
Pre-procedure imaging planning for dialysis access in patients with end-stage renal disease using ultrasound and upper extremity computed tomography angiography: a narrative review
Introduction
In patients suffering from end-stage renal disease (ESRD), functioning access for hemodialysis is a life sustaining requirement. The access can serve either as a bridge to renal transplant or as a long-term solution in non-transplant candidates.
Vascular access modalities for long-term hemodialysis include tunneled dialysis catheter placement and surgical access creation [arteriovenous fistula (AVF), arteriovenous graft (AVG) and percutaneous arteriovenous fistula (pAVF)]. The purely autologous AVF is considered first vascular access option of choice (1) and preferred to a polytetrafluoroethylene (PTFE)-graft (AVG). However, creation of early cannulation AVGs in patients with poor native options is gaining increasing popularity, as they show reasonable short-term patency. Long-term patency of the graft is limited by thrombotic occlusion requiring frequent endovascular interventions as well as increased risk of infection. Although central venous catheters (CVCs) are regarded as tertiary options due to their significantly higher morbidity and mortality rate (2,3), they are frequently used if hemodialysis needs to be initiated immediately and as a definitive solution in a subset of patients with poor native vessels and significant comorbidities. Furthermore, a delay in access planning and the rising occurrence of acute kidney injury on chronic kidney disease stage 5 requiring urgent dialysis initiation leads to increased reliance on CVC (1).
To create the proper vascular access for each individual patient (considering a patient-centered approach) detailed preoperative planning, including workup of the access history, transplant candidacy, vessel status and comorbidities/risk factors is mandatory.
A cornerstone of this planning procedure is the assessment of the patients’ vasculature to identify those potentially suitable for the creation of a hemodialysis access. The two main elements of this evaluation are the physical exam and imaging. Traditionally, the physical exam has been the primary assessment tool for access planning and imaging was only reserved in cases of equivocal physical exam. The newer guidelines incorporate routine diagnostic imaging prior to access placement (3,4). This paradigm shift was driven by the intention to decrease the number of CVCs and AGV and to increase AVF creation. The different imaging modalities help to identify suitable autologous veins and determine pathologic findings (both arterial and venous) which may impair fistula maturation or increase the risk of access failure.
This manuscript aims to provide an overview of the different imaging modalities that can be utilized for vascular access planning and creation. Advantages and drawbacks for each modality will be discussed along with the implications for daily clinical practice. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-797/rc).
Methods
A literature search was performed in PubMed and Cochrane database of systematic review covering publications up to 2021. We used the following combination of keywords: (I) imaging modalities and vascular access; (II) preoperative planning and vascular access; (III) vein mapping; (IV) duplex ultrasound for vascular access planning; (V) DSA and vascular access planning; (VI) MRA and vascular access planning; (VII) CTA and vascular access planning; (VIII) clinical practice guidelines vascular access. Current guidelines, meta-analyses and prospective studies were included, however, most of provided data/references are derived from observational studies. The search was restricted to guidelines, original research and papers published in English language.
Key inclusion criterion was the description of one of the following imaging modalities, examined/analyzed for feasibility with respect to vascular access creation for hemodialysis: (I) duplex ultrasound; (II) CTA; (III) DSA; (IV) MRI. Papers, that dealt with imaging modalities, used for maintenance and revision of vascular access, were not included. The initial literature search was performed by SR, MA and DS. They independently assessed the methodological quality of the studies prior to their final inclusion (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | July and August 2021 |
| Databases and other sources searched | PubMed, Cochrane database |
| Search terms used | (I) Imaging modalities and vascular access; (II) preoperative planning and vascular access; (III) vein mapping; (IV) duplex ultrasound for vascular access planning; (V) DSA and vascular access planning; (VI) MRA and vascular access planning; (VII) CTA and vascular access planning; (VIII) clinical practice guidelines vascular access |
| Timeframe | 1.1.1990 to 31.08.2021 |
| Inclusion and exclusion criteria | The search was restricted to guidelines, original research and papers published in English language |
| Key inclusion criterion was the description of one of the following imaging modalities, examined/analyzed for feasibility with respect to vascular access creation for hemodialysis: (I) duplex ultrasound; (II) CTA; (III) DSA; (IV) MRI. Papers, that dealt with imaging modalities, used for maintenance and revision of vascular access, were not included | |
| Selection process | The initial literature search was performed by SR, MA and DS. They independently assessed the methodological quality of the studies prior to their final inclusion |
DSA, digital subtraction angiography; MRA, magnetic resonance angiography; CTA, computed tomography angiography; NA, not applicable.
Duplex ultrasound
According to current guidelines, pre-operative ultrasound of bilateral upper extremity arteries and veins is recommended in all patients in the workup of vascular access creation (Class I, Level A) (3).
After a dedicated clinical examination to exclude overt pathologies and assess for venous chest wall collaterals, the arterial vasculature of the upper extremity is scanned from the subclavian artery to the radial and ulnar arteries at the level of the wrist to evaluate inflow criteria (Table 2).
Table 2
| VA modalities | (Arterial) inflow | (Venous) outflow |
|---|---|---|
| AVF | ||
| Snuffbox/radio-cephalic | ≥2–2.5 mm | No central venous stenosis/occlusion |
| No significant stenosis | Inner diameter: 2–2.5 mm | |
| No circumferential calcification at intended anastomotic site | No phlebitis/postphlebitic alterations | |
| Course of cephalic vein | ||
| Distance vein to skin surface (preferably <6 mm) | ||
| Brachio-cephalic | ≥3 mm | No central venous stenosis/occlusion |
| No significant stenosis | Inner diameter: 3 mm | |
| No circumferential calcification at intended anastomotic site | No phlebitis/postphlebitic alterations | |
| Anatomic variant (high bifurcation of brachial artery) | Course of cephalic vein | |
| Distance vein to skin surface (preferably <6 mm) | ||
| Brachio-basilic | ≥3 mm | No central venous stenosis/occlusion |
| No significant stenosis | 3 mm | |
| No circumferential calcification at intended anastomotic site | No phlebitis/postphlebitic alterations | |
| Anatomic variant (high bifurcation of brachial artery) | Course of basilic vein | |
| Level of junction with brachial vein-transposition possible? | ||
| AVG | ||
| Forearm straight graft | ≥2–2.5 mm | Adequate deep cubital outflow |
| No significant stenosis | No central venous stenosis/occlusion | |
| No circumferential calcification | ||
| Forearm loop graft | ≥3 mm | Adequate deep cubital outflow |
| No significant stenosis | No central venous stenosis/occlusion | |
| No circumferential calcification | ||
| Anatomic variant (high bifurcation of brachial artery) | ||
| Upper arm straight graft | ≥3 mm | Adequate outflow (basilic/brachial vein) |
| No significant stenosis | No central venous stenosis/occlusion | |
| No circumferential calcification | ||
| Anatomic variant (high bifurcation of brachial artery) | ||
| pAVF | ||
| WavelinQ | ≥2 mm (ulnar or radial artery) | 2 mm (ulnar or radial vein) |
| Distance between ulnar/radial artery and ulnar/radial vein <2 mm | ||
| Ellipsys | ≥3 mm | Distance between proximal radial artery and perforating vein of the elbow <1.5 mm |
VA, vascular access; AVF, arteriovenous fistula; AVG, arteriovenous graft; pAVF, percutaneous arteriovenous fistula.
The vessel diameters of the radial and brachial arteries are measured in the longitudinal and/or cross-sectional view on B-mode ultrasound and assessment for atherosclerotic involvement of these arteries is pursued (Figure 1). Concerning surgical planning, the most crucial part of the exam is the color and pw-Doppler evaluation to exclude stenotic disease or occlusion within the subclavian, axillary, brachial and radial as well as ulnar arteries. Anatomical variants, such as high brachial bifurcation need to be reported, due to their potential to alter the surgical approach.
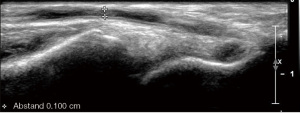
For pAVF creation, specific information on the cubital (Ellipsys® vascular access system) and wrist (WavelinQ®) vessel anatomy is of relevance, particularly related to the arterio-venous proximity and presence of perforators (5).
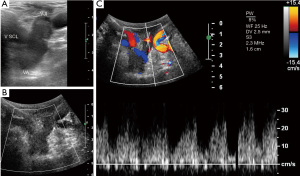
Using color- and pw-Doppler the central veins (subclavian, jugular internal and brachiocephalic vein) are examined to detect any occlusion, post-thrombotic alterations or anatomic abnormalities that might impede adequate venous outflow of the arm (Figure 2).
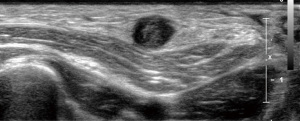
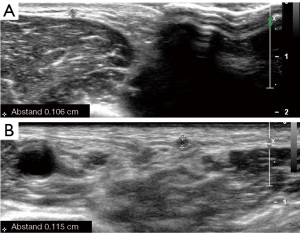
In case of a free central outflow, the superficial veins are scanned from the distal cephalic vein at the wrist to the subclavian vein (with and without tourniquet) (outflow criteria, Table 2). In case of a patent central venous system and absence of postphlebitic alterations or acute phlebitis (Figure 3), the measurement of the vein diameter in the cross-section view on B-mode ultrasound is performed (Figure 4). Additionally, the anatomic course of the vein (straight, <6 mm from the skin surface, side branches) is documented.
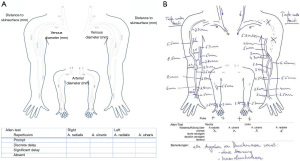
A descriptive report is generated for surgical planning purposes. Figure 5 provides a template of such report, showing the upper extremity arterial and venous tree, including documentation of vessel diameter and stenotic degree as well as venous outflow segments.
Digital subtraction angiography (DSA) and venography
DSA and venography (DSV) is considered the gold standard for visualization of the arterial and venous vasculature, especially for assessment of the central venous system. It enables identification of clinically occult central venous stenotic disease and occlusion (6,7).
In case of preoperative vascular access planning, the standard approach for visualization of the arterial vasculature includes a retrograde puncture of the brachial artery leading to visualization of the entire arterial inflow from the subclavian artery to the digital arterial supply. The technique enables detection of inflow stenosis and provides information on vessel diameter and anatomical abnormalities (Figure 6).
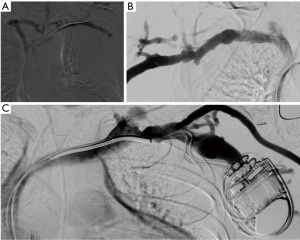
In order to visualize the venous outflow tree, a superficial forearm or hand vein is punctured in an antegrade approach. Venous punctures above the elbow should be avoided to preserve these veins for fistula and/or graft creation in the future.
Computed tomography angiography (CTA)
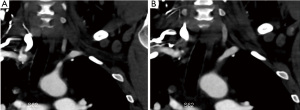
CTA is typically used for detection of pathology after creation of the vascular access. Alternatively, it may be used for mapping the arterial anatomy. However, similar to DSA this modality requires the use of iodinated contrast agents which should be avoided in pre-dialysis patients to prevent further decline/deterioration of their already markedly impaired renal function. Although, modern multidetector CT scanners have extremely short acquisition times with consecutive reduction of radiation dose, the use of ionizing radiation needs to be considered. CTA provides 3D reconstructions and maximum intensity projection (MIP) images, which can be helpful in vascular access planning. CTA can also detect extrinsic compression as an underlying cause of central venous stenosis (8) and as a possible cause of stenosis of the central arterial vasculature including the supra-aortic vessels (Figure 7).
Magnetic resonance angiography (MRA)
MRA is a non-invasive modality offering complete overview of the arterial (MR arteriography) and venous (MR venography) anatomy of the upper extremity in a one-exam session. For vascular access planning it is usually performed as contrast-enhanced MRA (CE-MRA), using a gadolinium-based agent. The application of gadolinium does not bear the risk of an acute deterioration of the renal function, which is an important aspect in (pre-dialysis) patients with marginal renal function. Nevertheless, the risk of developing a nephrogenic systemic fibrosis (NSF) secondary to gadolinium needs to be considered but is exceedingly low and can be almost neglected with newer macrocyclic ionic contrast media gadolinium based MR contrast agents (9-11). In chronic renal failure the half-life of gadolinium is prolonged to 12–30 hours and the combination of metabolic acidosis and inadequate clearance triggers the development of dermopathy with older MR contrast agents (11).
Intravascular ultrasound (IVUS)
Although this imaging modality does not play any role in pre- or postoperative vascular access imaging yet, its use in assessing central venous pathologies (thrombus, stenosis, occlusion, dissection) is gaining increased attention. Data suggest that IVUS is superior to DSA in detecting post angioplasty recoil and persistent intraluminal webs (12).
The use of this relatively new modality is limited to the post interventional setting and its use is not incorporated yet into the most recent guidelines.
Discussion
This manuscript provides an overview of the different imaging modalities, which can be applied for planning to create a vascular access for hemodialysis focusing on the advantages and limitations of the different modalities and the specific requirements for each type of access (AV fistula, AV graft, CVC).
DSA/DSV is considered the gold standard to assess the central venous system, but is also a valuable tool for the arterial vasculature due to the possibility of immediate percutaneous intervention (e.g., angioplasty/stenting). However, in clinical practice, its application is usually reserved for postoperative assessment and treatment of non-maturing or failing fistulas. The downside of this modality is the required iodinated contrast agent administration and the radiation exposure (13). In patients in whom iodinated contrast should be avoided, CO2 as contrast agent can be used and has a similar performance compared to conventional venography with iodine-based contrast agents (14).
The clinical practice guidelines for vascular access for hemodialysis recommend DSA/DSV in a preoperative assessment only in patients with a certain likelihood of central venous stenosis secondary to previous catheter placements (7). It needs to be noted that European guidelines are even more restrictive when recommending DSA/DSV solely in patients in whom a subsequent intervention is anticipated (Class I, Level C) (3).
CTA and MRA play an inferior role as imaging modalities in pre-procedural planning and are more often used to detect pathologies in patients with already established vascular accesses.
CTA imaging provides an overview of the arterial vasculature of the upper extremity and potential anatomic abnormalities. It reveals highly calcified arterial segments and detects inflow limiting stenoses (>50%) or occlusions (3,15). Depending on the examination protocol related to timing of image acquisition it can also visualize central venous pathologies.
The current literature does not provide any comparative data between duplex ultrasound and CTA for preoperative vascular access planning, and the 2018 Clinical Practice guidelines recommend to consider CTA only in patients with inconclusive ultrasonographic or angiographic/venographic results related to the degree of stenotic disease (Class IIb, Level C) (3).
MRA provides excellent information on central venous pathologies, e.g., in patients with a history of recurrent CVC placements, but its use is limited by accessibility, costs and local expertise. Furthermore, the MRA exam is time consuming.
Another important drawback in the antecedent was the risk of the development of a gadolinium associated NSF. This severe complication can be avoided by performing non-contrast enhanced MRA (NCE-MRA).
Published data (9,16) demonstrated this technique to be inferior compared to conventional gadolinium based MRA in terms of total numbers of visible arterial segments and image quality. NCE-MRA can be useful in the depiction of venous structures (9).
Compared to duplex ultrasound, MRA has a superior performance with regard to detection of arterial and venous stenoses (17) and provides a comprehensive overview of the vascular anatomy.
Despite these advantages, the ESVS Guidelines do not recommend CE-MRA in patients with end-stage renal disease (Class III, Level C) (3) for pre-access evaluation, although actual recommendations of radiologic societies state that the risk for development of NSF is minimal at best.
The preferred imaging modality for performing vascular access planning is duplex ultrasound (4). This recommendation is mainly based on its advantage of wide accessibility, non-invasiveness and the absence of ionizing radiation without requiring contrast agent administration. However, clear evidence is lacking with regard to the usefulness of preoperative ultrasound translating into superior results in long-term access patency.
A meta-analysis by Georgiadis et al. (18), comparing preoperative physical examination versus duplex ultrasound, did not show significant differences in postoperatively fistula maturation/usability for hemodialysis after one and six months (19-22).
A Cochrane Database review from 2015 (23), analyzed four randomized controlled trials (RCTs), including over 400 participants, dealing with preoperative vascular access evaluation by ultrasound compared with standard preoperative care with clinical assessment alone. Results did not show a significant difference in both, the number of successfully created [RR 1.06 (95% CI: 0.95–1.18)] and successfully usable fistulas [RR 1.12 (95% CI: 0.99–1.28)]. Furthermore, the number of patients initiating dialysis with a catheter was equally in both groups based on one study [RR 0.66 (95% CI: 0.42–1.04].
Slightly more encouraging results were published by McGill et al. (24). They performed a retrospective analysis of more than 30,000 patients, initiating hemodialysis on a CVC with subsequent planning for AVF or AVG creation. They compared patients undergoing any preoperative imaging (Doppler imaging or venography) versus clinical evaluation before or after starting hemodialysis. Only 7.4% of the entire cohort underwent preprocedural imaging. Patients, who had imaging underwent more frequently fistula or graft creation (70.9% vs. 45.9%, P=0.002) and had lower rates of ongoing CVC use (41.5% vs. 71.0%) and death (39.4% vs. 50.6%, P<0.001). Those, who received preoperative imaging, were more likely to achieve a functioning AVF/AVG (71.3% vs. 69.7%, P=0.02). The numbers of surgical procedures post access creation were significantly higher in patients who received pre-access imaging (P=0.001) and pre-procedure imaging was not found to be an independent predictor for achieving a working AVF/AVG (OR 1.09, 95% CI: 1.02–1.16).
Finally, a recent retrospective cohort study by Fedorova et al., compared access configuration (AVF vs. AVG), location (forearm vs. upper arm), successful initiation of hemodialysis and secondary patency in 46,000 patients stratified by preoperative vein mapping or not. Data were derived from the Hemodialysis Access dataset of the Society for Vascular Surgery Vascular Quality Initiative. Results showed a significant higher creation of forearm access and better secondary patency rates (P<0.001) in those patients who underwent preoperative vein mapping (25).
Differences in society guidelines are understandable when considering these controversies. While the European Guidelines recommend a pre-access imaging exam for all patients undergoing vascular access creation (3), the 2019 KDOQI guidelines recommend preoperative vein mapping only in selected patients (26).
Based on our experience pre-access imaging might contribute to decision-making processes related to the type and localization of hemodialysis accesses in individual patients.
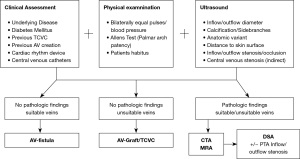
Based on our own experiences a preoperative planning algorithm is proposed in Figure 8. In the ESRD population with multiple comorbidities (diabetes, cardiovascular diseases, obesity) a comprehensive pre-access evaluation including physical examination and sonographic imaging of the bilateral upper extremities is considered institutional standard of care. A schematic drawing (Figure 5) based on the ultrasound examination serves as a fundamental prerequisite for interdisciplinary discussions in order to determine the most reasonable vascular access procedure for each individual patient. Additional preoperative imaging studies including CTA or MRA are pursued in selected cases. DSA/DSV can be performed if concomitant endovascular intervention is anticipated.
Summary
A single imaging modality cannot provide all necessary information prior to vascular access creation. The combination of patients’ medical history, physical examination and vascular ultrasound is widely accepted as the standard of care in access planning, whereas further modalities are preserved for selected patients and circumstances. An interdisciplinary institutional access team should develop a standard pre-access algorithm based on current evidence and guidelines as well as local expertise and abilities.
The following three considerations should be at the center of the planning and creation of a vascular access in each ESRD patient:
- The patient’s current condition (e.g., young, healthy, acute renal failure, no history of CVCs, palpable and easily identifiable veins vs. older, diabetic, chronic kidney disease, long-standing history of recurrent CVCs), before planning the diagnostic pathway.
- Adequate imaging modality for the required vascular access (e.g., ultrasound mapping prior to AV fistula creation in pre-dialysis patients with uneventful history vs. venography in patients with exhausted veins and long-standing history of CVCs in the setting of chronic kidney disease).
- Patient centered choice of the specific access (shared decision making, information of the patient on the pros and cons of all potential access options, considering the patients social and medical background; considering the impact of the vascular access on the patient’s quality of life).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-797/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-797/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. SP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023. SR receives grant by the Swiss Society of Vascular Surgery (SGG) 2020 of 10,000 CHF for conducting a quality of life study in patients with vascular access for haemodialysis. AI received scientific grant by Cantonal Hospital (30,000 CHF) for Compression Ultrasound Study, and scientific grant by SWF (10,000 CHF) for VascuQoL-6 study. AI received payment from Medtronic-Ellipsys pAVF workshop. AI has a participation in Vascular International School. AI is a tutor and is responsible for course organization. DS has a participation on the XATOA and XATOC study (Bayer AG) (payment to the institution). DS received honoraria for lectures from Bauerfeind, Bayer AG, Sanofi-Aventis AG (payment to the institution), honoraria for manuscript writing from Daiichi-Sankyo (payment to the institution). DS has a participation on Advisory Board of Bayer AG (payment to the institution). DS is the President of the union of vascular societies of Switzerland (unpaid). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alfano G, Fontana F, Iannaccone M, et al. Preoperative management of arteriovenous fistula (AVF) for hemodialysis. J Vasc Access 2017;18:451-63.
- Al-Jaishi AA, Liu AR, Lok CE, et al. Complications of the Arteriovenous Fistula: A Systematic Review. J Am Soc Nephrol 2017;28:1839-50. [Crossref] [PubMed]
- Schmidli J, Widmer MK, Basile C, et al. 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55:757-818. [Crossref] [PubMed]
- Access Vascular. 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006;48:S176-247. [PubMed]
- Popli K, Dittman JM, Amendola MF, et al. Anatomic suitability for commercially available percutaneous arteriovenous fistula creation systems. J Vasc Surg 2021;73:999-1004. [Crossref] [PubMed]
- Hyland K, Cohen RM, Kwak A, et al. Preoperative mapping venography in patients who require hemodialysis access: imaging findings and contribution to management. J Vasc Interv Radiol 2008;19:1027-33. [Crossref] [PubMed]
- Fluck R, Kumwenda M. Renal Association Clinical Practice Guideline on vascular access for haemodialysis. Nephron Clin Pract 2011;118:c225-40. [Crossref] [PubMed]
- Murphy EA, Ross RA, Jones RG, et al. Imaging in Vascular Access. Cardiovasc Eng Technol 2017;8:255-72. [Crossref] [PubMed]
- Bode AS, Planken RN, Merkx MA, et al. Feasibility of non-contrast-enhanced magnetic resonance angiography for imaging upper extremity vasculature prior to vascular access creation. Eur J Vasc Endovasc Surg 2012;43:88-94. [Crossref] [PubMed]
- Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007;243:148-57. [Crossref] [PubMed]
- Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21:1104-8. [Crossref] [PubMed]
- de Graaf R, van Laanen J, Peppelenbosch N, et al. The value of intravascular ultrasound in the treatment of central venous obstructions in hemodialysis patients. J Vasc Access 2016;17 Suppl 1:S12-5.
- Asif A, Cherla G, Merrill D, et al. Venous mapping using venography and the risk of radiocontrast-induced nephropathy. Semin Dial 2005;18:239-42. [Crossref] [PubMed]
- Heye S, Maleux G, Marchal GJ. Upper-extremity venography: CO2 versus iodinated contrast material. Radiology 2006;241:291-7. [Crossref] [PubMed]
- Heye S, Maleux G, Claes K, et al. Stenosis detection in native hemodialysis fistulas with MDCT angiography. AJR Am J Roentgenol 2009;192:1079-84. [Crossref] [PubMed]
- Gjesdal KI, Storaas T, Geitung JT. A noncontrast-enhanced pulse sequence optimized to visualize human peripheral vessels. Eur Radiol 2009;19:110-20. [Crossref] [PubMed]
- Planken NR, Tordoir JH, Duijm LE, et al. Magnetic resonance angiographic assessment of upper extremity vessels prior to vascular access surgery: feasibility and accuracy. Eur Radiol 2008;18:158-67. [Crossref] [PubMed]
- Georgiadis GS, Charalampidis DG, Argyriou C, et al. The Necessity for Routine Pre-operative Ultrasound Mapping Before Arteriovenous Fistula Creation: A Meta-analysis. Eur J Vasc Endovasc Surg 2015;49:600-5. [Crossref] [PubMed]
- Mihmanli I, Besirli K, Kurugoglu S, et al. Cephalic vein and hemodialysis fistula: surgeon's observation versus color Doppler ultrasonographic findings. J Ultrasound Med 2001;20:217-22. [Crossref] [PubMed]
- Nursal TZ, Oguzkurt L, Tercan F, et al. Is routine preoperative ultrasonographic mapping for arteriovenous fistula creation necessary in patients with favorable physical examination findings? Results of a randomized controlled trial. World J Surg 2006;30:1100-7. [Crossref] [PubMed]
- Ferring M, Claridge M, Smith SA, et al. Routine preoperative vascular ultrasound improves patency and use of arteriovenous fistulas for hemodialysis: a randomized trial. Clin J Am Soc Nephrol 2010;5:2236-44. [Crossref] [PubMed]
- Smith GE, Barnes R, Chetter IC. Randomized clinical trial of selective versus routine preoperative duplex ultrasound imaging before arteriovenous fistula surgery. Br J Surg 2014;101:469-74. [Crossref] [PubMed]
- Kosa SD, Al-Jaishi AA, Moist L, et al. Preoperative vascular access evaluation for haemodialysis patients. Cochrane Database Syst Rev 2015;CD007013. [Crossref] [PubMed]
- McGill RL, Ruthazer R, Lacson E Jr, et al. Vascular imaging for hemodialysis vascular access planning. Hemodial Int 2017;21:490-7. [Crossref] [PubMed]
- Fedorova E, Zhang GQ, Shireman PK, et al. Association of preoperative vein mapping with hemodialysis access characteristics and outcomes in the Vascular Quality Initiative. J Vasc Surg 2022;75:1395-1402.e5. [Crossref] [PubMed]
- Lok CE, Huber TS, Lee T, et al. 2019 Update. Am J Kidney Dis 2020;75:S1-S164. [Crossref] [PubMed]


