
Table 2
| Congenital heart defect | Number (%) of patients in 12/2018 | Number (%) of patients in 09/2020 |
|---|---|---|
| Post-tricuspid shunts | 325 (47.8) | 359 (46.7) |
| Ventricular septal defect | 199 (29.3) | 220 (28.6) |
| Atrioventricular septal defect | 79 (11.6) | 86 (11.2) |
| Patent Ductus arteriosus | 40 (5.9) | 45 (5.9) |
| Aorto-pulmonary window | 6 (0.9) | 6 (0.8) |
| Details not stated | 1 (0.1) | 2 (0.3) |
| Pre-tricuspid shunts | 213 (31.3) | 249 (32.4) |
| Atrial septal defect | 186 (27.4) | 215 (28.0) |
| Partial anomalous pulmonary venous return | 16 (2.4) | 20 (2.6) |
| Patent foramen ovale | 5 (0.7) | 7 (0.9) |
| Partial AV-septal defect | 4 (0.6) | 4 (0.5) |
| Total anomalous pulmonary venous return | 1 (0.1) | 2 (0.3) |
| Details not stated | 1 (0.1) | 1 (0.1) |
| Complex anomalies | 121 (17.8) | 132 (17.2) |
| Pulmonary atresia with VSD | 30 (4.4) | 34 (4.4) |
| Complete transposition | 19 (2.8) | 19 (2.5) |
| Double-inlet-ventricle | 13 (1.9) | 13 (1.7) |
| Fallot tetralogy | 13 (1.9) | 14 (1.8) |
| Congenitally corrected transposition | 12 (1.8) | 13 (1.7) |
| Tricuspid atresia | 12 (1.8) | 13 (1.7) |
| Double-outlet right ventricle—Fallot-type | 9 (1.3) | 9 (1.2) |
| Double-outlet right ventricle—TGA-type | 5 (0.7) | 7 (09) |
| Truncus arteriosus communis | 4 (0.6) | 5 (0.7) |
| Ebstein’s anomaly | 2 (0.3) | 3 (0.4) |
| Pulmonary atresia with intact ventricular septum | 1 (0.1) | 1 (0.1) |
| Details not stated | 1 (0.1) | 1 (0.1) |
| Left heart disease/aortic valve, and aortic anomalies | 9 (1.3) | 9 (1.2) |
| Aortic valve stenosis, congenital | 5 (0.7) | 5 (0.7) |
| Aortic coarctation | 2 (0.3) | 2 (0.3) |
| Subaortic stenosis | 1 (0.1) | 1 (0.1) |
| Aortic valve regurgitation, congenital | 1 (0.1) | 1 (0.1) |
| Other congenital heart anomalies | 12 (1.8) | 20 (2.6) |
| Pulmonary artery stenosis/RVOTO | 3 (0.4) | 6 (0.8) |
| AV valve anomalies, congenital | 2 (0.3) | 3 (0.4) |
| Details not stated | 7 (1.0) | 11 (1.4) |
| Total | 680 (100.0) | 769 (100.0) |
AV, aortic valve; VSD, ventricular septal defect; TGA, transposition of the great arteries; RVOTO, right ventricular outflow tract disruption.
At last follow-up, patients had either post-tricuspid shunts (n=359; 46.7%), pre-tricuspid shunts (n=249; 32.4%), complex CHD (n=132; 17.2%), congenital anomaly of the left heart, aortic valve, or aorta (n=9; 1.3%), or miscellaneous other types of CHD (n=20; 2.6%) (Table 2). Overall, the most prevalent CHDs were ventricular septal defects, atrial septal defects, atrioventricular septal defects, patent ductus arteriosus, and pulmonary atresia with ventricular septal defect. Compared with the 2018 COMPERA data, there were no significant differences in the type of CHD included (8).
Patient demographics and baseline characteristics
In the current analysis, 512 patients (66.6%) were female and 257 patients (33.4%) were male. A similar sex ratio was found in the subgroup analyses (Table 1). The mean age of all PAH-CHD patients was 45.3±16.8 years. The 9 patients with Fontan circulation were considerably younger (36.0±11.6 years) (Table 1). More than half of the patients were in their third, fourth, or fifth decade of life (n=429, 55.8%). Of the remaining patients, 169 (22.0%) were younger than 30 years and 171 (22.2%) were in the 6th decade of life. In comparison to the previous COMPERA analysis (8), there were no significant differences regarding gender or age distribution. Key demographic and clinical baseline data in comparison with the 2018 data are provided in Tables 1-3.
Table 3
| Baseline data | PAH-CHD total | EM without targeted PAH medication | EM with targeted PAH medication | Non-EM-PAH with targeted PAH medication | Fontan with targeted PAH medication | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||||
| Total | 769 | 79 | 279 | 212 | 9 | |||||||||
| Age | 45.3±16.8 | 40.2±15.2 | 43.2±15.0 | 48.4±17.7 | 36.0±11.6 | |||||||||
| Sex | ||||||||||||||
| Male | 257 | 33.4 | 25 | 31.6 | 92 | 33.0 | 70 | 33.0 | 5 | 55.6 | ||||
| Female | 512 | 66.6 | 54 | 68.4 | 187 | 67.0 | 142 | 67.0 | 4 | 44.4 | ||||
| FC | ||||||||||||||
| Unknown | 92 | 12.0 | 30 | 38.9 | 24 | 8.6 | 20 | 9.4 | 4 | 44.4 | ||||
| I | 26 | 3.4 | 1 | 1.3 | 9 | 3.2 | 8 | 3.8 | 0 | 0 | ||||
| II | 191 | 24.8 | 11 | 13.9 | 76 | 27.2 | 62 | 29.2 | 5 | 55.6 | ||||
| III | 431 | 56.0 | 34 | 43.0 | 158 | 56.6 | 118 | 55.7 | 0 | 0 | ||||
| IV | 29 | 3.8 | 3 | 3.8 | 12 | 4.3 | 4 | 1.9 | 0 | 0 | ||||
| 6-minute walk distance (meters) | 369±121 (n=508) | 386±120 (n=28) | 354±123 (n=192) | 390±120 (n=145) | 464±40 (n=3) | |||||||||
EM, Eisenmenger syndrome; FC, Functional class according to Perloff; Non-EM-PAH, patients with congenital heart disease-associated pulmonary arterial hypertension but without Eisenmenger syndrome; PAH, pulmonary arterial hypertension; PAH-CHD, Congenital heart disease-associated pulmonary arterial hypertension; PAH medication, targeted medication to treat PAH.
Additive clinical data
A total of 431 patients (56.0%) were in functional class III; the exercise capacity, measured as 6-minute walking distance (6MWD), averaged 369±121 meters in the actual analysis (Table 1). In comparison with the COMPERA data from 2018, there was no significant difference.
Furthermore, analysis of the 2020 data revealed the importance of cardiac and non-cardiac comorbidities found in 289 of 656 patients (44.1%); in particular, coronary artery disease, arterial hypertension, thromboembolism, sleep apnea syndrome, diabetes mellitus, or thyreopathies. Atrial fibrillation or flutter was described in 86 patients (11.2%), and syncope during the course of the disease was reported in 42 of 388 patients (10.8%). A pacemaker was needed by 25 of 418 patients (6.0%). Trisomy-21 was present in 100 of 448 patients (Figure 1).

PAH therapy
All currently available drugs for targeted PAH therapy were used in the patients with CHD included in COMPERA. Only in individual cases were calcium channel blockers part of the medication.
Among the 674 PAH-CHD patients with at least one follow-up, ERA were used in 450 (66.8%), a PDE5i in 416 (61.7%), prostanoids in 85 (12.6%), and a sGC stimulator in 36 (5.3%). In most diagnostic groups, ERA were used more frequently than PDE5i, prostanoids, or riociguat. Only in the very small Fontan cohort (n=8 with follow-up) was a PDE5i the preferred drug class (prescribed in seven out of eight patients; Figure 2). Within drug classes, bosentan (n=210) was more frequently used than ambrisentan (n=73), or macitentan (n=163) as the ERA, while sildenafil (n=280) was used more frequently than tadalafil (n=136) as the PDE5i (n=416).
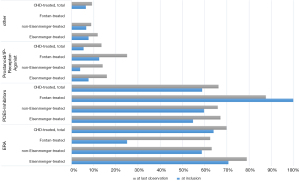
At the time of inclusion in the COMPERA-CHD registry, treatment was predominantly monotherapy (69.3%) in the overall population. Monotherapy was also preferred in the subgroups with Eisenmenger syndrome (65%), those with non-Eisenmenger syndrome with PAH (73.7%), and in Fontan patients (75%). The largest percentage of patients on combination therapy was seen in the Eisenmenger group (35.0%).
In the 674 patients who had at least one follow-up visit after enrollment in COMPERA-CHD, therapy shifted in favor of combination therapy (53%). The percentage of patients on combination therapy increased to 62.1% in the subgroup with Eisenmenger syndrome, to 46.9% in the group with “Non-Eisenmenger-PAH”; and also in the small Fontan group.
Even compared with the 2018 treatment data, there was another three percent increase in patients taking combination therapy.
Additive and supportive medical treatment
In addition to targeted PAH medication, additive or supportive medical treatment was deemed necessary in the majority of patients. Specifically, the following drugs were administered: diuretics (n=279 of 586; 47.6%), beta blockers (n=164 of 578; 28.4%), ACE inhibitors or angiotensin receptor blockers (ARBs) (n=100 of 570; 17.5%), digitalis (n=84 of 573; 14.7%), or class III antiarrhythmics (n=32 of 568; 5.6%).
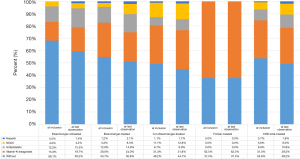
The use of anticoagulants varied considerably with regard to the type of agent used in the different disease groups. Antiplatelet agents, vitamin K antagonists, non-vitamin K antagonists (NOAC), and heparins were used. Of the 674 patients who had at least one follow-up visit, at the time of enrollment in the COMPERA registry, a large proportion of patients were without anticoagulation: 49 in the therapy-naive Eisenmenger group (68.1%), 133 in the treated Eisenmenger group (54.7%), and 88 in the “Non-Eisenmenger-PAH” group (49.2%). In contrast, five out of eight patients with Fontan circulation were on oral anticoagulation with vitamin K antagonists. During the follow-up period, only minor changes in the percentage distribution of anticoagulant medication were observed (Figure 3).
Discussion
PAH develops in 5–10% of patients with CHD, and accounts for 34–42% of all PAH cases (10-12). Despite improved therapeutic measures and a decreasing incidence in industrialized countries, PAH-CHD will remain a problem, especially in lower-income countries and in the future, the number of patients with complex CHD and pulmonary vascular disease, including those with Fontan circulation, will significantly increase (13).
In contrast to other forms of PAH, only limited data exist on targeted, diseaseoriented therapy for PAH-CHD (6,7,14).
Because most data on PAH therapy in CHD are derived from small, uncontrolled studies that typically include only a few patients and short observation periods, it is important to utilize data from registries that include patients with PAH-CHD; and to update these data regularly (15-19).
Nearly all other PH registries in Europe and the United States that also include CHD comprise all types of group 1 patients (PAH) but differentiate less precisely between clinical subtypes, treatment characteristics, and outcomes of distinct patient groups (20-30).
As one of the world’s largest PH registries, the international COMPERA registry provides comprehensive, clinically relevant information on targeted PAH treatment, including patients with PAH-CHD. Meanwhile, the COMPERA-CHD subregistry represents the largest registry of PAH in CHD (n=769), allowing sophisticated epidemiological and clinical analyses because of its sample size and detailed documentation (8).
The increase in the numbers of PAH-CHD patients is relatively less than the number of patients with other forms of PAH, whose numbers have increased by 22% over the same period. Within the past two years, the number of patients with PAH-CHD in COMPERA has increased by 13.4% (Table 1). Within this group the number of Eisenmenger patients rose by 39 (16.3%). Of particular note is the fact that in the PAH-CHD group, the proportion of patients with “Non-Eisenmenger PAH” has risen substantially (n=45; 26.9%). In parallel, the number of participating centers increased by 13 (26.5%). This may reflect a needed correction to a lack of awareness of the importance of systematic follow-up in patients with CHD, which has also been documented in contemporary studies (2,14). In this respect, particular attention must be paid to the “Non-Eisenmenger PAH” group in the years and decades to come.
Congenital heart anomalies and demographic data
Unlike other registries, COMPERA-CHD includes a high proportion of patients with complex CHD (n=121; 17.8%). These patients are often excluded from clinical trials. In addition, pre-tricuspid and post-tricuspid septal defects were enrolled; these typically account for the majority of CHD. Another unique feature of COMPERA-CHD is that patients with Eisenmenger’s syndrome who were not on targeted PAH therapy, as well as patients with Fontan circulation who were on targeted PAH therapy for PVD, were also included.
The age of patients included in COMPERA-CHD was 45±17 years. Notably, this is 5 to 13 years older than the patient ages in other international registries on PAH-CHD but younger than the patient ages in large registries on other forms of PAH (15-20). The sex ratio, with a preponderance of females, is similar to that of all other registries, with the exception of the German National Registry for CHD, in which more males than females with PAH-CHD were reported (Table 1) (21).
At the time of inclusion in the COMPERA-CHD registry, the majority of PAH-CHD patients were in functional class III, correlating with the limited 6MWD of 369±121 m, which is similar to limitations reported in other registries. There were no relevant differences in the data from 2020 compared with the previous registry data reported in 2018 for the following parameters: CHD diagnosis, age, sex, functional class, or 6MWD (Tables 1,2).
However, particularly striking is the high proportion of patients with cardiac and non-cardiac comorbidities, an entity that has been largely disregarded in previous studies and analyses. The analysis of the 2020 data revealed for the first time in the COMPERA-CHD-registry comorbidities of clinical relevance in 289 of 656 patients (44.1%), in particular coronary artery disease, arterial hypertension, thromboembolism, sleep apnea syndrome, diabetes mellitus, and thyreopathies. Some of these comorbidities certainly correspond to the usual distribution in the normal population. For thyreopathies, a correlation with the presence of trisomy 21 (n=100 of 448), the use of amiodarone (n=28 of 568), and the fact that Germany is an iodine-deficient area are potentially relevant factors (22).
Targeted medical PAH treatment
In COMPERA-CHD, all available targeted PAH drugs have been used, including ERAs, PDE5i, prostanoids, prostacyclin receptor agonists, and sGCs (Figure 2).
At the time of the last follow-up (data base freeze 09/2020), in COMPERA-CHD ERAs (n=454) and PDE5i (n=416) were preferentially administered in all groups. Prostanoids or an sGC stimulator were used in only a minority of patients; 14 (2.3%). Some preparations (e.g., the ERA sitaxentan) were withdrawn during the observation period and replaced by others. Calcium channel blockers are currently used only exceptionally (n=26) in responders after hemodynamic vasoreactivity testing.
At the time of inclusion in COMPERA-CHD, the majority of all CHD patients received monotherapy (69%), and distinctly less frequently combination therapy (31%). This is partly attributable to the fact that many patients were enrolled in the registry more than 10 years ago. At least at that time, there was a tendency to initiate combination therapy only in cases of symptomatic deterioration or when predefined and center-specific treatment goals were not achieved. This is in contrast to the current tendency to favor early combination therapy of two or even more PAH-active drugs, at least for some forms of PAH.
The reluctance to using combination therapies in PAH-CHD may originate from the fact that, particularly in Eisenmenger syndrome, evidence-based and consensus-driven treatment algorithms are not available, and there is no robust evidence to support the use of PAH combination therapy (23). However, in recent years, some study data and reports have suggested that adults with CHD may also benefit from targeted combination therapy (7,24-28). Accordingly, a more aggressive approach is also reflected in the COMPERA-CHD data, as the percentage of initially monotherapy (41%) versus combination therapy (53%) shifted during the observation period in favor of combination therapy.
Additive and supportive medical treatment
It is noteworthy that a large proportion of the included patients received supplementary drug treatment in addition to targeted PAH medication; preferably diuretics, beta-blockers, ACE inhibitors or AT blockers, digitalis, or class III antiarrhythmics. The application of these drugs should not be underestimated, as their use can be critical, especially in cyanotic patients, including Eisenmenger’s syndrome, and side effects can be expected. In particular, lowering of peripheral vascular resistance with consecutive deepening of cyanosis may occur with ACE inhibitors or AT blockers; hemoconcentration and enhancement of hyperuricemia may occur with thiazide diuretics; and higher-degree heart block may occur with beta blockers or digitalis (29,30).
As anticoagulants, vitamin K antagonists or direct-acting, non-vitamin K antagonists (NOAC) and platelet inhibitors were used (Figure 3). The indication for anticoagulants in PAH is still under debate, in particular in Eisenmenger syndrome, where there is, on the one hand, an increased risk of bleeding, and, on the other hand, an increased risk of thrombosis, from abnormalities in platelet function, platelet count, and other coagulation parameters (29,31-35). Because data are insufficient to determine whether oral anticoagulation has a positive effect on morbidity and mortality in Eisenmenger syndrome, current ACHD guidelines do not recommend the routine use of oral anticoagulants and explicitly address potential adverse effects (7,36,37).
The COMPERA-CHD data also reflect the fact that in Eisenmenger syndrome, oral anticoagulants are currently restricted to specific indications in patients without significant hemoptysis: the vast majority of PAH-CHD patients (53.6%) did not receive anticoagulants. However, while at the time of initial data entry in COMPERA-CHD, patients with Eisenmenger syndrome were mostly not anticoagulated, the number of anticoagulated patients has increased since. This may be explained by complications in the long-term course (e.g., atrial arrhythmias, thromboembolism, mechanical valve replacement) resulting in the need to use these drugs. Surprisingly, in both the Eisenmenger groups, with and without targeted PAH therapy, up to 15% of patients were treated with antiplatelets, despite the fact that almost all of them have a genuine disorder of platelet number and function (38).
In contrast, the small group of Fontan patients was predominantly treated with anticoagulants because of the high rate of thromboembolic events (up to 33%) expected due to the particular hemodynamic and coexistent coagulopathy and hypercoagulation states commonly seen after post-Fontan operation (37,39-42).
Strengths and limitations
Strengths of the presented registry study include the relatively large sample size of prospectively registered patients with PAH-CHD, the “real-world” conditions, and the long observation period.
Limitations include the voluntary nature of a registry, although COMPERA-CHD did enroll patients prospectively and consecutively, and implemented various control measures to ensure high data quality. Statistical measures to reduce confounding factors, such as propensity score matching or multivariable risk-adjusted modeling, were not performed. Therefore, group differences at baseline must be considered when evaluating all results and conclusions.
Finally, it should be emphasized that the registry study was performed at tertiary care centers for ACHD or for PAH. Thus, the distribution of patients in terms of type and severity of CHD does not resemble the typical patient population seen outside of these centers. Generalization of the conclusions and transfer of the findings to patients of different countries or cultures is potentially problematic. Further studies are needed in this regard.
Conclusions
Based on real-world data from the international COMPERA-CHD registry, our updated analysis provides a comprehensive overview about current management modalities and treatment concepts for PAH-CHD patients. While individual therapy was dependent on the underlying CHD, there was an overall trend towards more aggressive treatment strategies and combination therapies.
Hence, our data may help to uncover even closer associations between the type and severity of the particular CHD, treatment status, and outcome, and may also serve as a basis for further research. In this context, detailed long-term survival analyses should clarify the mortality in each patient group related to the treatment regimen. Potentially, registry data may continue to advance the management of PAH-CHD patients and aid in the development of screening and treatment protocols, and thereby further improve the management of affected individuals.
Acknowledgments
The authors are indebted to the COMPERA investigators and their staff. We explicitly thank Dr. Claudia S. Copeland for the professional editing of the final draft of the manuscript.
Funding: COMPERA is funded by unrestricted grants from Acceleron, Actelion Pharmaceuticals (Janssen), Bayer, OMT and GSK. These companies were not involved in data analysis or the writing of this manuscript.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yskert von Kodolitsch, Harald Kaemmerer, Koichiro Niwa) for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/cdt-21-351). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” was commissioned by the editorial office without any funding or sponsorship. Dr. DH reports non-financial support from Actelion, Boehringer-Ingelheim, and Shire, outside the submitted work; Dr. DP reports personal fees from Actelion, Biogen, Aspen, Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi, outside the submitted work; Dr. MD reports personal fees from Actelion, Bayer, GSK and MSD, outside the submitted work; Dr. HAG reports personal fees from Actelion, Bayer, Gilead, GSK, MSD, Pfizer and United Therapeutics, outside the submitted work; Dr. MG reports personal fees from Actelion, Bayer and GSK, outside the submitted work; Dr. MMH reports personal fees from Acceleron, Actelion, Bayer, MSD and Pfizer, outside the submitted work; Dr. CDV reports personal fees from Actelion, Bayer, GSK, MSD, Pfizer, and United Therapeutics, outside the submitted work; Dr. RE reports personal fees from Actelion, Boehringer Ingelheim, OMT, Bayer, and Berlin Chemie; grants from Actelion and Boehringer Ingelheim, outside the submitted work; Dr. MH reports grants and personal fees from Actelion, personal fees from Bayer, Berlin Chemie, Boehringer Ingelheim, GSK, Janssen, Novartis and MSD, outside the submitted work; Dr. MH reports personal fees from Acceleron, Actelion, AstraZeneca, Bayer, BERLIN CHEMIE, GSK, MSD, Novartis and OMT, outside the submitted work; Dr. HW reports personal fees from Action, Bayer, Biotest, Boehringer, GSK, Pfizer, and Roche, outside the submitted work; Dr. DS reports personal fees from Actelion, Bayer, and GSK, outside the submitted work; Dr. LS reports personal fees from Actelion, Bayer, and MSD, outside the submitted work; Dr. SU reports grants from Swiss National Science Foundation, Zurich Lung, Swiss Lung, and Orpha Swiss, grants and personal fees from Actelion SA/Johnson & Johnson, Switzerland, and MSD Switzerland, outside the submitted work; Dr. TJL reports personal fees from Actelion, Janssen-Cilag, BMS, MSD, and OMT GmbH, outside the submitted work; Dr. LB reports personal fees from Actelion, outside the submitted work; Dr. MC reports personal fees from Boehringer Ingelheim Pharma GmbH, Roche Pharma, and Boehringer Ingelheim, outside the submitted work; Dr. HW reports personal fees from Boehringer Ingelheim, and Roche, outside the submitted work. Dr. EG reports personal fees from Actelion, Janssen, Bayer, MSD, Bial, OrPha Swiss GmbH, OMT and Medscape, outside the submitted work; Dr. SR reports personal fees from Actelion, Bayer, GSK, Pfizer, Novartis, Gilead, MSD, and United Therapeutics, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The registry has been approved by the institutional review boards of all contributing centers and written informed consent was obtained from all participating patients before the start of documentation (lead ethics committee at Technical University Dresden, Germany: approval EK 129052007 as of 22 May 2007). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neidenbach R, Niwa K, Oto O, et al. Improving medical care and prevention in adults with congenital heart disease-reflections on a global problem-part I: development of congenital cardiology, epidemiology, clinical aspects, heart failure, cardiac arrhythmia. Cardiovasc Diagn Ther 2018;8:705-15. [Crossref] [PubMed]
- Neidenbach R, Achenbach S, Andonian C, et al. Systematic assessment of health care perception in adults with congenital heart disease in Germany. Cardiovasc Diagn Ther 2021;11:481-91. [Crossref] [PubMed]
- Mandalenakis Z, Giang KW, Eriksson P, et al. Survival in Children With Congenital Heart Disease: Have We Reached a Peak at 97%? J Am Heart Assoc 2020;9:e017704. [Crossref] [PubMed]
- Perloff JK, Warnes CA. Challenges posed by adults with repaired congenital heart disease. Circulation 2001;103:2637-43. [Crossref] [PubMed]
- Kaemmerer H, Mebus S, Schulze-Neick I, et al. The adult patient with eisenmenger syndrome: a medical update after dana point part I: epidemiology, clinical aspects and diagnostic options. Curr Cardiol Rev 2010;6:343-55. [Crossref] [PubMed]
- Hoeper MM, Apitz C, Grünig E, et al. Targeted therapy of pulmonary arterial hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018;272S:37-45. [Crossref] [PubMed]
- Kaemmerer H, Apitz C, Brockmeier K, et al. Pulmonary hypertension in adults with congenital heart disease: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018;272S:79-88. [Crossref] [PubMed]
- Kaemmerer H, Gorenflo M, Huscher D, et al. Pulmonary Hypertension in Adults with Congenital Heart Disease: Real-World Data from the International COMPERA-CHD Registry. J Clin Med 2020;9:1456. [Crossref] [PubMed]
- Coats AJ, Shewan LG. Statement on authorship and publishing ethics in the International Journal of Cardiology. Int J Cardiol 2011;153:239-40. [Crossref] [PubMed]
- van Riel AC, Schuuring MJ, van Hessen ID, et al. Contemporary prevalence of pulmonary arterial hypertension in adult congenital heart disease following the updated clinical classification. Int J Cardiol 2014;174:299-305. [Crossref] [PubMed]
- van Riel AC, Blok IM, Zwinderman AH, et al. Lifetime Risk of Pulmonary Hypertension for All Patients After Shunt Closure. J Am Coll Cardiol 2015;66:1084-6. [Crossref] [PubMed]
- van der Velde ET, Vriend JW, Mannens MM, et al. CONCOR, an initiative towards a national registry and DNA-bank of patients with congenital heart disease in the Netherlands: rationale, design, and first results. Eur J Epidemiol 2005;20:549-57. [Crossref] [PubMed]
- Kempny A, Dimopoulos K, Gatzoulis MA. Declining incidence and prevalence of Eisenmenger syndrome in the developed world: a triumph of modern medicine. Heart 2017;103:1313-4. [Crossref] [PubMed]
- Seidel L, Nebel K, Achenbach S, et al. Facts about the General Medical Care of Adults with Congenital Heart Defects: Experience of a Tertiary Care Center. J Clin Med 2020;9:1943. [Crossref] [PubMed]
- Ghofrani HA, Distler O, Gerhardt F, et al. Treatment of pulmonary arterial hypertension (PAH): updated Recommendations of the Cologne Consensus Conference 2011. Int J Cardiol 2011;154:S20-33. [Crossref] [PubMed]
- Favilli S, Spaziani G, Ballo P, et al. Advanced therapies in patients with congenital heart disease-related pulmonary arterial hypertension: results from a long-term, single center, real-world follow-up. Intern Emerg Med 2015;10:445-50. [Crossref] [PubMed]
- Alonso-Gonzalez R, Lopez-Guarch CJ, Subirana-Domenech MT, et al. Pulmonary hypertension and congenital heart disease: An insight from the REHAP National Registry. Int J Cardiol 2015;184:717-23. [Crossref] [PubMed]
- Jansa P, Jarkovsky J, Al-Hiti H, et al. Epidemiology and long-term survival of pulmonary arterial hypertension in the Czech Republic: a retrospective analysis of a nationwide registry. BMC Pulm Med 2014;14:45. [Crossref] [PubMed]
- Van de Bruaene A, Delcroix M, Pasquet A, et al. The Belgian Eisenmenger syndrome registry: implications for treatment strategies? Acta Cardiol 2009;64:447-53. [Crossref] [PubMed]
- Idrees M, Al-Najashi K, Khan A, et al. Pulmonary arterial hypertension in Saudi Arabia: Patients' clinical and physiological characteristics and hemodynamic parameters. A single center experience. Ann Thorac Med 2014;9:209-15. [Crossref] [PubMed]
- Diller GP, Körten MA, Bauer UM, et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects. Eur Heart J 2016;37:1449-55. [Crossref] [PubMed]
- Rosenkranz S, Howard LS, Gomberg-Maitland M, et al. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation 2020;141:678-93. [Crossref] [PubMed]
- Nashat H, Kempny A, Harries C, et al. A single-centre, placebo-controlled, double-blind randomised cross-over study of nebulised iloprost in patients with Eisenmenger syndrome: A pilot study. Int J Cardiol 2020;299:131-5. [Crossref] [PubMed]
- D'Alto M, Constantine A, Balint OH, et al. The effects of parenteral prostacyclin therapy as add-on treatment to oral compounds in Eisenmenger syndrome. Eur Respir J 2019;54:1901401. [Crossref] [PubMed]
- Durongpisitkul K, Chungsomprasong P, Vijarnsorn C, et al. Improved low-risk criteria scores for combination therapy of sildenafil and generic bosentan in patients with congenital heart disease with severe pulmonary hypertension: A prospective open label study. JRSM Cardiovasc Dis 2021;10:2048004020982213. [Crossref] [PubMed]
- D'Alto M, Romeo E, Argiento P, et al. Bosentan-sildenafil association in patients with congenital heart disease-related pulmonary arterial hypertension and Eisenmenger physiology. Int J Cardiol 2012;155:378-82. [Crossref] [PubMed]
- Iversen K, Jensen AS, Jensen TV, et al. Combination therapy with bosentan and sildenafil in Eisenmenger syndrome: a randomized, placebo-controlled, double-blinded trial. Eur Heart J 2010;31:1124-31. [Crossref] [PubMed]
- Manes A, Palazzini M, Leci E, et al. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: a comparison between clinical subgroups. Eur Heart J 2014;35:716-24. [Crossref] [PubMed]
- Grünig E, Benjamin N, Krüger U, et al. General measures and supportive therapy for pulmonary arterial hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018;272S:30-6. [Crossref] [PubMed]
- Oechslin E, Mebus S, Schulze-Neick I, et al. The Adult Patient with Eisenmenger Syndrome: A Medical Update after Dana Point Part III: Specific Management and Surgical Aspects. Curr Cardiol Rev 2010;6:363-72. [Crossref] [PubMed]
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493-537. [Crossref] [PubMed]
- Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014;129:57-65. [Crossref] [PubMed]
- Kempny A, Hjortshøj CS, Gu H, et al. Predictors of Death in Contemporary Adult Patients With Eisenmenger Syndrome: A Multicenter Study. Circulation 2017;135:1432-40. [Crossref] [PubMed]
- Hjortshøj CMS, Kempny A, Jensen AS, et al. Past and current cause-specific mortality in Eisenmenger syndrome. Eur Heart J 2017;38:2060-7. [Crossref] [PubMed]
- Lill MC, Perloff JK, Child JS. Pathogenesis of thrombocytopenia in cyanotic congenital heart disease. Am J Cardiol 2006;98:254-8. [Crossref] [PubMed]
- Baumgartner H, De Backer J. The ESC Clinical Practice Guidelines for the Management of Adult Congenital Heart Disease 2020. Eur Heart J 2020;41:4153-4. [Crossref] [PubMed]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:1494-563. [Crossref] [PubMed]
- Pujol C, Stöckl A, Mebus S, et al. Value of Rotational Thromboelastometry and Impedance Aggregometry for Evaluating Coagulation Disorders in Patients With Cyanotic and Nongenetic Congenital Heart Disease. Am J Cardiol 2019;123:1696-702. [Crossref] [PubMed]
- Balling G, Vogt M, Kaemmerer H, et al. Intracardiac thrombus formation after the Fontan operation. J Thorac Cardiovasc Surg 2000;119:745-52. [Crossref] [PubMed]
- Balling G. Fontan Anticoagulation: A Never-Ending Debate? J Am Coll Cardiol 2016;68:1320-2. [Crossref] [PubMed]
- Khairy P, Poirier N, Mercier LA. Univentricular heart. Circulation 2007;115:800-12. [Crossref] [PubMed]
- Viswanathan S. Thromboembolism and anticoagulation after Fontan surgery. Ann Pediatr Cardiol 2016;9:236-40. [Crossref] [PubMed]


