
Role of stenting for maintenance of the extremity fistula/graft overview
The use of stents in the management of hemodialysis access failure has been a controversial topic since the 1980s. Initially, angioplasty was noted to decrease surgical interventions by 30% and increase the longevity of dialysis shunts by up to 1 year (1). With time, it became clear that while angioplasty could alleviate immediate dysfunction in a dialysis access, the stenoses that were treated with angioplasty were prone to restenosis related to intimal-medial hyperplasia (2). Stents offered the promise of minimally invasive therapy that would keep the angioplasty site open for longer and lead to longer periods without intervention. The purpose of this review is to summarize the data on stent usage in hemodialysis access. This will include distinction between stents and stent-grafts, and we will summarize the data related to dialysis grafts dysfunction, arteriovenous (AV) fistula dysfunction and treatment of the more central aspects of the dialysis circuit including the cephalic arch.
The early data: non-covered stents in hemodialysis grafts
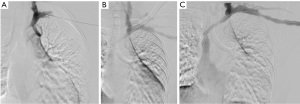
Beathard described the first series of stent use in hemodialysis grafts in 1993 (3). In this trial, 30 Gianturco Z stents (Cook, Inc., Bloomington, IN, USA) were placed after angioplasty in stenoses associated with PTFE grafts while 30 patients had angioplasty alone. Primary patency was compared between the two groups and correlated with historical patency data from another 185 previously angioplastied patients. Primary patency at 1 year was 17% in the stented group and 28% in the angioplasty control group. Kaplan-Meier analysis revealed that the stent offered little advantage over angioplasty alone. In 1995, Quinn et al. reported the outcomes of 50 patients treated with stents that were randomized to angioplasty. 44 of these were Gianturco stents, 1 was a Palmaz stent (Johnson & Johnson), and 5 were Wallstents (Boston Scientific) used for central stenoses. Again, the patency of stents was not superior to angioplasty alone. One-year primary patency was 10% in the stented patients and 11% in the angioplasited patients (4). In 1997, Hoffer et al. reported the first randomized trial of the Wallstent vs. angioplasty in hemodialysis access (5). The hypothesis was that the greater flexibility of the Wallstent would enable successful stenting without as much neointimal hyperplasia as was seen with the Gianturco stents. Thirty-seven grafts were evaluated in 34 patients. Seventeen received Wallstents and 20 received angioplasty alone. Primary and secondary patency was 128 and 431 days without significant difference in either group despite a decrease in the number of interventions needed to achieve secondary patency in the angioplasty group (1.8 vs. 0.8). In 2005, Vogel et al. performed a prospective study of the SMART stent to test the hypothesis that the SMART stent (Cordis) used in salvage situations (rupture, elastic recoil, or rapid restenosis) would provide a better 6-month patency than successful angioplasty (6). This was a non-randomized trial in which stents were used when recoil resulted in a 30% stenosis after angioplasty; the vessel ruptured after angioplasty, or the patient had a stenosis that recurred after angioplasty within 3 months for central lesions and 2 months for peripheral lesions. Twenty-five patients received SMART stents, and 35 patients were treated with angioplasty alone. The primary patency was 5.6 months for angioplasty and 8.2 months for the SMART stent group (P=0.05). The secondary patencies were not statistically significant. An example of a non-covered SMART stent is shown in Figure 1.
A better tool: the use of stent-grafts for dialysis grafts
In 2010, Haskal et al. reported the first prospective randomized trial of treatment for dialysis graft venous anastomotic stenoses with angiographic follow-up at 2 and 6 months (7). In this, the RENOVA trial, 190 patients with venous anastomotic stenoses of expanded polytetrafluoroethylene (ePTFE) grafts were randomized to angioplasty or treatment with ePTFE covered stent grafts (Flair, Bard Peripheral Vascular). Mandatory venography was required at 2- and 6-month follow-up for this trial to determine the angiographic restenosis rate. In this trial, the stent-graft group had a primary patency of 51%, and the angioplasty group had a primary patency rate of 23% which was noted to be lower than the estimate based on prior small nonrandomized studies. Furthermore, the access circuit patency was better at 38% vs. 20%. The 2-year follow-up data was presented in 2016 (8) and demonstrated a 2-fold increase in patency when stent grafts are used. A similar study was completed in 2016 comparing the Viabahn (Gore) stent-graft to angioplasty in the outflow stenoses in grafts. This study differed from the RENOVA study in that is used functional endpoints for dialysis access failure rather than forced angiographic measurements and it included thrombosed and stenotic access circuits. The outcomes were similar with 51.6% patency in the stent-graft arm and 34.2% in the angioplasty arm. Furthermore, Yang et al. performed a similar study in Taiwan in which 98 patients were randomized to either angioplasty or Viabahn stent-graft at the venous anastomosis of grafts. Three- and 6-month patency was significantly higher at 69% and 72% vs. 9% and 29% from angioplasty (9).
Additional small studies: specialized anatomy: cephalic arch, native fistulae, and the use of stent-grafts to revise previous stents
The cephalic arch can be a particularly treacherous area to manage in the hemodialysis patient. It tends to narrow as it joins the axillary vein, is curved, and is prone to rupture. Multiple investigators have evaluated the use of stent-grafts in this location but the size of the trials tends to be small. Rajan reported a randomized trial between stent graft and angioplasty in 14 patients in 2016. In this small study, the primary target lesion and access patency for stent grafts was significantly greater with stent grafts than with angioplasty. At 3, 6, and 12 months, the primary access patency was 100%, 67%, and 22% for stent grafts and only 20% for angioplasty at 3 months with 0 primary patency at 6 and 12 months (10). In 2019, D’cruz et al. reported a meta-analysis of randomized control trials and observational studies that involved interventions in the cephalic arch in patients with brachiocephalic fistulae (11). The included 9 studies involved 473 patients. Two of these were randomized controlled studies (10,12) that include a total of 30 patients. Only one of these randomized controlled trials (RCTs) was presented as published manuscript and it only included 14 patients (10). The other RCT was presented as an abstract in 2016 and included 16 patients (12). The remaining 427 patients were from retrospective observational trials. In the meta-analysis, stent-grafts had greater primary patency at 6 and 12 months than both bare metal stents (relative risk =0.3).
For general use in native fistulae, Bent et al. did report patency rates in an observational study of 17 patients with native fistula stenosis (13). Three-, 6-, and 12-month patency in this small cohort was 94.1%, 88.2%, and 88.2% suggesting that stent-grafts in native fistulae maintain their patency in the short term.
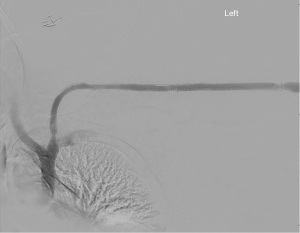
In 2016, Falk et al. reported results of the RESCUE trial which sought to test the hypothesis that stent-grafts would have better patency than angioplasty for in-stent restenosis. In this trial, 275 patients were randomized between stent-graft with the Fluency device (Bard Peripheral Vascular) and angioplasty. There was a much greater patency after stent graft with a 66% and 16% 6- and 12-month patency with the device, and only 12% and 2.2% 6- and 12-month patency for angioplasty (14). An example of a stent-graft is shown in Figure 2.
Discussion
AV access for hemodialysis is the lifeline for most patients with end-stage renal disease (ESRD). Angioplasty is still the most commonly performed treatment for access related stenoses. Stents were initially used because they addressed the rapid recoil often seen with angioplasty. Molecularly, angioplasty remodels the vein wall, stripping the endothelium and cracking the intima-media of the vessel to allow healing at a larger diameter. With rapid recoil, that diameter is not maintained. Stents prevent the loss of diameter from recoil but the intimal hyperplasia associated with stents creates a long channel of narrowed intima/media inside the stent struts that can be very difficult to manage with angioplasty alone.
Currently, the data favors the use of stent-grafts in specific situations for the management of dialysis access failure. Namely, in venous outflow stenoses from grafts, stent-grafts perform better than angioplasty alone and will yield a longer primary patency and freedom from intervention. Furthermore, cephalic arch stenoses seem to do better with stent-grafts than with angioplasty despite the small number of patients in the prospective randomized studies performed. Also, in-stent restenosis in bare metal stents has an excellent 6-month patency of 66% after stent-graft placement through the in-stent restenosis. By 12 months, however, the primary patency falls to 12% due to the “candy-wrapper” stenoses often seen within stent grafts. Table 1 summarizes the current data about stent-grafts in dialysis access intervention.
Table 1
| Year | Author | Trial type | Trial | N | Target lesion | Study device | Device patency (%) | PTA patency (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 12 mo | 24 mo | 3 mo | 6 mo | 12 mo | 24 mo | ||||||||
| 2010 | Bent | Observational | – | 17 | Native fistula stenosis | Flair | 94.1 | 88.2 | 88.2 | – | – | – | – | – | |
| 2013 | Verstandig | Observational | – | 52 | Central veins | Mixed | 60 | 40 | 28 | – | – | – | – | – | |
| 2010 & 2016 | Haskal | RCT | RENOVA | 190 | Graft anastamosis | Flair | – | 51 | 47.6 | 26.9 | – | 23 | 24.8 | 13.5 | |
| 2015 | Rajan | RCT | – | 14 | Cephalic arch; circuit | Viabahn | 100 | 67 | 22 | – | 20 | 0 | 0 | – | |
| 2015 | Rajan | RCT | – | 14 | Cephalic arch; target | Viabahn | 100 | 100 | 29 | – | 60 | 0 | 0 | – | |
| 2017 | Yang | RCT | – | 98 | Graft anastamosis | Viabahn | 69 | 72 | – | – | 9 | 29 | – | – | |
| 2016 | Vesely | RCT | REVISE | 293 | Graft anastamosis | Viabahn | – | 51.6 | – | – | – | 34.2 | – | – | |
| 2016 | Falk | RCT | RESCUE | 275 | In-Stent restenosis | Fluency | – | 66 | 16 | – | – | 12 | 2.2 | – | |
RCT, randomized controlled trial; mo, month.
Practically, many interventionalists will approach stents with some caution because once a stent is placed, the flexibility of the vein and the need to manage in-stent restenosis becomes the main technical issue to overcome. If one can “get away with” angioplasty alone and get more than 3 months of patency until the next intervention, many practitioners will consider that acceptable despite the better primary and secondary patency from stent grafts. Once the interval between intervention becomes frequent, the stent-graft becomes a tool that can be used to limit the number of interventions per year to keep the access open. However, even stents and stent-grafts will not keep an access open indefinitely without intervention. As the number of interventions in a stented access increase, it becomes critically important to judge when it is time to plan for a new access or a different approach to managing the ESRD needs of the patient. This reinforces the critical nature of developing an access plan for each patient and tailoring the interventions to promoting the best quality of life for each patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sasan Partovi and Lee Kirksey) for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-9/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. The author has stock options with Trisalus, Inc. for services. The author performed from 2006–2012 on their Scientific Advisory Board. The author currently serves as Chair of the Renal Diseases Specialty Council of the Society of Interventional Radiology. The author receives grants from Trisalus, Inc., and payments are made to institution. The author receives speaker fees from General Electric and Honorarium from Instylla, Inc. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burger H, Zijlstra JJ, Kluchert SA, et al. Percutaneous transluminal angioplasty improves longevity in fistulae and shunts for haemodialysis. Nephrol Dial Transplant 1990;5:608-11. [Crossref] [PubMed]
- Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 2006;17:1112-27. [Crossref] [PubMed]
- Beathard GA. Gianturco self-expanding stent in the treatment of stenosis in dialysis access grafts. Kidney Int 1993;43:872-7. [Crossref] [PubMed]
- Quinn SF, Schuman ES, Demlow TA, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol 1995;6:851-5. [Crossref] [PubMed]
- Hoffer EK, Sultan S, Herskowitz MM, et al. Prospective randomized trial of a metallic intravascular stent in hemodialysis graft maintenance. J Vasc Interv Radiol 1997;8:965-73. [Crossref] [PubMed]
- Vogel PM, Parise C. Comparison of SMART stent placement for arteriovenous graft salvage versus successful graft PTA. J Vasc Interv Radiol 2005;16:1619-26. [Crossref] [PubMed]
- Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med 2010;362:494-503. [Crossref] [PubMed]
- Haskal ZJ, Saad TF, Hoggard JG, et al. Prospective, Randomized, Concurrently-Controlled Study of a Stent Graft versus Balloon Angioplasty for Treatment of Arteriovenous Access Graft Stenosis: 2-Year Results of the RENOVA Study. J Vasc Interv Radiol 2016;27:1105-14.e3. [Crossref] [PubMed]
- Yang HT, Yu SY, Su TW, et al. A prospective randomized study of stent graft placement after balloon angioplasty versus balloon angioplasty alone for the treatment of hemodialysis patients with prosthetic graft outflow stenosis. J Vasc Surg 2018;68:546-53. [Crossref] [PubMed]
- Rajan DK, Falk A. A Randomized Prospective Study Comparing Outcomes of Angioplasty versus VIABAHN Stent-Graft Placement for Cephalic Arch Stenosis in Dysfunctional Hemodialysis Accesses. J Vasc Interv Radiol 2015;26:1355-61. [Crossref] [PubMed]
- D'cruz RT, Leong SW, Syn N, et al. Endovascular treatment of cephalic arch stenosis in brachiocephalic arteriovenous fistulas: A systematic review and meta-analysis. J Vasc Access 2019;20:345-55.
- Gogna A, Chong C, Irani F, et al. Randomized controlled trial comparing standard balloon angioplasty, placement of drug-eluting stent versus stent graft for the treatment of cephalic arch stenosis in patients with hemodialysis access stenoses. J Vasc Interv Radiol 2016;3:S140-1. [Crossref]
- Bent CL, Rajan DK, Tan K, et al. Effectiveness of stent-graft placement for salvage of dysfunctional arteriovenous hemodialysis fistulas. J Vasc Interv Radiol 2010;21:496-502. [Crossref] [PubMed]
- Falk A, Maya ID, Yevzlin AS, et al. A Prospective, Randomized Study of an Expanded Polytetrafluoroethylene Stent Graft versus Balloon Angioplasty for In-Stent Restenosis in Arteriovenous Grafts and Fistulae: Two-Year Results of the RESCUE Study. J Vasc Interv Radiol 2016;27:1465-76. [Crossref] [PubMed]


