
Distal transradial access for coronary procedures: a prospective cohort of 3,683 all-comers patients from the DISTRACTION registry
Introduction
Percutaneous proximal transradial access (pTRA) for coronary angiography and percutaneous coronary intervention (PCI) was firstly described by Campeau (1) and by Kiemeneij et al. (2), respectively. Subsequentially, Amato et al. (3) and Pyles et al. (4) reported the cannulation of the radial artery on the dorsum of the hand for perioperative monitoring. Firstly described by Babunashvili et al. (5) for early retrograde recanalization and reuse of ipsilateral proximal radial arteries, the distal transradial access (dTRA) in the anatomical snuffbox for coronary angiography and interventions was reported in details by Kiemeneij (6) and has gained large popularity around the world.
As an improvement of the conventional pTRA, this technique has potential advantages in terms of both operator and patient comfort, accelerated haemostasis, and lower rates of proximal radial artery occlusion (RAO) (7-9), the most frequent complication of pTRA, which occurs in 7.5% and 5.5% at one and 30-day of follow-up, respectively (10,11).
Importantly, radial artery preservation and patency after coronary angiography and PCI is mandatory for its reuse for repeated transradial procedures, coronary artery bypass grafting (CABG) and hemodialysis fistula creation (12).
In the last five years, dTRA has gradually become familiar to interventional cardiologists around the world and several systematic reviews and meta-analysis have suggested its benefits over pTRA, mainly lower rates of proximal RAO and faster hemostasis (13-15). For this quality improvement (16), beyond operators adequate training with dTRA, it is of paramount importance the establishment of a standardized cath lab protocol, from patient’s preparation set up to hemostasis and post-procedural care.
The adoption by our group of dTRA as standard for routine coronary angiography and PCI has been published elsewhere (17,18). We herein aim to describe the feasibility and safety of a real-world prospective experience with dTRA for routine coronary angiography and PCI in a broader sample of all-comers consecutive patients along with a standardized dTRA protocol. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-542/rc).
Methods
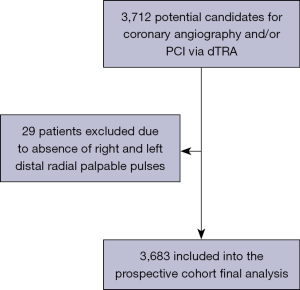
From February 2019 to January 2022, 3,683 consecutive patients underwent coronary angiography and/or PCI via dTRA at Hospital Regional do Vale do Paraíba and Hospital Universitário I, Escola Paulista de Medicina, Universidade Federal de São Paulo have been continuously enrolled in the DIStal TRAnsradial access as default approach for Coronary angiography and intervenTIONs (DISTRACTION) prospective cohort registry (ensaiosclinicos.gov.br; identifier: RBR-7nzxkm). The presence of any (even weak) palpable pulses at both anatomical snuffbox and wrist was the unique eligibility criterion for enrollment. Of note, patients with unstable hemodynamic conditions were not excluded (Figure 1). The study was approved by the Research Ethics Committee of the Hospital Universitário I of the Universidade Federal de São Paulo (protocol 4.071.731, CAAE 30384020.5.0000.5505). Informed consent was given as a prerequisite before enrolling each subject in this prospective registry. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Continuous variables were described as mean ± standard deviation and categorical data as numbers and percent ages. All analyses were performed with the Research Electronic Data Capture (REDCap) version 10.6.5© 2021 Vanderbilt University.
Standardized protocol for dTRA procedures
Patient positioning and preparation (nursing staff and operating physician)
As recently described elsewhere in details (17,18), for left dTRA (ldTRA), patient’s upper arm is placed over the abdomen into the direction of the operator. For right dTRA (rdTRA), patient’s arm is positioned with hand in a neutral position. In order to bring the distal radial artery to the surface of the radial fossa, the patient was requested to grip the thumb under the other four fingers, with the hand slightly abducted. Following detailed evaluation of patient’s pulses and medical records, the site (left or right) of dTRA was decided according to operating physician’s discretion and patient preference.
Distal radial artery puncture (operating physician)
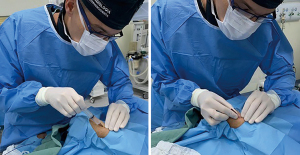
After subcutaneous injection of lidocaine, distal radial artery was punctured with a 20G plastic cannula-over-needle (Seldinger’s technique), from lateral to medial, under an angle of 30–45 degrees, into the direction of the wrist course of the radial artery (Figure 2). Since contact of the needle with scaphoid and trapezium bones periosteum can be painful, the “through-and-through” puncture (operators’ preference) was always performed with special caution. Despite several advantages of ultrasound (US), such as precise assessment of radial artery dimensions and evaluation of postprocedural patency, this resource was not routinely used.
Distal radial artery cannulation (operating physician)
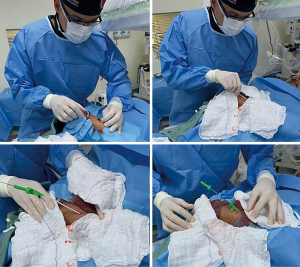
Following arterial puncture, with brisk back flow, a straight, soft and flexible 0.021” hydrophilic guidewire was advanced to guide further sheath insertion (Figure 3). The 10 cm hydrophilic radial 6 Fr sheath Radifocus® Introducer II Standard Kit (Terumo Corp., Tokyo, Japan) was the default device. Exceptionally, different sheaths might be chosen: 4 or 5 Fr, Glidesheath Slender 5/6 Fr or 6/7 Fr (Terumo Corp., Tokyo, Japan) and 7 Fr for complex bifurcations, for example (Figure 4).
Procedure execution (operating physician)
After arterial waveform confirmation and intra-arterial administration of 200 µg of nitroglycerin and unfractionated heparin (50 U/kg), coronary angiography and/or PCI were then performed as the usual fashion. Weight-adjusted additional doses of unfractionated heparin were administered in case of PCI, wire-based physiological assessments or intracoronary imaging.
Hemostasis (operating physician and nursing staff)
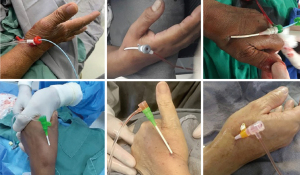
At the end of the procedure, a standard radial compression device (TR band®, preludeSYNC® or Seal-one®) was placed over puncture site (Figure 5). For patients with a large wrist circumference and/or if device unavailability, a simple “handmade haemostatic pad of wrapped gauze” using adhesive tape with enough pressure for compression was placed over puncture site (Figure 5). In general, following the concept of patent hemostasis, hemostatic devices could be completely removed within 1 and 2 h in all coronary angiography and PCI patients, respectively.
Post-procedure care (operating physician and nursing staff)
Just after hemostasis and at discharge, distal and proximal radial artery pulses were assessed by nursing staff and assistant physician. Access site-related bleeding were classified according to EASY hematoma classification (19).
Results
Table 1 summarizes baseline patients features and Table 2, procedural characteristics of all 3,683 consecutive all-comers patients enrolled.
Table 1
| Patient characteristics (total n=3,683 patients) | N (%) |
|---|---|
| Age | 63.3±13.5 |
| Height (m) | 1.66±0.14 |
| Weight (kg) | 76.2±15 |
| BMI (kg/m²) | 27.5±4.6 |
| Men | 2,434 (66.1) |
| Hypertension | 2,852 (77.5) |
| Diabetes mellitus | 1,460 (39.7) |
| Current smoking | 814 (22.1) |
| Former smoking | 1,079 (29.3) |
| Obesity | 919 (25.5) |
| Previous stroke | 84 (2.3) |
| Heart failure with reduced ejection fraction | 291 (7.9) |
| Severe aortic valvar disease | 114 (3.1) |
| Severe mitral valvar disease | 61 (1.7) |
| Known coronary artery disease | 1,325 (36.0) |
| Previous PCI | 952 (25.9) |
| Previous CABG | 143 (3.9) |
| Previous ipsilateral pTRA sheath insertion | 314 (8.5) |
| Previous ipsilateral dTRA sheath insertion | 401 (10.9) |
| Chronic kidney disease without dialysis (eGFR <60) | 228 (6.2) |
| Chronic kidney disease under dialysis | 66 (1.8) |
| Indication for coronary angiography and/or intervention | |
| Chronic coronary syndromes | 1,497 (40.7) |
| Unstable angina | 267 (7.3) |
| NSTEMI | 738 (20.0) |
| Anterior STEMI | 412 (11.2) |
| Inferior STEMI | 325 (8.8) |
| Infero-lateral STEMI | 77 (2.1) |
| Lateral STEMI | 28 (0.8) |
| Severe aortic disease | 94 (2.6) |
| Severe mitral disease | 54 (1.5) |
| Other reasons | 164 (4.5) |
| Cardiogenic shock at cath lab presentation | 95 (2.6) |
Data presented as mean ± standard deviation or number (percentage). M, meter; kg, kilogram; BMI, body mass index; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; pTRA, proximal transradial access; dTRA, distal transradial access; eGFR, estimated glomerular filtration rate; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Table 2
| Procedural characteristics (total n=3,683 patients) | N (%) |
|---|---|
| Coronary angiography | 3,277 (89.0) |
| Elective coronary angiography | 1,376 (37.4) |
| Urgency coronary angiography | 1,901 (51.7) |
| PCI | 2,210 (60.0) |
| Elective PCI | 381 (10.4) |
| Primary PCI | 767 (20.8) |
| Rescue PCI | 24 (0.7) |
| Ad hoc PCI | 1,050 (28.5) |
| Wire-based intracoronary physiological assessment | 25 (0.7) |
| Intravascular imaging (IVUS or OCT) | 63 (1.7) |
| Rotational atherectomy | 9 (0.2) |
| Chronic total occlusion PCI | 84 (2.3) |
| Target coronary artery territory | |
| Left main | 70 (1.9) |
| Left anterior descending artery and/or diagonal branches | 1,069 (29.0) |
| Left circumflex artery and/or obtuse marginal branches | 492 (13.4) |
| Ramus intermedius | 16 (0.4) |
| Right coronary artery and/or branches | 716 (19.5) |
| SVG-RCA | 6 (0.2) |
| VG-LAD | 5 (0.1) |
| SVG-LCx | 8 (0.2) |
| LIMA-LAD | 5 (0.1) |
| Type of dTRA | |
| ldTRA | 315 (8.6) |
| Redo ldTRA | 12 (0.3) |
| rdTRA | 2,952 (80.2) |
| Redo rdTRA | 376 (10.2) |
| Simultaneous bilateral dTRA (ldTRA and rdTRA) | 27 (0.7) |
| Sheath size | |
| 4 Fr | 1 (<0.1) |
| 5 Fr | 17 (0.5) |
| 5/6 Fr | 1 (<0.1) |
| 6 Fr | 3,629 (98.6) |
| 6/7 Fr | 4 (0.1) |
| 7 Fr | 28 (0.8) |
| Hemostasis of dTRA | |
| Radial compression device | 3,594 (97.7) |
| Gauze compressive bandage | 89 (2.3) |
| dTRA-related complications | |
| Minor hematoma (EASY classification <2) | 18 (0.5) |
| Crossover to another access site | 92 (2.5) |
| ldTRA failure → rdTRA successful | 8 (0.2) |
| rdTRA failure → ldTRA successful | 7 (0.2) |
| rdTRA failure → right pTRA successful | 48 (1.3) |
| ldTRA failure → right pTRA successful | 1 (<0.1) |
| ldTRA failure → TFA successful | 4 (0.1) |
| ldTRA failure → left pTRA successful | 3 (0.1) |
| ldTRA failure → left pTRA failure > left transulnar successful | 2 (0.1) |
| ldTRA failure → left pTRA (LIMA-LAD) failure → TFA successful | 6 (0.2) |
| rdTRA failure → right pTRA failure → right transulnar successful | 2 (0.1) |
| rdTRA failure → TFA successful | 2 (0.1) |
| rdTRA failure → right pTRA failure → TFA successful | 7 (0.2) |
| rdTRA failure → right pTRA failure → ldTRA failure → TFA successful | 1 (<0.1) |
| Successful dTRA sheath insertion | 3,606 (97.9) |
Data presented as mean ± standard deviation or number (percentage). PCI, percutaneous coronary intervention; IVUS, intravascular ultrasound; OCT, optical coherence tomography; SVG-RCA, saphenous vein graft-right coronary artery; SVG-LAD, saphenous vein graft-left anterior descending; SVG-LCx, saphenous vein graft-left circumflex; LIMA-LAD, left internal mammary artery-left anterior descending; dTRA, distal transradial access; ldTRA, left distal transradial access; rdTRA, right distal transradial access; Fr, French; pTRA, proximal transradial access; TFA, transfemoral access.
Mean patient age was 63.3±13.5 years old, most male (66.1%), with hypertension (77.5%) and acute coronary syndromes (ACS) (50.2%); overall, 842 (22.9%) patients had ST-elevation myocardial infarction (STEMI). Ninety-five (2.6%) presented to the catheterization laboratory in cardiogenic shock and were submitted to coronary angiography and/or PCI via dTRA (Figure 6). Of note, 39.7% of patients had diabetes; 25.5%, obesity; 36%, known coronary artery disease; 25.9%, previous PCI; 8.5% and 10.9%, previous ipsilateral pTRA and dTRA sheath insertions, respectively. Sixty-six (1.8%) patients were already under hemodialysis and 228 (6.2%) were prone to that due to significant chronic kidney impairment (Table 1).
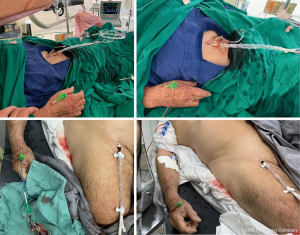
Out of all 3,277 coronary angiographies, the majority (58%) were performed on an urgent basis, mainly due to ACS. For 60.4% of all patients, PCI was undertaken. Among all 2,222 PCI procedures, 1,050 (47.3%) were ad hoc; 767 (34.5%), primary; 381 (17.1%), elective; 24 (0.1%), rescue; and 84 (3.8%), recanalization of chronic total occlusions. Left anterior descending and its branches were the most prevalent (29%) target coronary territory, followed by right coronary artery and its branches (19.5%) and left circumflex and its branches (13.4%) (Table 2).
There were only 2.5% access site crossovers (failed wiring and sheath insertion despite successful distal radial artery puncture), 16% of those successfully executed via contralateral dTRA. Successful any dTRA sheath insertion was then achieved in 3,606 (97.5%) of all 3,683 patients. rdTRA was the most frequent (80.2%) primary access site, followed by redo rdTRA (10.2%), ldTRA (8.6%), simultaneous bilateral dTRA (0.7%) (Figure 7) and redo ldTRA (0.3%). Standard 6 Fr radial sheaths and regular radial compression devices were used for most patients (98.6% and 97.7%, respectively) (Table 2). No differences at occurrence of bleeding or any access site-related complications were observed among the multiple hemostasis strategies.
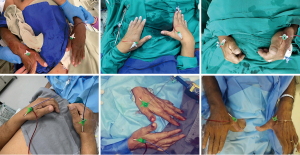
In three patients, concomitant diagnostic cerebral angiography was successfully performed via rdTRA and, in two others, rdTRA was used as ancillary (coronary angiography and aortograms) arterial access for transcatheter aortic valve replacement.
There were neither major complications nor major adverse cerebrovascular and cardiac events directly related to dTRA were recorded. No access site-related hematoma type ≥2, according to EASY classification (19), was recorded. Also, none hand/thumb dysfunction after any procedure was documented. One patient developed a pseudoaneurysm after successful 6 Fr rdTRA coronary angiography and ad hoc PCI, successfully managed by US-guided very prolonged TR band® neck compression (20) and another one, a guidewire-induced forearm radial artery perforation, spontaneously sealed after coronary angiography and ad hoc PCI via ldTRA (21). Despite not very reliable due to absence of US evaluation, proximal and distal radial artery pulses were palpable (by operating physician and nursing staff) in all patients after hemostasis and at hospital discharge.
Discussion
The present study evaluated the real-world large experience results with dTRA for routine coronary procedures in a broad and unselected sample of all-comers patients, encompassing all presentations of coronary artery disease. Also, our standardized protocol for dTRA procedures is summarized. Data were obtained from the DISTRACTION (ensaiosclinicos.gov.br; identifier: RBR-7nzxkm), the first Brazilian observational registry to assess dTRA as standard for routine coronary angiography and/or PCI. In our early experience, there was only 3% of access site crossovers, mostly executed via contralateral dTRA (53.8%) (17). The present updated analysis with eight and a half-fold the number of patients confirmed the maintenance of low rate (2.5%) of access site crossover (successful distal radial artery puncture, but failed wire and sheath advancement). No specific features or factors were evaluated for dTRA failure.
Contrary to most data published so far (6,8,13-15), which essentially included patients at stable conditions, we included patients with any (even weak) distal radial artery palpable pulses, regardless the clinical scenario. Only 29 patients were excluded from the registry, due to absence of any bilateral distal radial pulse (Figure 1). Of note, the majority (50.2%) of our patients had ACS, 22.9% had STEMI and 2.6% presented to the cath lab in cardiogenic shock. We have been publishing some examples of challenging coronary interventions via dTRA such as complex bifurcation (22,23),unprotected left main (23,24), cardiogenic shock (23,24), STEMI (23-25), chronic total occlusion recanalization and other complex PCI (25-27) (Figure 8), and post-CABGinterventions (18). It is important to highlight that after dTRA sheath insertion, coronary angiography and/or PCI can be performed exactly like as for pTRA.
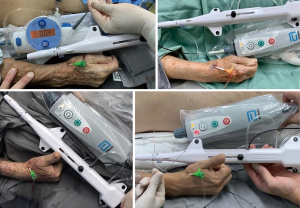
Eight percent of our patients were already under hemodialysis or were prone to that due to significant chronic kidney impairment. In such patients, pTRA has been traditionally averted in order to retain radial artery for future arteriovenous fistulae confection.
Only few (0.5%) of all 3,683 patients experienced some minor [EASY(19) <2] local dTRA hematoma after hemostasis. None hand/thumb dysfunction after any procedure was documented. One interesting case of J-tip 0.035” guidewire-induced forearm radial artery perforation (not directly related to dTRA) spontaneously sealed by a 6 Fr guiding catheter via ldTRA was documented (21),as well as another rare and isolated rdTRA-related local pseudoaneurysm, successfully managed by prolonged (4 h) US-guided TR band® neck compression (20). Albeit not so trusty due to the absence of post-procedural routine Doppler US evaluation, proximal and distal RA pulses were present in all patients after hemostasis and at hospital discharge.
The cath lab staff early perception (after first few cases) of the advantages and potential benefits of this new approach was crucial for their support and commitment for adoption of dTRA as default in our daily practice.
Meta-analysis and systematic review addressing coronary angiography and interventions via dTRA
In a systematic scoping review of 4,212 participants submitted to coronary angiography and interventions via dTRA, mean patient age was 63.8 years old (similar to ours) and 23% were female (less than our 33.5% of women). dTRA was primarily chosen for chronic coronary disease (87.6%, more than two-fold ours 41.2%), with 41.7% for diagnostic and 46.9% for therapeutic procedures (less than our 60% of PCI). The overall success was 95.4%, compared to our analysis of 97.6%. The authors reported complications in 2.4% of cases, mainly (18.2%) hemorrhagic (13). Unlike our analysis, none of those included centres detailed their experience with dTRA as standard approach.
Compared to standard pTRA Liang et al. (15), in a recent updated meta-analysis of 9,054 patients from 14 studies, did not find significant differences in canulation/puncture failures [odds ratio (OR) =1.94; 95% confidence interval (CI): 0.97–3.86; P=0.06], hematomas (OR =0.97; 95% CI: 0.55–1.73; P=0.926), radial artery spasms (OR =0.76; 95% CI: 0.43–1.36; P=0.354), total procedural time (standardized mean difference =0.23; 95% CI: −0.21 to 0.68; P=0.308), or radiation dose area products (weighted mean difference =216.88 Gy/cm2; 95% CI: −126.24 to 560.00; P=0.215). In turn, dTRA had significant less proximal RAO (OR =0.39; 95% CI: 0.23–0.66; P<0.001), faster hemostasis (weighted mean difference =−66.62 min; 95% CI: −76.68 to −56.56; P<0.001), longer time to access (standardized mean difference =0.32; 95% CI: 0.08–0.56; P=0.008), and higher fluoroscopy time (standardized mean difference =0.16; 95% CI: 0.00 to 0.33; P=0.05).
RAO after dTRA versus pTRA
Eid-Lidt et al. (8) reported the first randomized comparison of pTRA versus dTRA for coronary angiography and/or PCI in 282 patients, evaluating the rates of proximal RAO documented by Doppler US. In an intention-to-treat assessment, the 24 h and 30 days rates of proximal RAO were 8.8% and 6.4% for pTRA and 1.2% and 0.6% in the dTRA group (24 h: OR =7.4, 95% CI: 1.6–34.3, P=0.003; 30 days: OR =10.6, 95% CI: 1.3–86.4, P=0.007). Mizuguchi et al. (28) evaluated 228 patients submitted to coronary interventions via dTRA, and only one patient (0.4%) presented proximal RAO by Doppler US. In a meta-analysis by Hamandi et al. (14) assessing 5 studies (6,746 patients), the authors reported statistically significant lower rates of proximal RAO with dTRA compared with pTRA by Doppler US (2.3% versus 4.9%; P=0.004).Finally,in an updated meta-analysis of 9,054 patients from 14 studies by Liang et al. (15), dTRA, compared to pTRA, had significant less rates of proximal RAO (OR =0.39; 95% CI: 0.23–0.66; P<0.001).
Potential advantages of dTRA
dTRA represents a contemporary access site, with the current literature demonstrating favorable success versus complications rates—global procedure metrics comparable to pTRA (7-9,13-16). Despite requiring more puncture attempts, due to operator inexperience and/or smaller vessel diameter, dTRA provides relevant advantages over pTRA, including faster hemostasis and less proximal RAO (7-9,13-16). The updated observational and randomized evidences indicate dTRA is reliable and safe (7-9,13-16).Larger randomized clinical trials are warranted to further examine the superiority of dTRA versus pTRA regarding RAO and others outcomes.
Study limitations
This is a two-centres observational and prospective registry, in which procedures were performed by two experienced interventional cardiologists with pTRA. Thus, the results of the present study cannot be extrapolated and generalized to other centres and to interventional cardiologists unfamiliar with the technique. The absence of a control group restrains our suppositions. dTRA puncture and cannulation attempted as well as fluoroscopy and procedure times were not systematically recorded. In one hand, despite the presence of proximal and distal radial artery pulses after hemostasis and at discharge, the absence of routine post-procedure Doppler US evaluation might have underestimated the vascular complications rates. On the other hand, by performing successful dTRA approach without US guidance might help to disseminate this novel technique.
Conclusions
The incorporation of dTRA as standard of care for routine coronary angiography and PCI in a real-world fashion of all-comers patients by proficient transradial operators appears to be safe and feasible. Future randomized trials are warranted in order to corroborate the safety and the clinical benefits of this relatively new and potentially disruptive technique.
Acknowledgments
We would like to extend our appreciation to our cath lab team, whose compromise with the incorporation of dTRA was indispensable to the feasibility of this work.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-542/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-542/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-542/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn 1989;16:3-7. [Crossref] [PubMed]
- Kiemeneij F, Laarman GJ. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn 1993;30:173-8. [Crossref] [PubMed]
- Amato JJ, Solod E, Cleveland RJ A. “second” radial artery for monitoring the perioperative pediatric cardiac patient. J Pediatr Surg 1977;12:715-7. [Crossref] [PubMed]
- Pyles ST, Scher KS, Vega ET, et al. Cannulation of the dorsal radial artery: a new technique. Anesth Analg 1982;61:876-8. [Crossref] [PubMed]
- Babunashvili A, Dundua D. Recanalization and reuse of early occluded radial artery within 6 days after previous transradial diagnostic procedure. Catheter Cardiovasc Interv 2011;77:530-6. [Crossref] [PubMed]
- Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention 2017;13:851-7. [Crossref] [PubMed]
- Corcos T. Distal radial access for coronary angiography and percutaneous coronary intervention: A state-of-the-art review. Catheter Cardiovasc Interv 2019;93:639-44. [Crossref] [PubMed]
- Eid-Lidt G, Rivera Rodríguez A, Jimenez Castellanos J, et al. Distal Radial Artery Approach to Prevent Radial Artery Occlusion Trial. JACC Cardiovasc Interv 2021;14:378-85. [Crossref] [PubMed]
- Oliveira MD, Caixeta A. Distal transradial access to prevent proximal radial artery occlusion: what is really known? J Transcat Intervent 2021;29:Ea202102. [Crossref]
- Rashid M, Kwok CS, Pancholy S, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2016;5:002686. [Crossref] [PubMed]
- Bernat I, Aminian A, Pancholy S, et al. Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention: An International Consensus Paper. JACC Cardiovasc Interv 2019;12:2235-46. [Crossref] [PubMed]
- Sgueglia GA, Di Giorgio A, Gaspardone A, et al. Anatomic Basis and Physiological Rationale of Distal Radial Artery Access for Percutaneous Coronary and Endovascular Procedures. JACC Cardiovasc Interv 2018;11:2113-9. [Crossref] [PubMed]
- Coomes EA, Haghbayan H, Cheema AN. Distal transradial access for cardiac catheterization: A systematic scoping review. Catheter Cardiovasc Interv 2020;96:1381-9. [Crossref] [PubMed]
- Hamandi M, Saad M, Hasan R, et al. Distal Versus Conventional Transradial Artery Access for Coronary Angiography and Intervention: A Meta-Analysis. Cardiovasc Revasc Med 2020;21:1209-13. [Crossref] [PubMed]
- Liang C, Han Q, Jia Y, et al. Distal Transradial Access in Anatomical Snuffbox for Coronary Angiography and Intervention: An Updated Meta-Analysis. J Interv Cardiol 2021;2021:7099044. [Crossref] [PubMed]
- Oliveira MD, Caixeta A. Distal Transradial Access (dTRA) for Coronary Angiography and Interventions: A Quality Improvement Step Forward? J Invasive Cardiol 2020;32:E238-9. [PubMed]
- Oliveira MD, Navarro EC, Kiemeneij F. Distal transradial access as default approach for coronary angiography and interventions. Cardiovasc Diagn Ther 2019;9:513-9. [Crossref] [PubMed]
- Oliveira MD, Navarro EC, Caixeta A. Distal transradial access for post-CABG coronary and surgical grafts angiography and interventions. Indian Heart J 2021;73:440-5. [Crossref] [PubMed]
- Bertrand OF, De Larochellière R, Rodés-Cabau J, et al. A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation 2006;114:2636-43. [Crossref] [PubMed]
- Oliveira MD, Alves de Sá G, Navarro EC, et al. Pseudoaneurysm After Distal Transradial Coronary Intervention Successfully Managed by Prolonged Pneumatic Compression: Simple Solution for a Rare and Challenging Problem. J Invasive Cardiol 2021;33:E836-8. [PubMed]
- Oliveira MD, Barros TR, Caixeta A. Spontaneously Sealed Forearm Radial Artery Perforation During a Left Distal Transradial Coronary Intervention. J Invasive Cardiol 2020;32:E303-4. [PubMed]
- Oliveira MD, Navarro EC, Caixeta A. IVUS-guided DK-crush left anterior descending-diagonal complex bifurcation PCI via redo distal transradial access. J Xiangya Med 2020;5:20. [Crossref]
- Oliveira MD, Navarro EC, Tavares F, et al. Ostial left anterior descending (unprotected left main) primary percutaneous coronary intervention via distal transradial access in the setting of cardiogenic shock due to anterior ST-segment elevation myocardial infarction. J Transcath Interv 2020;28:Ea2020000017. [Crossref]
- Oliveira MD, Navarro EC, de Sá GA, et al. Unprotected Left Main Primary PCI via Distal Transradial Access in the Setting of STEMI-Related Cardiogenic Shock. Heart Views 2021;22:146-9. [PubMed]
- Oliveira MD, Navarro EC, de Sá GA, et al. Chronic Total Occlusion Recanalization Concurrent to Culprit Primary Percutaneous Coronary Intervention via Distal Transradial Access: Maximizing Revascularization Through Minimalist Approach. Heart Views 2021;22:150-3. [PubMed]
- Oliveira MD, Lyra FG, Neto VTC, et al. Bilateral Distal Transradial Access for Ostial Left Anterior Descending Chronic Total Occlusion Recanalization. J Invasive Cardiol 2021;33:E138. [PubMed]
- Oliveira MD, Navarro EC, Alves de Sá G, et al. Complex Coronary Intervention Via Right Distal Transradial Access With Lusoria Subclavian Artery Under Refractory Electrical Storm: A Really Challenging Case. J Invasive Cardiol 2021;33:E65-6. [PubMed]
- Mizuguchi Y, Izumikawa T, Hashimoto S, et al. Efficacy and safety of the distal transradial approach in coronary angiography and percutaneous coronary intervention: a Japanese multicenter experience. Cardiovasc Interv Ther 2020;35:162-7. [Crossref] [PubMed]



