
Endovascular creation of hemodialysis arteriovenous fistulae: the current status and future perspective—a literature review
Introduction
There has been a steady rise of end-stage renal disease (ESRD) patients over the past few decades, with an annual incidence of 124,500 and prevalence of 750,000 Americans in 2017. The majority of ESRD patients are managed with hemodialysis while a minority of patients receive peritoneal dialysis or renal transplants (1). Worldwide, around 2 million patients are managed with hemodialysis (2).
Hemodialysis access options include central venous catheters (CVCs), arteriovenous grafts (AVGs) and arteriovenous fistulae (AVF). Among those, AVF and AVG are preferred over CVC due to the lower risk of infection (3). AVF also have an advantage over AVG with respect to primary and secondary patency rates. However, there are many barriers to AVF use, including long surgical-wait times for creation as well as variable primary failure rates, defined as AVF that are never usable, or fail within the first three months. The primary failure rate ranges between 20–70%. Even with the use of vein mapping and risk stratification, failure rate has been as high as 25% (4). The pathogenesis of primary AVF failure is complex but can be secondary to surgical trauma, technique, or pre-existing lesions.
In 2015, the introduction of percutaneously or endovascularly created AVF (endoAVF) offered another option for AVF creation. The short-term data on technical success and maturation rates have been promising. However, many questions remain about the long-term outcomes, re-intervention rates, ease of cannulation, cost burden, and patient experience. In this review, we aim to discuss the history, technique, outcomes, and future direction of endoAVF. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-600/rc).
Methods
A literature search was performed on the electronic databases including MEDLINE and Embase, from 2015 to the 2021 to identify all relevant studies. A combination of the search terms included “endoAVF”, “endovascular arteriovenous fistulae”, and “percutaneous AVF” (Table 1). The reference list also included studies identified manually, and studies referenced for other purposes.
Table 1
| Items | Specification |
|---|---|
| Date of search | 09/01/2021 |
| Databases and other sources searched | EMBASE, MEDLINE |
| Search terms used | endoAVF, endovascular arteriovenous fistula, percutaneous AVF |
| Timeframe | 2015–2021 |
| Inclusion and exclusion criteria | Inclusion: English language literature only |
| Selection process | Literature review performed by XL, VA, and SR |
endoAVF, endovascular creation of arteriovenous fistula.
History of AVF
Early progress in the development of hemodialysis was tempered by the challenge of durable long-term vascular access for dialysis. Willem Kolff made significant progress over prior attempts at hemodialysis by developing the “rotating drum kidney” (5). Despite improvements in technique, Dr. Kolff could only complete 12 hemodialysis sessions in one patient because the patient lost all vascular access options as each session required a surgical cut-down to the artery. Vascular access for long-term hemodialysis remained a challenge until the 1960s, when the concept of placing a “dialysis shunt” between the artery and vein, termed Scribner’s shunt, emerged. Initial vascular shunts were part-internal and part-external with tapered Teflon cannulas surgically inserted in the radial artery and adjacent cephalic vein. A portion of these cannulas would course subcutaneously before exiting the skin and the external portions of these cannulas were then connected by additional Teflon and/or silicon tubing while not being used for hemodialysis. These internal-external prosthetic shunts had many challenges, including frequent clotting, infection, cannula dislodgement, pressure erosions of the skin over the cannulas and limited patency, which was measured in months (6).
Recognition of these short-comings led to Brescia and Cimino’s landmark technique paper in 1966 describing the first cohort of patients with surgically created AVF for hemodialysis (7). They described a side-to-side anastomosis created between the radial artery and adjacent forearm vein using local anesthesia and a 3-cm incision. The fistulae were accessed using a tourniquet to distend the veins and could be used as soon as one day after surgery. This technique was successful in 13 of 16 patients in their series and those patients were dialyzed for 110 dialysis months at the time of the publication. The authors noted that all fistulae that were successfully used after surgery continued to be used at flow rates of 250–300 mL/min without clotting or infection.
In the years following Brescia and Cimino’s publication, many surgical techniques emerged for AVF, including end-to-end anastomoses and radial artery side-to-vein end anastomoses. As forearm AVF were increasingly used for maintenance hemodialysis, alternative access creation sites were investigated in patients with inadequate forearm vasculature. In 1977, Gracz et al. described their experience with creating an anastomosis between the perforating vein and brachial artery or radial artery at the antecubital fossa (8). A similar concept was proposed by Toledo-Pereyra et al. to create proximal forearm AVF between the radial artery and cephalic vein, or venous perforator (9). Konner et al. proposed a modified Gracz technique, where the anastomosis was made using the venous perforator (10). Advantages of this technique were that it could be used in patients without adequate distal forearm veins, the smaller diameter of the perforating vein could prevent a high-flow fistula, multiple upper arm veins could serve as access veins, and the anastomosis lay deep in the antecubital fossa such that it was unlikely to be inadvertently punctured during hemodialysis access. Ultimately, the Gracz fistulae and proximal radial artery fistulae laid the conceptual groundwork for endoAVF.
Challenges of surgical AVF
Primary failure accounts for 20–70% of surgical AVF (4). Furthermore, AVF require on average 1 to 2 interventions per patient year to assist with maturation and maintenance (11,12). The pathogenesis of surgical AVF failure is complex and can be broadly divided into pre-existing lesions, acquired lesions, and suboptimal remodeling. Pre-existing lesions, such as pre-existing venous and arterial stenosis, can be identified with pre-procedural imaging. De novo lesions, most often occurring in the juxta-anastomotic segment, may be caused by increased shear stress, surgical technique, or vessel manipulation during fistula creation. These factors trigger a pro-inflammatory cascade, involving upregulations of leukotrienes, chemokines, and vasoactive molecules. The pro-inflammatory factors in turn cause adventitial fibroblast activation, smooth muscle cell migration, and unfavorable vessel remodeling. The cascade of events culminates in neointimal hyperplasia, leading to luminal narrowing and primary AVF failure and can be a cause for delayed maturation (13).
The consequence of primary failure and long maturation times of AVF is increased initiation of hemodialysis with CVC, longer indwelling CVC time, patient resistance to surgical AVF, and associated increased morbidity and mortality secondary to longer CVC use (3). These issues remain even more relevant in present times as the proportion of patients who remain on CVC for dialysis has been increasing (1).
Inception of the endovascular AVF
Since the initial published experience of proximal radial artery fistulae and antecubital fistulae by Gracz and Toledo-Pereyra, several studies have validated the benefits of these fistulae over conventional upper arm fistulae with respect to relatively improved primary failure rates, high primary patency, and decreased risk of steal and high-output cardiac failure (14-16). The motivation to develop a fistula percutaneously or endovascularly was to minimize surgical trauma to the vessels during creation of the anastomosis, which was hypothesized to result in improved primary patency and decreased primary failure rates.
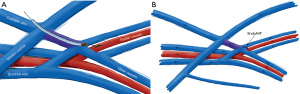
EndoAVF design is based heavily on the experience of Gracz and Toledo-Pereyra with respect to superficial and deep arm venous anatomy (Figure 1), and further characterized by Hull et al. (17). Briefly, the superficial venous system of the arm is comprised of the cephalic, basilic, and cubital veins, and the deep venous drainage comprised of paired ulnar, radial, and brachial veins as well as single central veins including the subclavian and brachiocephalic veins. The superficial and deep venous systems communicate via single or multiple perforators, located at the antecubital fossa. Both currently FDA approved endoAVF devices rely on the perforator to divert flow to the upper arm superficial veins, which can then be used for hemodialysis cannulation.
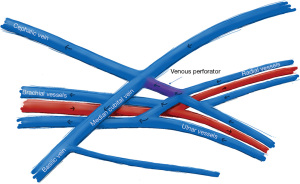
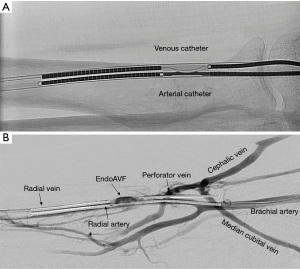
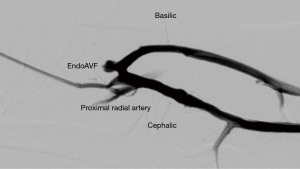
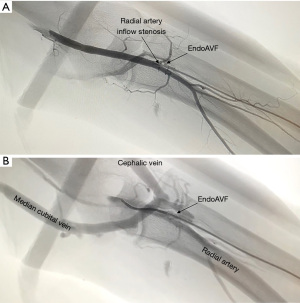
The WavelinQ device [Becton, Dickinson and Company (BD), Franklin Lakes, NJ] is a dual catheter system, which deploys parallel catheters in adjacent artery and vein (Figure 2). Under ultrasound guidance, venous access is introduced into the brachial or forearm vein. The guidewire is then advanced into the planned medial/lateral radial or ulnar vein. Arterial access is introduced via a sheath in the brachial artery (on-label) or via the distal radial/ulnar artery (off-label). A guidewire is advanced through the access either antegrade (via brachial access) or retrograde (via wrist access), into the proximal forearm radial or ulnar artery. Venous catheter with electrode and arterial catheter with ceramic saddle are then advanced over the wires. Under fluoroscopic guidance, catheters are aligned such that the rare-earth magnets contained within the catheters attract and pull the parallel vessels into contact. Then, the radiofrequency electrode within the venous catheter is used to create a side-to-side anastomosis into the magnetically coupled proximal radial or ulnar artery. After creation, blood flows through the freshly created anastomosis, towards the perforator, and drains via the upper arm superficial veins (cephalic and/or basilic), in addition to draining through the upper arm deep veins (Figures 2-5).
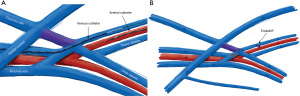

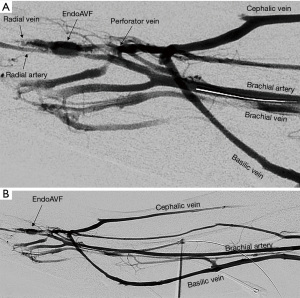
The Ellipsys (Medtronic, Minneapolis, MN) device is a single-catheter system, which creates a direct anastomosis between the proximal radial artery and perforator (Figure 6). Under ultrasound guidance, initial venous access is gained through a cubital (median cubital or cephalic) vein into the perforating vein. Then, under continuous ultrasound guidance, the micropuncture needle is advanced through the perforator and into the proximal radial artery. The micropuncture needle is exchanged for a sheath over a 0.018 wire. The Ellipsys catheter is then advanced over the wire until the tip of the catheter is within the radial artery and the base is within the vein. At this point, the tip and the base of the catheter contract to bring the opposing artery and vein into contact. The puncture site is then fused with thermal energy to create an elliptical side-to-side anastomosis. The end result is similar in that blood flows through the anastomosis and into the upper arm superficial veins via the perforator (Figures 7,8).
Anatomic suitability and candidacy for EndoAVF
The perforator is the keystone of both devices and serves to divert flow to the upper arm cephalic and/or basilic veins. Candidacy for endoAVF via either device, therefore, requires an adequate perforator >2 mm in diameter, as well as upper arm superficial veins >2–2.5 mm in diameter. The Ellipsys system additionally requires the perforating vein and proximal radial artery to be less than 1.5 mm from each other, and the WavelinQ system requires brachial and radial/ulnar arteries >2 mm in diameter, and <1 mm distance between anastomosing artery and vein. Recent studies have shown that approximately half of the screened patient population might be eligible for either endoAVF system based on anatomy (18). Observational studies have also shown that approximately 30% and 60% of the screened patients were eligible for WavelinQ and Ellipsys systems, respectively (19,20). Regardless of candidacy for endoAVF, patients who are eligible for a Brescia-Cimino (forearm radiocephalic) fistula should undergo creation of this fistula prior to proceeding to a proximal forearm or upper arm fistula as per Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (unless preferred otherwise) (3).
Endovascular AVF trial outcomes
Initial trials with WavelinQ
In 2015, Rajan et al. reported early results of the initial 6-Fr EverlinQ device, which was later modified to a 4-Fr system and known as WavelinQ (Table 2). In this study of 33 patients, technical success was 97% and physiologic maturation (defined as brachial artery flow >500 cc/min and vein diameter >4 mm) was achieved in 28 patients (85%). Hemodialysis was achieved in 24 of 27 patients at 6 months and cumulative patency was 96.2% at 6 months. Six device-related adverse events were reported (21).
Table 2
| Study | Device | Follow-up duration | Primary patency rate | Cumulative patency rate | Intervention rate | Maturation rate | Dialysis rate |
|---|---|---|---|---|---|---|---|
| Rajan et al. (21) | 6 Fr WavelinQ | 6 months | – | 96% at 6 months | 0.6 per patient | 96% | 96% |
| Lok et al. (22) | 6 Fr WavelinQ | 12 months | 69% at 1 year | 84% at 1 year | 0.46 per patient-year | 87% | 64% |
| Berland et al. (23) | 4 Fr WavelinQ | 180 days | 83% at 6 months | 87% at 6 months | 0.21 per patient-year | 91% | 78% |
| Hull et al. (24) | Ellipsys | Up to 24 months | – | 75% at 1 year | 1.57 per patient-year | 77% at 6 weeks | – |
| Hull et al. (25) | Ellipsys | 12 months | – | 87% at 1 year | 2.7 per patient-year | 86% | 88% |
endoAVF, endovascular creation of arteriovenous fistula.
Early success led to the prospective, single-arm, multicentric novel endovascular access trial (NEAT), which assessed the efficacy and safety of the 6-Fr EverlinQ system. In this study, embolization of one of the paired brachial veins was routinely performed at the index procedure to divert flow from the deep system and into the upper arm superficial veins. This was also performed to decrease number of secondary procedures required for maturation. The technical success was 98%. In addition, 64% of the mature fistulae were dialyzable (defined as two-needle cannulation in two-thirds or more of dialysis sessions over 4 consecutive weeks), and the primary and cumulative patency rates were 69% and 84% at 12 months. The re-intervention rate was 0.46 per patient-year of dialysis over 12 months. 75% of pre-dialysis patients in this study were able to initiate dialysis with an endoAVF. Eight serious procedure-related adverse events were reported (22).
In a smaller trial, Berland et al. evaluated the modified 4-Fr WavelinQ system in the Endovascular Access System Enhancement (EASE) trial. This study was a prospective, single arm, multi-operator study including a total of 32 patients in South America. The primary and cumulative patencies at 6 months were 83% and 87%, respectively. The re-intervention rate was 0.21 per patient-year at 6 months. Average time to cannulation of the fistulae was 43 days, and 78% of fistulae were dialyzable by 90 days. 74% of indwelling CVCs were able to be removed before 180 days. No device-related serious adverse events were reported (23). The EASE trial demonstrated short-term patency and functional cannulation rates, comparable to the original 6-Fr device. In addition, the smaller profile of the device allowed for multiple access options to reduce the rate of peri-procedural complications.
Initial trials with Ellipsys
Hull et al. reported the early results of the Ellipsys percutaneous AVF creation device in 2017. A total of 26 patients were prospectively recruited from 2014–2015. Technical success was achieved in 88% of the patients. At follow-up, 77% of patients met the primary end points defined as brachial artery flow >400 cc/min, dialyzable fistula, or patent fistula by US assessment at 6 weeks. The cumulative patency rates were 88% and 75% at 6 and 12 months. No device-related major anastomosis complications were reported (24).
Subsequently, a multicenter, multi-operator trial was published, assessing the efficacy and safety of the Ellipsys system in a total of 107 patients. Technical success was 95% with technical failures mostly attributed to access difficulty. At follow-up, 98.4%, 98.4% and 92.3% of the fistulae were functionally patent at 90, 180, and 360 days, respectively. Mean time to 2-needle dialysis was 100 days. A total of 271 secondary procedures were performed during the 12 months, with 205 procedures performed to increase and divert flow towards the superficial system, and 66 procedures to maintain the fistula patency. Specifically, 28 patients (26%) required surgical transposition to superficialize the target vein. In total, the secondary re-intervention rate was 2.7 per patient-year of dialysis. No device-related serious adverse events were reported (25).
Follow-up endovascular AVF outcomes
Short-term Ellipsys outcomes
Multiple small studies have provided more insights on short-term patency rates, maturation times, and secondary intervention rates (Table 3).
Table 3
| Study | Device | Primary patency rate | Cumulative patency rate | Intervention rate | Maturation rate | Dialysis rate |
|---|---|---|---|---|---|---|
| Short-term | ||||||
| Mallios et al. (26) | Ellipsys | 82% | 92% | 0.24 per patient | – | 100%* |
| Hebibi et al. (27) | Ellipsys | – | – | 0.53 per patient** | – | 71% |
| Sultan et al. (28) | Ellipsys | 83% at 6 weeks | – | 0.73 per patient | 47% | 20% |
| Hull et al. (29) | Ellipsys | 7% at 6 months | 96% at 6 months | 2.3 per patient** | 93% | 87% |
| Zemela et al. (30) | WavelinQ | – | 88% | 0.53 per patient | – | 48% |
| Mid-term | ||||||
| Mallios et al. (31) | Ellipsys | 54% at 1 year | 96% at 1 year | 0.52 per patient** | – | – |
| Beathard et al. (32) | Ellipsys | – | 91.6% at 2 years | – | 98% | 95% |
*, all patients on catheter dialysis were successfully dialyzed via endoAVF; **, estimated from provided data. endoAVF, endovascular creation of arteriovenous fistula.
With Ellipsys endoAVF, Mallios et al. reported improved maturation rates and patency when percutaneous balloon angioplasty of the anastomotic site was performed at the time of AVF creation. Authors reported a cumulative patency rate of 92%, and 69% of the patients were successfully dialyzed within 6 months. No adverse event was reported (26). Similarly, Hebibi et al. reported that a large percentage of patients were able to initiate two-needle cannulation between 10 days to 4 weeks, with the majority of patients initiated within 60 days. Procedure-related complications were not reported (27). In contrast, Sultan et al. reported that only 7 out of 15 Ellipsys endoAVF were either dialyzable or meeting the physiologic maturation criteria by US (blood flow >500 cc/min in either outflow vein) at 6 months. Primary failure (thrombosis), and additional need for secondary intervention were primary reasons to account for non-use or non-maturation. Procedure-related complications were not reported (28). The authors suggested that earlier follow-up and prompt secondary interventions were needed to prevent early endoAVF failure.
In a recent study by Hull et al., an aggressive post-operative protocol was adopted. All patients were evaluated at 4-week post-op appointment, and all non-mature fistulae underwent secondary interventions, resulting in a re-intervention rate of 67% for assisted maturation. In addition, 63% of the 60 patients required additional maintenance interventions, resulting in a total of 2.3 re-interventions per patient. The cumulative patency rate was 96% at 180 days. Authors noted 2 early fistula thrombosis, among other complications (29). However, it should be noted that the high secondary intervention rate was partially attributed to the cannulation protocol, where only one target outflow vein (cephalic vs. basilic vs. transposed brachial) was selected for cannulation. Therefore, secondary interventions were needed to achieve two-needle cannulation. This is in contrast to protocols commonly used in European centers, where US guided fistula access may permit catheterization of two different outflow vessels.
Short-term WavelinQ outcomes
With regards to WavelinQ endoAVF, Zemela et al. reported a technical success rate of 100%. The cumulative patency rate was 88%, and 48% of the patients were successfully dialyzed. More importantly, 47% of the patients required secondary interventions, including side-branch coiling, angioplasty, and thrombectomy. 8 out of 35 patients reported peri-operative complications (30).
In general, the short-term data with either device was limited due to the heterogeneity in study designs but suggested progressive improvement in technique. For example, some studies used outflow vein flow rate (>500 mL/min) as the marker for physiologic maturation while some others used inflow artery flow rate (>500 mL/min). In addition, some studies performed concurrent balloon angioplasty at the time of endoAVF creation while others opted for close follow-up and re-intervention if indicated. Nearly all studies reported secondary intervention rates less than 1 per patient, noting that Hull et al. reported high re-intervention rates using an aggressive follow-up protocol. In addition, the re-intervention rate of 2.3 per patient-year in the Hull et al. study was lower than the previous 2.7 per patient-year in the pivotal Ellipsys trial, suggesting that improvement in the technique, experience, and protocol could reduce the number of interventions. Further, as previously discussed, the high re-intervention rates in the U.S. cohort (24,29) were partially attributed to the differences in cannulation protocol between American and European centers.
Mid-term outcomes
There have been a few mid-term studies using the Ellipsys system. In a recent study, a total of 234 fistulae were created over 2-year period using the Ellipsys system. The technical success rate was 99%. The authors have found the 1-year primary, primary assisted and secondary patency rates were 54%, 85% and 96%, respectively. The secondary intervention rate was 35%. No procedure-related significant adverse event was reported (31). Similarly, Beathard et al. reported the 2-year follow-up data on a total of 105 patients with the Ellipsys endoAVF. Remarkably, nearly all endoAVF (95%) were functional, defined as an access capable of undergoing two-needle cannulation. The 1- and 2-year cumulative patency rates were 92.8% and 91.6%, respectively. The secondary intervention rate was not reported. Procedure-related complications were not reported. Interestingly, the authors also included a patient experience survey in the study design. In general, the patient reception was positive, noting that the response rate was only 39% (32).
With regards to the WavelinQ device, no recent mid-term (>1 year) result has been reported, although a post-market registry has recently completed and results are pending (33).
Comparison of EndoAVF systems
To date, there has been one study retrospectively comparing the two endoAVF systems (20). Shahverdyan et al. conducted a retrospective review of 100 patients who underwent 4-Fr WavelinQ and Ellipsys endoAVF placement. During the index procedure, brachial vein embolization was performed in the WavelinQ cohort, and balloon angioplasty of the anastomosis was performed in the Ellipsys cohort. No significant baseline differences were observed between the two cohorts.
A total of 34 WavelinQ and 65 Ellipsys endoAVF were created. The technical success rates were 97% and 100% in WavelinQ and Ellipsys cohort, respectively. At follow-up, 8.5% of WavelinQ patients and 1.5% of Ellipsys patients reported serious adverse events. The cumulative patency rates were 60% and 82% at 12 months (HR 0.42, 95% CI: 0.19–0.97), and the maturation (brachial artery >500 mL/min and target (cephalic and/or basilic) vein >5 mm) rates were 54% and 68% for WavelinQ and Ellipsys endoAVF, respectively. The secondary intervention rates were 0.46 and 0.96 per patient-years for WavelinQ and Ellipsys endoAVF, although the authors did not report whether that was a statistically significant difference. This study suggested that Ellipsys is associated with higher cumulative patency rates but similar maturation rates and dialysis rates in comparison to WavelinQ.
Specific clinical applications
More recent data suggests that Ellipsys endoAVF can be used for a variety of specific clinical applications. For example, early cannulation (within 4 weeks of creation) of endoAVF has been reported in selected cases to avoid CVC placement for either dialysis initiation or catheter exchange. In a recent study, successful 2-needle cannulation was achieved at 10-day post-creation to avoid CVC placement (19). In addition, a recent study reported 14 patients were able to initiate early dialysis with a range of 1–12 days post-endoAVF creation, and a mean time-to-dialysis of 8 days (34). Although authors did not explore the difference between early and late cannulation cohorts, the results demonstrated that early cannulation may be feasible in selected patients.
In addition, endoAVF can be used for a two-stage brachial vein elevation procedure in selected patients. Mallios et al. reported that successful two-stage procedures were performed in 8 patients with no suitable superficial outflow veins (basilic or cephalic). As the first-stage procedure, Ellipsys endoAVF was created per manufacturer’s instruction for use between the proximal radial vessels. Then, 4-to-12-week post-creation, the downstream brachial vein was elevated as the secondary procedure. Technical success was 100% and cumulative patency rate was 100% at 6 months (35).
Lastly, recent data have suggested that percutaneous radiocephalic AVF can be created in selected patient populations with Ellipsys system. In a small case series, 4 endo-radiocephalic fistulae were created. The anatomic criteria were similar to that of proximal radial endoAVF, namely the presence of a perforating vein >2 mm, target vessels >2 mm and close proximity of target vessels. Successful two-needle dialysis was performed in 3 patients while the last patient did not require dialysis. No interventions were needed in the small cohort (36).
These early experiences suggest that clinical applications of endoAVF may be broadened to incorporate a collaboration between percutaneous and surgical techniques.
Comparison with surgical data
Functional outcome comparison
Table 4 summarizes patency, re-intervention rates, maturation rates, and time to successful dialysis for endoAVF and surgical AVF (Table 4). In a recent retrospective observational study by Inston et al., a matched comparative analysis was carried out between WavelinQ and surgical radiocephalic AVF. The authors have found that the endoAVF cohort was comparable to the surgical cohort in time-to-dialysis, primary patency rate, cumulative patency rate, and secondary intervention rate. The two groups had comparable time to indwelling CVC removal. However, the endoAVF cohort had a significantly shorter wait time to the index procedure (endoAVF or surgical creation) than the surgical group (33 vs. 86 days, P<0.0001) (37). Similarly, Harika et al. compared Ellipsys endoAVF to radiocephalic, brachiocephalic, or brachiobasilic surgical fistulae. The authors have found that the Ellipsys endoAVF cohort was associated with a higher rate of maturation at 6 weeks (65% vs. 50%, P=0.02), lower primary patency rate (61% vs. 86%, P=0.01), as well as a higher rate of intervention (50% vs. 34%, P=0.013) at 1 year. However, there was no significant difference in cumulative patency rates at 1 or 2 years, or intervention rates at 2 years between the cohorts. In a sub-group analysis, endoAVF cohort had similar maturation rate at 6 weeks in comparison to elbow surgical group, and better maturation rate at 6 weeks in comparison to wrist surgical group (65.4% vs. 59.6%, P=0.48, and 65.4% vs. 43.3%, P=0.005) (38).
Table 4
| Study | Type of EndoAVF | Type of surgical AVF | Primary patency rate˚ | Secondary patency rate˚ | Intervention rate˚ | Maturation rate˚ | Dialysis rate˚ |
|---|---|---|---|---|---|---|---|
| Inston et al. (37) | WavelinQ | RC AVF | 65.5% vs. 53.4% at 6 months; 56.5% vs. 44% at 1 year | 75.8% vs. 66.7% at 6 months; 69.5% vs. 57.6% at 1 year* | 0.402 vs. 0.273 per patient-year | – | –** |
| Harika et al. (38) | Ellipsys | RC, BC, and BB AVF | 61% vs. 86% at 1 year*; 55% vs. 52% at 2 years | 91% vs. 90% at 12 months; 91% vs. 88% at 2 years | 0.50 vs. 0.34 per patient at 1 year; 0.65 vs. 0.73 per patient at 2 years. | 65% vs. 50% at 6 weeks* | – |
| Shahverdyan et al. (39) | Ellipsys | Gracz AVF | 64% vs. 47% primary patency failure at 1 year | 20% vs 12% secondary patency failure at 1 year | 0.86 vs. 0.66 per patient-year | 85% vs. 79% at 6 months | –*** |
| Osofsky et al. (40) | Ellipsys | BC AVF | 39% vs. 10% primary failure rate* | – | 1.04 vs. 0.29 per patient* | 52% vs. 87%* | – |
*, findings with statistically significant difference, P<0.05; **, mean time to 2 needle cannulation was similar between the two groups; ***, time to 2 needle cannulation was similar between the two groups; ˚, EndoAVF versus surgical AVF. endoAVF, endovascular creation of arteriovenous fistula; RC, radiocephalic; BC, brachiocephalic; BB, brachiobasilic.
In another study comparing Ellipsys endoAVF to Gracz surgical AVF, Ellipsys endoAVF was associated with lower procedure time (14 vs. 70 min, P<0.001). The two groups were comparable in physiological maturation rate at 6 months (79% vs. 85%), time to dialysis initiation (68 vs. 57 days), secondary intervention rates (0.66 vs. 0.86 per patient-year), cumulative primary failure incidence rate at 12 months (47% vs. 64%), and cumulative patency rates at 12 months (80% vs. 88%), when comparing the Gracz cohort to the endoAVF cohort (39).
In contrast, Osofsky et al. compared brachiocephalic surgical AVF to Ellipsys endoAVF. The authors have found that the endoAVF group was associated with a statistically lower rate of clinical maturation (52% vs. 87%, P=0.003), and statistically higher primary failure rate (39% vs. 10% P=0.003). In addition, endoAVF took longer to mature (29 vs. 10 weeks, P<0.001), and needed higher number of secondary interventions (1.1 vs. 0.3, P<0.001) (40). Possible explanations for worse outcomes with endoAVF include difficulties in patient follow-up in a rural environment, initial steep learning curve with the new device, and multiple operators creating endoAVF.
The recent comparative data between surgical and endoAVF groups on functional outcomes have shown varying results. Some of the variations may be explained by the inherent difference in various surgical AVF, which ranged from radiocephalic AVF, proximal radial artery AVF, and Gracz fistulae in the forearm to brachiocephalic AVF and brachiobasilic AVF in the upper arm in the included studies. In general, upper extremity surgical fistulae tend to mature quicker, need fewer interventions, and remain patent longer, noting that brachiocephalic AVF is prone to develop cephalic arch stenosis and high flow steal syndrome (15). Therefore, the differences in outcomes between endoAVF and various surgical AVF may be partially attributed to the baseline differences in surgical outcomes. Secondly, differences in end points, follow-up protocols, and other patient-oriented factors may have played a significant role as well.
Cost comparison
In addition to outcome measures, recent data have shown that endoAVF may be associated with lower costs than surgical AVF. In a study by Yang et al., authors have found that the secondary intervention rate was 0.59 per patient year in endoAVF groups and 3.43 per patient year in surgical AVF, respectively (P<0.05). Accordingly, the estimated total post-procedural costs were $1,794 and $13,033 per patient year for endoAVF and surgical AVF cohorts, respectively (41). Similarly, Arnold et al. have found that the total event rates (post-procedural infection and secondary intervention) and costs were 0.74 per patient year and $815 vs. 7.22 per patient year and $17,443 for incident endoAVF and surgical AVF groups, respectively (P<0.0001). Similarly, the total event rates and costs were 0.46 per patient year and $1,134 vs. 4.1 per patient year and $14,523 for prevalent endoAVF and surgical AVF groups, respectively (P<0.0001) (42). However, this remains to be investigated further as re-intervention rates and need for surgical revision after endoAVF creation can be significantly variable across institutions, operators, and populations.
Future direction
Current endoAVF data is derived from single-arm trials and observational studies, which are heavily influenced by selection bias and operator experience. In particular, non-randomized and retrospective study design carries an inherent risk of selection and confounding biases. In addition, many of the European and multi-centric studies included patient populations that were different from typical United States patient population, including a lack of African Americans, and relatively younger age (~60 years old) (Table 5). Thus, results of non US studies may not be widely applicable to the general population.
Table 5
| Study | Selection | Comparability | Outcome | Total score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representative of the exposed cohort | Selection of eternal control | Ascertainment of exposure | Outcome of interest not present at the start of the study | Main factor | Additional factor | Assessment of outcome | Sufficient follow-up time (≥12 months) | Adequacy of follow-up | ||||
| Rajan et al. 2015, (21) | 0 | 0 | * | * | 0 | 0 | * | 0 | * | 4/9 | ||
| Lok et al. 2017, (22) | 0 | 0 | * | * | 0 | 0 | * | * | * | 5/9 | ||
| Berland et al. 2019, (23) | 0 | 0 | * | * | 0 | 0 | * | 0 | * | 4/9 | ||
| Hull et al. 2017, (24) | * | 0 | * | * | 0 | 0 | * | * | * | 6/9 | ||
| Hull et al. 2018, (25) | * | 0 | * | * | 0 | 0 | * | * | * | 6/9 | ||
| Mallios 2018, (26) | 0 | 0 | * | * | 0 | 0 | * | 0 | * | 4/9 | ||
| Hebibi et al. 2019, (27) | * | 0 | * | * | 0 | 0 | * | 0 | * | 5/9 | ||
| Sultan et al. 2020, (28) | 0 | 0 | * | * | 0 | 0 | * | 0 | * | 5/9 | ||
| Hull et al. 2020, (29) | * | 0 | * | * | 0 | 0 | * | 0 | * | 5/9 | ||
| Zemela et al. 2021, (30) | * | 0 | * | * | 0 | 0 | * | 0 | * | 5/9 | ||
| Mallios et al. 2020, (31) | 0 | 0 | * | * | 0 | 0 | * | * | * | 5/9 | ||
| Beathard et al. 2020, (32) | * | 0 | * | * | 0 | 0 | * | * | * | 6/9 | ||
| Shahverdyan et al. 2020, (20) | 0 | * | * | * | 0 | 0 | * | 0 | * | 5/9 | ||
| Inston et al. 2020, (37) | 0 | * | * | * | 0 | 0 | * | 0 | * | 5/9 | ||
| Harika et al. 2021, (38) | 0 | * | * | * | 0 | 0 | * | * | * | 6/9 | ||
| Shahverdyan et al. 2021, (39) | * | * | * | * | 0 | 0 | * | 0 | * | 6/9 | ||
| Osofsky et al. 2021, (40) | 0 | * | * | * | 0 | 0 | * | 0 | * | 5/9 | ||
*, the criteria that was met for each study.
Long-term outcomes of endoAVF are awaited, particularly in relation to secondary patency rates, re-intervention rates, long term/rare complications, and its applicability in the broader ESRD population. To date, the longest follow-up study has reported the 2-year cumulative outcomes, in comparison to decades of experience in surgical AVF. Secondly, long term complications such as steal syndrome, pseudoaneurysm, and high output cardiac failure need further follow-up and characterization. For example, a recent paper reported a case of ischemic monomelic neuropathy in a WavelinQ endoAVF patient, although definitive correlation has not been established (43). Increased creation and use of endoAVF may additionally reveal other challenges, including the need for widespread education at hemodialysis centers regarding differences in cannulation technique. Additionally, although multiple draining veins of endoAVF may increase the chance of cannulation and increase the “venous capital”, it may also cause pathological remodeling both superficial and deep arm veins. Although studies have suggested improved maturation rates with coiling of deep veins, the long-term impact of doing so is yet to be determined. Lastly, previous endoAVF studies included patients who were relatively young (~60 years old) with selective vascular anatomy (>2 mm). Although elderly (>65) patients have been included in previous studies, the utility of endoAVF in elderly patients with sub-optimal vasculature requires further assessment.
There is an ongoing need for head-to-head comparison between endoAVF and surgical AVF. It is particularly important to define targets of comparison between the two approaches. For example, one perceived benefit of endoAVF over surgical AVF is its ease of use and accessibility as well as the ability to perform endoAVF without general anesthesia or monitored anesthesia care. In comparison, surgical AVF outcomes are heavily influenced by surgeons’ experience, which requires a large volume of cases for proficiency (44,45). While experienced vascular surgeons focused on surgical AVF creation are relatively limited in supply, endoAVF can potentially be created successfully by vascular surgeons, interventional radiologists, and interventional nephrologists. However, current data have also suggested that operator experience influences endoAVF outcomes. In the NEAT trial, the roll-in patient cohort was done by less experienced operators while the study cohort was performed by experienced operators (defined as >5 independent or observed cases). Although the technical success rates were comparable between the two groups, 63% of the roll-in cohort had mature fistula at 3 months versus 87% in the study cohort (22). Therefore, a comparison study assessing whether endoAVF can be easily and proficiently created is potentially useful.
On the other hand, the differences in patient acceptance and experience need to be explored further. A major roadblock to higher surgical AVF acceptance is patients’ concern over appearance, “surgical fatigue”, and wait time (46). Evidence has shown that endoAVF was associated with shorter wait time than comparable surgical AVF (37), and selected patients reported high satisfaction with the procedure (32).
Perhaps the greatest obstacle to widespread endoAVF adaptation in the United States will be challenges with cannulation. Unlike surgical AVF, endoAVF have no scars to guide cannulation, often need a tourniquet during initiation of cannulation, and can be accessed along the median cubital vein as well as along the upper arm cephalic/basilic veins. The majority of cannulation centers in the United States do not utilize ultrasound for access cannulation and there is a steep learning curve with accessing endoAVF. Inexperienced cannulation of an endoAVF can result in infiltration, massive bleeding, and a failed access. Unfortunately, the majority of patients undergo cannulation in independent and free-standing centers, which are not staffed by physicians who create the access. Despite support from cannulation specialists, there is significant hesitation by staff at dialysis centers to access endoAVF. While an optimistic goal, dialysis units dedicated to patients with endoAVF may be critical to the sustainability of the technology.
It is also critical to define the role of endoAVF in the dialysis access algorithm. A functional AVF can withstand repeated cannulation, while a suboptimal AVF requires repeated intervention, longer CVC indwelling time, and longer maturation time. In turn, a suboptimal AVF can lead to increased patient morbidity and mortality due to prolonged CVC use, particularly in the elderly patients, whose life expectancy may be limited and the chance for surgical AVF maturation is low (47,48). Therefore, a better access algorithm may be the “best-vessel-first” rather than “distal-first” approach to create AVF that have the best chance to mature (49). Such algorithms in conjunction with shared decision making can optimize a patient’s “ESKD Life Plan” as suggested by 2019 KDOQI (3). EndoAVF may increase the total venous “capital” and offer flexibility in vessel selection. In addition, endoAVF does not exclude future surgical AVF creation, therefore offering patients more access options.
Lastly, better physiological maturation criteria are needed. Various maturation criteria have been used in previous studies, including brachial artery flow >500 mL/min and vein diameter >4 mm, outflow vein flow >500 mL/min, or vein diameter >5 mm. The heterogeneities in maturation criteria can be partially attributed to the unique properties of endoAVF, where one inflow artery is shared by 2–3 outflow veins, versus the typical one inflow and one outflow surgical AVF configuration. Further, endoAVF may mature unpredictably, with preferential flow into one of the outflow veins. Thus, the typical 500 mL/min inflow artery cut-off may not be applicable in the endoAVF patient population. Indeed, in a recent ASDIN white paper, a cut-off brachial flow >800 mL/min has been suggested based on expert opinion (50). Overall, more standardized maturation criteria are needed and need to be validated, particularly as early intervention may salvage problematic endoAVF.
Conclusions
ESRD remains a significant burden on the healthcare system. The majority of ESRD patients are managed with hemodialysis, with surgical AVF being the preferred access option. However, surgical AVF are associated with variable failure rates and influenced by operator experience. EndoAVF have been introduced as an alternative to surgical AVF with potential advantages including shorter wait times to access creation, and decreased vessel trauma during creation. Recent data have been promising in its short and midterm success. However, several challenges remain, including optimizing post-procedure follow-up, educating dialysis units on cannulation of endoAVF, and assessing real-world and long-term experience with endoAVF relative to surgical AVF. Additional studies and published experiences may shed light on the role of endoAVF in the management of the hemodialysis patient population for the full potential of these technologies to be realized.
Acknowledgments
We wish to thank Medtronics for the permission to adapt some of the artwork.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sasan Partovi and Lee Kirksey) for the series “Endovascular and surgical interventions in the end stage renal disease population” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-600/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-600/coif). The series “Endovascular and surgical interventions in the end stage renal disease population” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2019;73:A7-A8. [Crossref] [PubMed]
- Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258-70. [Crossref] [PubMed]
- Lok CE, Huber TS, Lee T, et al. 2019 Update. Am J Kidney Dis 2020;75:S1-S164. [Crossref] [PubMed]
- Lok CE, Allon M, Moist L, et al. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 2006;17:3204-12. [Crossref] [PubMed]
- Kolff WJ. First clinical experience with the artificial kidney. Ann Intern Med 1965;62:608-19. [Crossref] [PubMed]
- Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs 1960;6:104-13. [PubMed]
- Brescia MJ, Cimino JE, Appel K, et al. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med 1966;275:1089-92. [Crossref] [PubMed]
- Gracz KC, Ing TS, Soung LS, et al. Proximal forearm fistula for maintenance hemodialysis. Kidney Int 1977;11:71-5. [Crossref] [PubMed]
- Toledo-Pereyra LH, Kyriakides GK, Ma KW, et al. Proximal Radial Artery-Cephalic: Vein Fistula Hemodialysis. Arch Surg 1977;112:226-7. [Crossref] [PubMed]
- Konner K, Hulbert-Shearon TE, Roys EC, et al. Tailoring the initial vascular access for dialysis patients. Kidney Int 2002;62:329-38. [Crossref] [PubMed]
- Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol 2006;17:807-13. [Crossref] [PubMed]
- Biuckians A, Scott EC, Meier GH, et al. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008;47:415-21; discussion 420-1. [Crossref] [PubMed]
- Viecelli AK, Mori TA, Roy-Chaudhury P, et al. The pathogenesis of hemodialysis vascular access failure and systemic therapies for its prevention: Optimism unfulfilled. Semin Dial 2018;31:244-57. [Crossref] [PubMed]
- Wu CC, Jiang H, Cheng J, et al. The outcome of the proximal radial artery arteriovenous fistula. J Vasc Surg 2015;61:802-8. [Crossref] [PubMed]
- Al-Jaishi AA, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2014;63:464-78. [Crossref] [PubMed]
- Almasri J, Alsawas M, Mainou M, et al. Outcomes of vascular access for hemodialysis: A systematic review and meta-analysis. J Vasc Surg 2016;64:236-43. [Crossref] [PubMed]
- Hull JE, Balakin BV, Kellerman BM, et al. Computational fluid dynamic evaluation of the side-to-side anastomosis for arteriovenous fistula. J Vasc Surg 2013;58:187-93.e1. [Crossref] [PubMed]
- Popli K, Dittman JM, Amendola MF, et al. Anatomic suitability for commercially available percutaneous arteriovenous fistula creation systems. J Vasc Surg 2021;73:999-1004. [Crossref] [PubMed]
- Franco G, Mallios A, Bourquelot P, et al. Feasibility for arteriovenous fistula creation with Ellipsys®. J Vasc Access 2020;21:701-4.
- Shahverdyan R, Beathard G, Mushtaq N, et al. Comparison of Outcomes of Percutaneous Arteriovenous Fistulae Creation by Ellipsys and WavelinQ Devices. J Vasc Interv Radiol 2020;31:1365-72. [Crossref] [PubMed]
- Rajan DK, Ebner A, Desai SB, et al. Percutaneous creation of an arteriovenous fistula for hemodialysis access. J Vasc Interv Radiol 2015;26:484-90. [Crossref] [PubMed]
- Lok CE, Rajan DK, Clement J, et al. Endovascular Proximal Forearm Arteriovenous Fistula for Hemodialysis Access: Results of the Prospective, Multicenter Novel Endovascular Access Trial (NEAT). Am J Kidney Dis 2017;70:486-97. [Crossref] [PubMed]
- Berland TL, Clement J, Griffin J, et al. Endovascular Creation of Arteriovenous Fistulae for Hemodialysis Access with a 4 Fr Device: Clinical Experience from the EASE Study. Ann Vasc Surg 2019;60:182-92. [Crossref] [PubMed]
- Hull JE, Elizondo-Riojas G, Bishop W, et al. Thermal Resistance Anastomosis Device for the Percutaneous Creation of Arteriovenous Fistulae for Hemodialysis. J Vasc Interv Radiol 2017;28:380-7. [Crossref] [PubMed]
- Hull JE, Jennings WC, Cooper RI, et al. The Pivotal Multicenter Trial of Ultrasound-Guided Percutaneous Arteriovenous Fistula Creation for Hemodialysis Access. J Vasc Interv Radiol 2018;29:149-158.e5. [Crossref] [PubMed]
- Mallios A, Jennings WC, Boura B, et al. Early results of percutaneous arteriovenous fistula creation with the Ellipsys Vascular Access System. J Vasc Surg 2018;68:1150-6. [Crossref] [PubMed]
- Hebibi H, Achiche J, Franco G, et al. Clinical hemodialysis experience with percutaneous arteriovenous fistulas created using the Ellipsys® vascular access system. Hemodial Int 2019;23:167-72. [Crossref] [PubMed]
- Sultan S, Langsfeld M, Chavez L, et al. Initial 6-month quality review of a percutaneous endovascular arteriovenous fistula program. J Vasc Access 2021;22:540-6.
- Hull J, Deitrick J, Groome K. Maturation for Hemodialysis in the Ellipsys Post-Market Registry. J Vasc Interv Radiol 2020;31:1373-81. [Crossref] [PubMed]
- Zemela MS, Minami HR, Alvarez AC, et al. Real-World Usage of the WavelinQ EndoAVF System. Ann Vasc Surg 2021;70:116-22. [Crossref] [PubMed]
- Mallios A, Bourquelot P, Franco G, et al. Midterm results of percutaneous arteriovenous fistula creation with the Ellipsys Vascular Access System, technical recommendations, and an algorithm for maintenance. J Vasc Surg 2020;72:2097-106. [Crossref] [PubMed]
- Beathard GA, Litchfield T, Jennings WC. Two-year cumulative patency of endovascular arteriovenous fistula. J Vasc Access 2020;21:350-6.
- everlinQ endoAVF Post Market Study [Internet]. Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02682420
- Mallios A, Beathard GA, Jennings WC. Early cannulation of percutaneously created arteriovenous hemodialysis fistulae. J Vasc Access 2020;21:997-1002.
- Mallios A, Bourquelot P, Harika G, et al. Percutaneous creation of proximal radio-radial arteriovenous hemodialysis fistula before secondary brachial vein elevation. J Vasc Access 2021;22:238-42.
- Mallios A, Nelson PR, Franco G, et al. Creating percutaneous radiocephalic arteriovenous fistulas at the wrist. J Vasc Access 2021;22:299-303.
- Inston N, Khawaja A, Tullett K, et al. WavelinQ created arteriovenous fistulas versus surgical radiocephalic arteriovenous fistulas? A single-centre observational study. J Vasc Access 2020;21:646-51.
- Harika G, Mallios A, Allouache M, et al. Comparison of surgical versus percutaneously created arteriovenous hemodialysis fistulas. J Vasc Surg 2021;74:209-16. [Crossref] [PubMed]
- Shahverdyan R, Beathard G, Mushtaq N, et al. Comparison of Ellipsys Percutaneous and Proximal Forearm Gracz-Type Surgical Arteriovenous Fistulas. Am J Kidney Dis 2021;78:520-529.e1. [Crossref] [PubMed]
- Osofsky R, Byrd D, Reagor J, et al. Initial Outcomes Following Introduction of Percutaneous Arteriovenous Fistula Program with Comparison to Historical Surgically Created Fistulas. Ann Vasc Surg 2021;74:271-80. [Crossref] [PubMed]
- Yang S, Lok C, Arnold R, et al. Comparison of post-creation procedures and costs between surgical and an endovascular approach to arteriovenous fistula creation. J Vasc Access 2017;18:8-14.
- Arnold RJG, Han Y, Balakrishnan R, et al. Comparison between Surgical and Endovascular Hemodialysis Arteriovenous Fistula Interventions and Associated Costs. J Vasc Interv Radiol 2018;29:1558-1566.e2. [Crossref] [PubMed]
- Chorney MA, Marino AG, Perez Lozada JCL. Ischemic Monomelic Neuropathy after Percutaneous Arteriovenous Fistula Creation. J Vasc Interv Radiol 2021;32:624-6. [Crossref] [PubMed]
- Shahinian VB, Zhang X, Tilea AM, et al. Surgeon Characteristics and Dialysis Vascular Access Outcomes in the United States: A Retrospective Cohort Study. Am J Kidney Dis 2020;75:158-66. [Crossref] [PubMed]
- Saran R, Elder SJ, Goodkin DA, et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg 2008;247:885-91. [Crossref] [PubMed]
- Quinn RR, Lamping DL, Lok CE, et al. The Vascular Access Questionnaire: assessing patient-reported views of vascular access. J Vasc Access 2008;9:122-8.
- Lee T, Thamer M, Zhang Q, et al. Vascular Access Type and Clinical Outcomes among Elderly Patients on Hemodialysis. Clin J Am Soc Nephrol 2017;12:1823-30. [Crossref] [PubMed]
- Drew DA, Lok CE, Cohen JT, et al. Vascular access choice in incident hemodialysis patients: a decision analysis. J Am Soc Nephrol 2015;26:183-91. [Crossref] [PubMed]
- Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol 2007;2:1043-53. [Crossref] [PubMed]
- Wasse H, Alvarez AC, Brouwer-Maier D, et al. Patient selection, education, and cannulation of percutaneous arteriovenous fistulae: An ASDIN White Paper. J Vasc Access 2020;21:810-7.


