
Atherosclerotic plaque composition and specific endovascular considerations in the end stage renal disease patients: a narrative review
Introduction
Background
Approximately 850 million persons worldwide have chronic kidney disease (CKD), and almost 4 million receive renal replacement therapy. Remodeling of the blood vessels and the myocardium leads to various cardiovascular complications such as atherosclerosis with the consequence of progression of renal disease including end stage renal disease (ESRD) as well as cardiovascular and cerebrovascular death. CKD patients present with a high incidence of cardiovascular morbidity and mortality that cannot be fully explained by traditional cardiovascular risk factors of cardiovascular diseases (CVDs). As such CKD is an established independent risk factor for peripheral arterial disease (PAD) which is distinct from traditional factors such as age, diabetes mellitus, hypertension, hyperuricemia and dyslipidemia (1). Cardiovascular mortality in patients with advanced CKD (stage 4) and end-stage kidney disease (stage 5) accounts for about 40% to 50% of all deaths (2,3). The high prevalence of atherosclerotic lesions and the high degree of vascular calcification (VC) pose specific challenges to endovascular treatment and result in a high number of reinterventions and treatment failure.
Besides high rates of treatment failure of endovascular therapy, CKD patients are at a higher risk of developing additional contrast-induced nephropathy (CIN) resulting in progressive decline of kidney function. Carbon dioxide (CO2) angiography is one option to potentially provide a safer and equally effective alternative to iodine-based contrast media in patients with CKD or iodine-based contrast media allergy (4-7).
Despite the unfavorable prognosis of CKD-associated vascular disease, improvements were made in the recent years. Pharmacological strategies are intended to slow the progression of CVD, and to reduce the CKD-associated morbidity and mortality (8,9). Furthermore, the renal replacement therapies performed today offer increased filtering and purification capacities with simultaneously reduced side effects. Novel endovascular methods like the aggressive use of endoprosthesis and braided nitinol stents pave the way to deal with VCs of CKD-associated PAD (10-12). We present the following article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-53/rc).
Objectives
The objective of this review is to summarize basics regarding atherosclerotic plaque formation, composition and prevalence in patients with CKD and to explain the differences to patients with peripheral atherosclerosis without pre-existing CKD. In addition, specific endovascular techniques available in the setting of extensively calcified vessels and impaired renal function will be discussed.
Methods
A computerized literature search was conducted in PubMed as well as manual search of reference lists for relevant English language articles in conjunction with discussions with experts in the field. The literature search covers publications up to September 2021. The following combinations of keywords were used: Angioplasty, atheromatosis, atherosclerosis, vascular calcification, carbon dioxide angiography, CO2 angiography, cardiovascular disease, chronic kidney disease, CKD, chronic kidney failure, chronic kidney insufficiency, chronic renal failure, chronic renal insufficiency, contrast-induced nephropathy, CIN, contrast induced acute kidney injury, drug-coated balloon, DCB, drug-eluting balloon, DEB, directional atherectomy, endovascular treatment, end stage renal disease, ESRD, endothelial damage, hemodialysis, iodinated-contrast media, inflammation, medial artery calcification, mineral bone disorder, nephropathy, outcome, peripheral arterial disease, peripheral artery disease, peripheral angiography, peritoneal dialysis, revascularization, pave-and-crack technique, uremia, vascular access, vascular calcification, intravascular ultrasound and IVUS. Two authors (i.e., CR and GG) assessed independently the methodological quality of the studies prior to their inclusion in this review. A summary of the search strategy is provided in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 16/09/2021 |
| Databases and other sources searched | PubMed |
| Search terms used | Angioplasty, atheromatosis, atherosclerosis, vascular calcification, carbon dioxide angiography, CO2 angiography, cardiovascular disease, chronic kidney disease, CKD, chronic kidney failure, chronic kidney insufficiency, chronic renal failure, chronic renal insufficiency, contrast-induced nephropathy, CIN, contrast induced acute kidney injury, drug-coated balloon, DCB, drug-eluting balloon, DEB, directional atherectomy, endovascular treatment, end stage renal disease, ESRD, endothelial damage, hemodialysis, iodinated-contrast media, inflammation, medial artery calcification, mineral bone disorder, nephropathy, outcome, peripheral arterial disease, peripheral artery disease, peripheral angiography, peritoneal dialysis, revascularization, pave-and-crack technique, uremia, vascular access, vascular calcification, intravascular ultrasound and IVUS |
| Timeframe | 01/01/1980–16/09/2021 |
| Inclusion and exclusion criteria | inclusion criteria: English language |
| Selection process | Two authors (CR, GG) as well as internal discussion with experts in the field |
CKD, chronic kidney disease; CIN, contrast-induced nephropathy; DCB, drug-coated balloon; DEB, drug-eluting balloon; ESRD, end stage renal disease; IVUS, intravascular ultrasound.
Discussion
Atherosclerotic disease in CKD patients
General considerations of VCs in patients with CKD
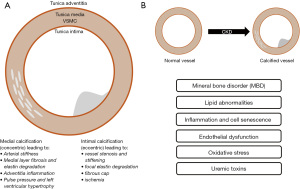
A high prevalence of atherosclerosis in patients with CKD has been documented in numerous autopsy studies and clinical registers (13,14). Development of VC shows similarities in both the onset and progression compared to the bone metabolism. VC in CKD begins early in the development of chronic renal failure and is promoted by various factors such as Ca/Pi (calcium/inorganic phosphate) imbalance, uremic toxins, oxidative stress, chronic and low-grade inflammation as well as activation of various signaling pathways in endothelial and vascular smooth muscle cells (VSMCs), among others (15,16). In particular, VC is associated with elevated cardiovascular risk in CKD patients (17). Oxidative stress is the result of an imbalance between the production and elimination of reactive oxygen species (ROS) in CKD and is considered a hallmark feature of CKD-associated CVDs. Compared to pre-dialysis uremic patients, ESRD patients on both peritoneal dialysis (PD) and hemodialysis (HD) express enhanced oxidative stress (18-20). In CKD patients, calcifications are severe in the media of the vessel wall, which is documented in several clinical and autopsy studies (21,22). Intimal and medial calcification frequently co-exists in CKD patients and their burden is greater than in non-CKD patients. Calcification in large arteries is associated with the presence of atheroma plaques whereas calcification in VSMC rich small arteries is often observed in the Tunica media (23). Mineral bone metabolism derangements and disorder contributes to CKD-related vascular injury and VC (24,25). Causal factors for VC are likely to be the instability of calcium and phosphate ions respectively the limited calcium-phosphate homeostasis due to impaired renal function and the abnormal differentiation of VSMC to osteoblast/chondroblast-like cells.
These findings were clinically confirmed by the results of the NEFRONA study (26,27). This study showed a tendency towards a higher prevalence of plaques in advanced CKD categories. The prevalence of femoral artery plaques, for example, are significantly associated with CKD (28,29). These results suggest that the severity of CKD is an independent factor influencing subclinical atheromatosis (27). Furthermore, the NEFRONA study was able to show that the cause of CKD influences the prevalence of atheromatosis. Diabetic nephropathy, for example, results in a higher risk of subclinical atheromatosis (30). Given the increased incidence of PAD in CKD and its association with increased mortality in dialysis patients, screening for and early diagnosis of PAD is important (31). Figure 1 gives a schematic overview of both the distribution of VCs in CKD patients and factors and mechanisms that potentially contribute to atherosclerosis.
Specific considerations in the treatment of PAD in the ESRD patients
Outcome after revascularization and endovascular treatment in patients with CKD
The outcome after vascular interventions is worse in patients with CKD compared to patients without CKD among others due to a higher incidence of perioperative mortality, limb loss after revascularization and delayed wound healing (32,33). Furthermore, some studies conclude that revascularization outcomes of peripheral vessels are worse in individuals with CKD including ESRD (34-36). In the retrospective study by Meyer et al. including 102 ESRD and 674 non-ESRD patients with critical limb ischemia (CLI), ESRD patients showed significantly worse results at 2 years with regard to amputation-free survival (AFS, ESRD 35.4% vs. non-ESRD, 67.2%), major amputation (ESRD 24.5% vs. non-ESRD 15.8%) and death (ESRD 55.0% vs. non-ESRD, 20.7%) (35).
To date, there are no prospective data to help select CKD patients for revascularization. However, a study found that subcohorts of CKD patients without extensive tissue necrosis or uncontrolled infection may benefit from revascularization, particularly in the ambulatory setting (37). On the other hand, worse outcomes are described in dialysis patients and, therefore, careful consideration and patient selection is mandatory. However, there are only few alternative management strategies for patients with CKD and symptomatic PAD. Endovascular therapy nowadays is a minimally invasive therapy that can be pursued with moderate sedation [fentanyl and versed (midazolam)] and does not require general anesthesia.
Interventional therapy always should be accompanied by aggressive medical therapy. The COMPASS and VOYAGER PAD trial demonstrated that the combination of antiplatelet and antithrombotic medications (including rivaroxaban) improve the vascular outcome. Furthermore, hypercholesteremia should be controlled with statins and/or PCSK9 inhibitors and more aggressive control of diabetes mellitus should be considered with GLP-1 and/or SGLT2 inhibitors (8,9).
Influence of calcium burden on interventional treatment options of PAD
The calcium burden represents one of the most widely accepted predictors of failure of endovascular treatment of PAD (38). The PACSS score is probably the most widely used DSA and high-intensity fluoroscopy based system to score the amount and distribution of intimal and medial vessel wall calcification at the target lesion (39). In the case of heavily calcified arteries, technical success and patency rates are lower (40-43). The mechanism of angioplasty [percutaneous transluminal angioplasty (PTA)] is based on enlarging the lumen by vessel wall stretching and plaque fracture. The effect of both angioplasty and stenting is limited by the alteration of the morphology and the compliance of the vessel wall caused by a high calcium burden (44). Flow limiting dissections after PTA and acute vessel recoil is thereby increased as a result (45). For pure vessel recoil, drug-coated balloons (DCB) can be considered. The risk of stent malposition, subexpansion and fractures is also increased by a high calcium burden (42,43,46). A large number of studies have been carried out using different devices to overcome these difficulties. Strategies included the use of atherectomy devices, scoring balloons or cutting balloons with the aim to improve angiographic results and patency rates. However, the reported patency rates vary considerably between studies (41,46-49).
Using scoring-balloons is that the entire force is focused on a wire or blade edge mounted on the balloon which allows a controlled plaque incision or dissection. In bifurcation and ostial lesions, scoring or cutting balloons may be the first consideration with the idea of minimizing an expected plaque shift. In the study by Kronlage et al., it was shown that scoring balloon angioplasty did, however, not significantly improve vessel patency—both when used as an adjunctive in preparation for stenting and as stand-alone treatment (50).
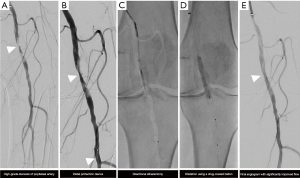
DCBs represent very promising devices for the treatment of femoro-popliteal and below-the-knee lesions (38,51-54). Paclitaxel interferes with microtubule interactions during mitosis, effectively halting mitosis and ultimately resulting in apoptosis, reducing the rate of neointimal hyperplasia. However, in order for paclitaxel to exert this effect, a deep penetration of the drug into the media layers of the vessel wall is required (38). VC may counteract this effect and the efficacy of DCB. Fanelli et al. could demonstrate that these are particularly limited only in completely circularly calcified arteries (38). As such strategies of vessel preparation are recommended to overcome the barrier set by the VC. Besides the application of scoring balloon angioplasty to crack the calcified plaques atherectomy has been proposed as a tool for vessel preparation. In this concept atherectomy and antirestenotic therapy with DCBs is combined to achieve synergistic effects. Atherectomy is expected prepare the vessel for drug delivery and subsequent absorption of the paclitaxel into the vessel wall combined with debulking and enhancement of vessel compliance (10,46,55). Figure 2 shows a case of a CKD patient with high-grade stenosis of the popliteal artery where this technique has been used. The prospective, multicenter and randomized controlled DEFINITIVE AR study investigated the safety and the effect of treating vessels with directional atherectomy (DA) before DCB angioplasty (DA + DCB) as compared with DCB angioplasty alone (10). This study with up to 15% CKD patients in each arm showed that even in the non-randomized arm with heavy calcifications the strategy of directional atherectomy and antirestenotic therapy (DAART) was feasible and effective. Calcification burden and lesion length >10 cm were identified as potential predictors for superior outcomes for the combination therapy. These results were confirmed by the VIVA REALITY study which assessed the safety and efficiency of a vessel preparation strategy with DA prior to DCB angioplasty in patients with symptomatic severely calcified femoropopliteal PAD (56). However, in both studies the subgroups with up to 15% CKD patients were not separately evaluated. DA is usually non-feasible in extensive calcium load [grade 4 according to the Peripheral Arterial Calcium Scoring System (PACSS score)] combined with long lesion lengths.
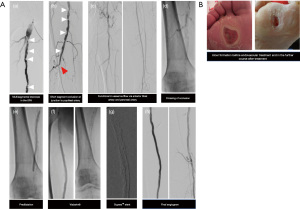
In order to overcome these severe calcifications, specific techniques were developed. One strategy is to exclude the vascular calcium by using long stent-grafts. Hinchliffe et al. described the more aggressive “pave-and-crack” technique to facilitate the introduction of aortic stent-grafts through stenosed iliac arteries (57). In femoro-popliteal arteries, this technique is used to achieve luminal gain by lining these vessels with a covered stent and subsequent aggressive balloon dilation to. Dias-Neto et al. provide a description of a version of this technique in the setting of femoropopliteal disease (11). Hereby, aggressive pre-dilatation is followed by implantation of nitinol-woven SuperaTM stents (Abbott Vascular, Santa Clara, CA, USA). Dias-Neto et al. described the use of SuperaTM stents after implantation of a Viabahn® stent-graft (Gore Medical, W. L. Gore & Associates Inc., Newark, Delaware, USA) which provides a mechanical barrier to prevent rupture and to facilitate exaggerated balloon dilatation. The interwoven nitinol stent is used to overcome elastic recoil and maintain flexibility. In this study, a 12-month primary patency rate of 79% was achieved which is in line or even superior compared to studies using Viabahn® alone in long calcified lesions (72% to 75%) (58-61). In their study, more than half of the cohort of patients with extreme calcium load suffered from CKD. Figure 3 illustrates a case of a patient with CKD who underwent endovascular therapy using the “pave-and-crack” technique.
Iodine-based contrast media in CKD
Patients with pre-existing CKD are especially vulnerable to the risk of renal adverse events when using iodine-based contrast media. The exact definition of the high-risk population as well as the appropriate scheme of renal protective medication before application of iodine-based contrast media are subjects of debate and research. The highest risk of iodine-based contrast media administration exists when the estimated glomerular filtration rate (eGFR) is less than 30 mL/min/1.73 m2 (62-65). With eGFR of 30 mL/min/1.73 m2 or greater, studies found no benefit of prophylactic i.v. fluids over no prophylaxis for CKD patients (64,66-68). Therefore, prophylactic i.v. fluids are recommended in current guidelines for patients with acute kidney injury (AKI) or with CKD with eGFR less than 30 mL/min/1.73 m2 who are not undergoing maintenance dialysis (69). These patients represent 0.1% to 0.5% of the general population and 0.05% to 1.8% of patients undergoing elective contrast procedures (70-73).
CIN is an AKI which might lead to a permanent loss of renal function. Cardiovascular interventional studies in CDK patients observed CIN in up to 15% of patients and 40–50% of patients at high risk for contrast-induced AKI (preexisting eGFR less than 30 mL/min/1.73 m2) (74,75).
Compared to the intra-arterial administration of iodine-based contrast media, the intravenous injection might reduce AKI by avoiding microembolization and by reducing peak contrast media concentration in the renal arteries (76-79). The randomized, so called Coronary Artery Disease Management study (CAD-MAN) compared the procedural complications of cardiac catheterization (intra-arterial) to CT angiography (intravenous) in patients with low-to-intermediate pre-test probability for obstructive coronary artery disease (CAD) (80). After intra-arterial contrast media administration, both AKI (13.2% vs. 5.6%) and CKD in patients with postcontrast AKI (12-fold greater risk) were more likely compared to intravenous administration (81). The non-randomized study by Moore et al. also showed a higher incidence of AKI after invasive cardiac catheterization with intra-arterially injected contrast media compared to CT with intravenous administration (4.4% vs. 1.2%). These results are in line with large observational studies showing a higher probability of AKI after intra-arterial than after intravenous contrast media administration (14.4% vs. 6.4%) (82-84). In contrast, a paired cohort study found only minor, non-significant differences between intravenously and intra-arterially administered iodine-based contrast media regarding AKI frequencies (9.9% vs. 11%) (85). Comparable results were reported by Karlsberg et al. for the intraindividual comparison between peripheral CT angiography and peripheral angiography (7.6% vs. 8.7%, P=0.641) (86).
In clinical routine, different strategies are used to prevent CIN. However, the level of evidence supporting these renal protective strategies is low. A randomized controlled study comparing different strategies of prophylactic hydration in patients at high risk of CIN found that none of the strategies (oral hydration vs. intravenous hydration) to be non-inferior in preventing CIN (67,87). Another study by Weisbord et al. showed that among patients at high risk for renal complications, there was no additional benefit of oral acetylcysteine, intravenous sodium bicarbonate or intravenous sodium chloride compared to placebo concerning the persistent decline in kidney function, need for dialysis and the prevention of CI-AKI (88). The PRESERVE trial as the largest study including 5,177 patients with high risk for renal complications examined the effect of intravenous sodium bicarbonate, intravenous sodium chloride, oral acetylcysteine or placebo and could also not detect any significant differences between the study groups (88-90).
The European Society of Urogenital Radiology (ESUR) differentiates between patient-related and procedure-related risk factors for AKI. Patient-related risk factors include an eGFR less than 45 mL/min/1.73 m2 regarding intra-arterially administered iodine-based contrast media with first pass renal exposure and an eGFR less than 30 mL/min/1.73 m2 regarding intravenously or intra-arterially administered contrast media with second pass renal exposure. According to the ESUR, 75% of injected iodine-based contrast media is excreted within the following 4 hours in the presence of normal or moderately reduced renal function (GFR >30 mL/min/1.73 m2). Therefore, a timeframe of 4 hours between injections of iodine-based contrast media should be respected. In the presence of severely reduced renal function (GFR <30 mL/min/1.73 m2), repeated administration of iodine-based contrast media should be performed with at least 48 hours in-between. If patients are anuric and on dialysis in case of ESRD, repetitive iodine injections can be performed as long as the fluid status is closely monitored clinically. Iodine- and gadolinium-based contrast media can be removed by HD or peritoneal dialysis. There is, however, no evidence that HD prevents from post contrast AKI or nephrogenic systemic fibrosis (NSF) (91).
Endovascular therapy guided by intravascular ultrasound (IVUS)
IVUS guidance is a modern possibility to guide peripheral vascular interventions. However, the benefit of IVUS including patient outcome has been mostly examined in cardiac interventions.
Data for the use of IVUS in peripheral interventions is still sparse. However, there are some data showing that IVUS may improve endovascular procedures such as the detection rate of significant vascular lesions and the extent of dissection after angioplasty (PTA) (92,93). Two studies by Kawasaki et al. proposed that IVUS may reduce the amount of iodine-based contrast media used for endovascular procedures (94,95).
Endovascular therapy guided by CO2 angiography
Lowering the risk of CIN through minimizing the use of iodine-based contrast media during endovascular interventions is challenging when optimal diagnostic imaging and angiographic guidance should be maintained. Hawkins pioneered the intra-arterial application of CO2 angiography for high-risk patients in the 1970s (96). Current studies show that CO2 angiography may provide an effective and safe alternative to iodine-based contrast media in CKD patients or patients with allergy to iodine-based contrast media (4,97,98). CO2 provides a negative contrast image with digital subtraction angiography by displacing blood. CO2 angiography, however, has not been standardized yet. Specialist equipment is required in order to ensure safe delivery of CO2. The safety volume for CO2 injections is still not clarified and varies between studies. Diamantopoulos et al. limited the amount of total gas volume per time interval to no more than 100 cc of CO2 over a 2-minute interval (4). The inferior image quality of CO2 angiography is due to the lower viscosity of CO2 compared to iodine-based contrast media and due to possible motion artefacts from transient discomfort during injection by the expansion of the gas when it exits the catheter (5). In the study by Madhusudhan et al., severe and mild leg pain occurred in 4.8% respectively 28.6% of patients during the CO2 injection and 18% cent of examinations needed to be discontinued in case of high flow rates and volumes (99).
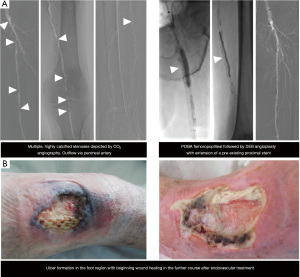
Despite CO2 being regarded as a kidney protective alternative to iodine-based contrast media, a recent meta-analysis comparing the incidence of AKI in angiography with CO2 versus iodine-based contrast media could show that its use is only associated with a modestly reduced rate of AKI of 6.2% (6). Other factors such as thromboembolic events during interventional procedures may, therefore, also contribute to renal impairment following peripheral angiography. Figure 4 illustrates a case of endovascular therapy guided by CO2 angiography.
Future implications and limitations of the review
Patients with severely impaired renal function are an underrepresented minority in many studies on endovascular techniques. The quality of data considering specific endovascular treatment options like CO2 angiography, is still one of the major limitations in patients with CDK. Therefore, more randomized trials would be an essential step to improve quality of data in the context of endovascular therapy with accompanying medical treatment for patients with impaired renal function.
Summary
CKD patients have a high cardiovascular-related risk with regard to morbidity and mortality. Onset and progression of VC begin early in the development of CKD and are promoted by various factors. VC in large arteries is associated with the presence of atheroma plaques whereas calcification in VSMC rich small arteries is often observed in the Tunica media. The high prevalence of atherosclerotic lesions and the rigidity of severe VCs in CKD patients with CVDs cause problems in endovascular therapy in the medium and long term despite new technologies and products. CKD patients are at risk of developing CIN. Despite limited evidence for specific renal protective measures, any action that may help reduce the risk of CIN should be considered. IVUS may reduce the amount of iodine-based contrast media used for endovascular procedures and CO2 angiography may provide an effective and safe alternative to iodine-based contrast media in selected patients with CKD. Given the limited outcomes in CKD patients following peripheral vascular interventions, aggressive approaches like the use of endoprosthesis or stents with high radial force are indicated. Additionally, vascular patients with CKD may benefit from a more aggressive medical management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sasan Partovi and Lee Kirksey) for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-53/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-53/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jankowski J, Floege J, Fliser D, et al. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021;143:1157-72. [Crossref] [PubMed]
- Thompson S, James M, Wiebe N, et al. Cause of Death in Patients with Reduced Kidney Function. J Am Soc Nephrol 2015;26:2504-11. [Crossref] [PubMed]
- Webster AC, Nagler EV, Morton RL, et al. Chronic Kidney Disease. Lancet 2017;389:1238-52. [Crossref] [PubMed]
- Diamantopoulos A, Patrone L, Santonocito S, et al. Carbon dioxide angiography during peripheral angioplasty procedures significantly reduces the risk of contrast-induced nephropathy in patients with chronic kidney disease. CVIR Endovasc 2020;3:9. [Crossref] [PubMed]
- Gupta A, Dosekun AK, Kumar V. Carbon dioxide-angiography for patients with peripheral arterial disease at risk of contrast-induced nephropathy. World J Cardiol 2020;12:76-90. [Crossref] [PubMed]
- Ghumman SS, Weinerman J, Khan A, et al. Contrast induced-acute kidney injury following peripheral angiography with carbon dioxide versus iodinated contrast media: A meta-analysis and systematic review of current literature. Catheter Cardiovasc Interv 2017;90:437-48. [Crossref] [PubMed]
- Sharafuddin MJ, Marjan AE. Current status of carbon dioxide angiography. J Vasc Surg 2017;66:618-37. [Crossref] [PubMed]
- Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219-29. [Crossref] [PubMed]
- Capell WH, Bonaca MP, Nehler MR, et al. Rationale and design for the Vascular Outcomes study of ASA along with rivaroxaban in endovascular or surgical limb revascularization for peripheral artery disease (VOYAGER PAD). Am Heart J 2018;199:83-91. [Crossref] [PubMed]
- Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional Atherectomy Followed by a Paclitaxel-Coated Balloon to Inhibit Restenosis and Maintain Vessel Patency: Twelve-Month Results of the DEFINITIVE AR Study. Circ Cardiovasc Interv 2017;10:e004848. [Crossref] [PubMed]
- Dias-Neto M, Matschuck M, Bausback Y, et al. Endovascular Treatment of Severely Calcified Femoropopliteal Lesions Using the "Pave-and-Crack" Technique: Technical Description and 12-Month Results. J Endovasc Ther 2018;25:334-42. [Crossref] [PubMed]
- Garcia L, Jaff MR, Metzger C, et al. Wire-Interwoven Nitinol Stent Outcome in the Superficial Femoral and Proximal Popliteal Arteries: Twelve-Month Results of the SUPERB Trial. Circ Cardiovasc Interv 2015;8:e000937. [Crossref] [PubMed]
- Clyne N, Lins LE, Pehrsson SK. Occurrence and significance of heart disease in uraemia. An autopsy study. Scand J Urol Nephrol 1986;20:307-11. [Crossref] [PubMed]
- Johansen KL, Chertow GM, Foley RN, et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2021;77:A7-8. [Crossref] [PubMed]
- Ramirez R, Carracedo J, Berdud I, et al. Microinflammation in hemodialysis is related to a preactivated subset of monocytes. Hemodial Int 2006;10:S24-7. [Crossref] [PubMed]
- Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol 2009;24:1445-52. [Crossref] [PubMed]
- London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol 2001;12:2759-67. [Crossref] [PubMed]
- Liakopoulos V, Roumeliotis S, Gorny X, et al. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxid Med Cell Longev 2017;2017:3494867. [Crossref] [PubMed]
- Liakopoulos V, Roumeliotis S, Zarogiannis S, et al. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial 2019;32:58-71. [Crossref] [PubMed]
- Liakopoulos V, Roumeliotis S, Bozikas A, et al. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxid Med Cell Longev. 2019;2019:9109473. [Crossref] [PubMed]
- Schwarz U, Buzello M, Ritz E, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 2000;15:218-23. [Crossref] [PubMed]
- Moe SM, O'Neill KD, Duan D, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 2002;61:638-47. [Crossref] [PubMed]
- Nelson AJ, Raggi P, Wolf M, et al. Targeting Vascular Calcification in Chronic Kidney Disease. JACC Basic Transl Sci 2020;5:398-412. [Crossref] [PubMed]
- Valdivielso JM, Rodríguez-Puyol D, Pascual J, et al. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler Thromb Vasc Biol 2019;39:1938-66. [Crossref] [PubMed]
- Staude H, Jeske S, Schmitz K, et al. Cardiovascular risk and mineral bone disorder in patients with chronic kidney disease. Kidney Blood Press Res 2013;37:68-83. [Crossref] [PubMed]
- Arroyo D, Betriu A, Martinez-Alonso M, et al. Observational multicenter study to evaluate the prevalence and prognosis of subclinical atheromatosis in a Spanish chronic kidney disease cohort: baseline data from the NEFRONA study. BMC Nephrol 2014;15:168. [Crossref] [PubMed]
- Betriu A, Martinez-Alonso M, Arcidiacono MV, et al. Prevalence of subclinical atheromatosis and associated risk factors in chronic kidney disease: the NEFRONA study. Nephrol Dial Transplant 2014;29:1415-22. [Crossref] [PubMed]
- Hsu S, Rifkin DE, Criqui MH, et al. Relationship of femoral artery ultrasound measures of atherosclerosis with chronic kidney disease. J Vasc Surg 2018;67:1855-1863.e1. [Crossref] [PubMed]
- Nezami N, Ghabili K, Shokouhi-Gogani B, et al. The Relationship between Carotid and Femoral Artery Intima-Media Thickness and Histopathologic Grade of Atherosclerosis in Patients with Chronic Kidney Disease. Nephron 2018;139:159-69. [Crossref] [PubMed]
- Barrios C, Pascual J, Otero S, et al. Diabetic nephropathy is an independent factor associated to severe subclinical atheromatous disease. Atherosclerosis 2015;242:37-44. [Crossref] [PubMed]
- Lok CE, Huber TS, Lee T, et al. 2019 Update. Am J Kidney Dis 2020;75:S1-S164. [Crossref] [PubMed]
- DeLoach SS, Mohler ER 3rd. Peripheral arterial disease: a guide for nephrologists. Clin J Am Soc Nephrol 2007;2:839-46. [Crossref] [PubMed]
- Hopley CW, Kavanagh S, Patel MR, et al. Chronic kidney disease and risk for cardiovascular and limb outcomes in patients with symptomatic peripheral artery disease: The EUCLID trial. Vasc Med 2019;24:422-30. [Crossref] [PubMed]
- Kim HO, Kim JM, Woo JS, et al. Effects of chronic kidney disease on clinical outcomes in patients with peripheral artery disease undergoing endovascular treatment: Analysis from the K-VIS ELLA registry. Int J Cardiol 2018;262:32-7. [Crossref] [PubMed]
- Meyer A, Fiessler C, Stavroulakis K, et al. Outcomes of dialysis patients with critical limb ischemia after revascularization compared with patients with normal renal function. J Vasc Surg 2018;68:822-829.e1. [Crossref] [PubMed]
- Negishi Y, Tanaka A, Ishii H, et al. Contrast-Induced Nephropathy and Long-Term Clinical Outcomes Following Percutaneous Coronary Intervention in Patients With Advanced Renal Dysfunction (Estimated Glomerular Filtration Rate <30 ml/min/1.73 m2). Am J Cardiol 2019;123:361-7. [Crossref] [PubMed]
- Jaar BG, Astor BC, Berns JS, et al. Predictors of amputation and survival following lower extremity revascularization in hemodialysis patients. Kidney Int 2004;65:613-20. [Crossref] [PubMed]
- Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol 2014;37:898-907. [Crossref] [PubMed]
- Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv 2014;83:E212-20. [Crossref] [PubMed]
- Schillinger M, Minar E. Percutaneous treatment of peripheral artery disease: novel techniques. Circulation 2012;126:2433-40. [Crossref] [PubMed]
- Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther 2012;19:480-8. [Crossref] [PubMed]
- Adlakha S, Sheikh M, Wu J, et al. Stent fracture in the coronary and peripheral arteries. J Interv Cardiol 2010;23:411-9. [Crossref] [PubMed]
- Halwani DO, Anderson PG, Brott BC, et al. The role of vascular calcification in inducing fatigue and fracture of coronary stents. J Biomed Mater Res B Appl Biomater 2012;100:292-304. [Crossref] [PubMed]
- Kashyap VS, Pavkov ML, Bishop PD, et al. Angiography underestimates peripheral atherosclerosis: lumenography revisited. J Endovasc Ther 2008;15:117-25. [Crossref] [PubMed]
- Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation 1992;86:64-70. [Crossref] [PubMed]
- Cioppa A, Stabile E, Popusoi G, et al. Combined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: One-year single centre clinical results. Cardiovasc Revasc Med 2012;13:219-23. [Crossref] [PubMed]
- Amighi J, Schillinger M, Dick P, et al. De novo superficial femoropopliteal artery lesions: peripheral cutting balloon angioplasty and restenosis rates--randomized controlled trial. Radiology 2008;247:267-72. [Crossref] [PubMed]
- Banerjee S, Das TS, Abu-Fadel MS, et al. Pilot trial of cryoplasty or conventional balloon post-dilation of nitinol stents for revascularization of peripheral arterial segments: the COBRA trial. J Am Coll Cardiol 2012;60:1352-9. [Crossref] [PubMed]
- Scheinert D, Peeters P, Bosiers M, et al. Results of the multicenter first-in-man study of a novel scoring balloon catheter for the treatment of infra-popliteal peripheral arterial disease. Catheter Cardiovasc Interv 2007;70:1034-9. [Crossref] [PubMed]
- Kronlage M, Werner C, Dufner M, et al. Long-term outcome upon treatment of calcified lesions of the lower limb using scoring angioplasty balloon (AngioSculpt™). Clin Res Cardiol 2020;109:1177-85. [Crossref] [PubMed]
- Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358:689-99. [Crossref] [PubMed]
- Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 2008;118:1358-65. [Crossref] [PubMed]
- Fanelli F, Cannavale A, Corona M, et al. The "DEBELLUM"--lower limb multilevel treatment with drug eluting balloon--randomized trial: 1-year results. J Cardiovasc Surg (Torino) 2014;55:207-16. [PubMed]
- Micari A, Cioppa A, Vadalà G, et al. Clinical evaluation of a paclitaxel-eluting balloon for treatment of femoropopliteal arterial disease: 12-month results from a multicenter Italian registry. JACC Cardiovasc Interv 2012;5:331-8. [Crossref] [PubMed]
- Lichtenberg M, Korosoglou G. Atherectomy plus antirestenotic therapy for SFA lesions: evolving evidence for better patency rates in complex lesions. J Cardiovasc Surg (Torino) 2019;60:205-11. [Crossref] [PubMed]
- Rocha-Singh KJ, Sachar R, DeRubertis BG, et al. Directional atherectomy before paclitaxel coated balloon angioplasty in complex femoropopliteal disease: The VIVA REALITY study. Catheter Cardiovasc Interv 2021;98:549-58. [Crossref] [PubMed]
- Hinchliffe RJ, Ivancev K, Sonesson B, et al. "Paving and cracking": an endovascular technique to facilitate the introduction of aortic stent-grafts through stenosed iliac arteries. J Endovasc Ther 2007;14:630-3. [Crossref] [PubMed]
- Mohr PJ, Oyama JK, Luu JT, et al. Clinical outcomes of endovascular treatment of TASC-II C and D femoropopliteal lesions with the Viabahn endoprosthesis. Cardiovasc Revasc Med 2015;16:465-8. [Crossref] [PubMed]
- Geraghty PJ, Mewissen MW, Jaff MR, et al. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg 2013;58:386-95.e4. [Crossref] [PubMed]
- Kruse RR, Poelmann FB, Doomernik D, et al. Five-Year Outcome of Self-Expanding Covered Stents for Superficial Femoral Artery Occlusive Disease and an Analysis of Factors Predicting Failure. J Endovasc Ther 2015;22:855-61. [Crossref] [PubMed]
- Lammer J, Zeller T, Hausegger KA, et al. Sustained benefit at 2 years for covered stents versus bare-metal stents in long SFA lesions: the VIASTAR trial. Cardiovasc Intervent Radiol 2015;38:25-32. [Crossref] [PubMed]
- Davenport MS, Khalatbari S, Cohan RH, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013;268:719-28. [Crossref] [PubMed]
- Davenport MS, Khalatbari S, Dillman JR, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology 2013;267:94-105. [Crossref] [PubMed]
- McDonald JS, McDonald RJ, Lieske JC, et al. Risk of Acute Kidney Injury, Dialysis, and Mortality in Patients With Chronic Kidney Disease After Intravenous Contrast Material Exposure. Mayo Clin Proc 2015;90:1046-53. [Crossref] [PubMed]
- McDonald JS, Leake CB, McDonald RJ, et al. Acute Kidney Injury After Intravenous Versus Intra-Arterial Contrast Material Administration in a Paired Cohort. Invest Radiol 2016;51:804-9. [Crossref] [PubMed]
- Martin-Moreno PL, Varo N, Martínez-Ansó E, et al. Comparison of Intravenous and Oral Hydration in the Prevention of Contrast-Induced Acute Kidney Injury in Low-Risk Patients: A Randomized Trial. Nephron 2015;131:51-8. [Crossref] [PubMed]
- Nijssen EC, Nelemans PJ, Rennenberg RJ, et al. Prophylactic Intravenous Hydration to Protect Renal Function From Intravascular Iodinated Contrast Material (AMACING): Long-term Results of a Prospective, Randomised, Controlled Trial. EClinicalMedicine 2018;4-5:109-16. [Crossref] [PubMed]
- Nijssen EC, Nelemans PJ, Rennenberg RJ, et al. Prophylaxis in High-Risk Patients With eGFR < 30 mL/min/1.73 m2: Get the Balance Right. Invest Radiol 2019;54:580-8. [Crossref] [PubMed]
- Davenport MS, Perazella MA, Yee J, et al. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020;294:660-8. [Crossref] [PubMed]
- Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1-12. [Crossref] [PubMed]
- de Jong PE, van der Velde M, Gansevoort RT, et al. Screening for chronic kidney disease: where does Europe go? Clin J Am Soc Nephrol 2008;3:616-23. [Crossref] [PubMed]
- From AM, Bartholmai BJ, Williams AW, et al. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc 2008;83:1095-100. [Crossref] [PubMed]
- Hill NR, Fatoba ST, Oke JL, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0158765. [Crossref] [PubMed]
- Rashid ST, Salman M, Myint F, et al. Prevention of contrast-induced nephropathy in vascular patients undergoing angiography: a randomized controlled trial of intravenous N-acetylcysteine. J Vasc Surg 2004;40:1136-41. [Crossref] [PubMed]
- Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation 2006;113:1799-806. [Crossref] [PubMed]
- Azzalini L, Candilio L, McCullough PA, et al. Current Risk of Contrast-Induced Acute Kidney Injury After Coronary Angiography and Intervention: A Reappraisal of the Literature. Can J Cardiol 2017;33:1225-8. [Crossref] [PubMed]
- Katzberg RW, Barrett BJ. Risk of iodinated contrast material--induced nephropathy with intravenous administration. Radiology 2007;243:622-8. [Crossref] [PubMed]
- Keeley EC, Grines CL. Scraping of aortic debris by coronary guiding catheters: a prospective evaluation of 1,000 cases. J Am Coll Cardiol 1998;32:1861-5. [Crossref] [PubMed]
- Gutierrez NM, Newhouse JH. Maximum Arterial Contrast Concentrations With Computed Tomography and Left Ventriculography: Implications for Contrast Nephrotoxicity Risk. J Comput Assist Tomogr 2017;41:976-82. [Crossref] [PubMed]
- Dewey M, Rief M, Martus P, et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ 2016;355:i5441. [Crossref] [PubMed]
- Schönenberger E, Martus P, Bosserdt M, et al. Kidney Injury after Intravenous versus Intra-arterial Contrast Agent in Patients Suspected of Having Coronary Artery Disease: A Randomized Trial. Radiology 2019;292:664-72. [Crossref] [PubMed]
- Moore RD, Steinberg EP, Powe NR, et al. Nephrotoxicity of high-osmolality versus low-osmolality contrast media: randomized clinical trial. Radiology 1992;182:649-55. [Crossref] [PubMed]
- Brown JR, MacKenzie TA, Maddox TM, et al. Acute Kidney Injury Risk Prediction in Patients Undergoing Coronary Angiography in a National Veterans Health Administration Cohort With External Validation. J Am Heart Assoc 2015;4:002136. [Crossref] [PubMed]
- Kooiman J, Pasha SM, Zondag W, et al. Meta-analysis: serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol 2012;81:2554-61. [Crossref] [PubMed]
- McDonald JS, Katzberg RW, McDonald RJ, et al. Is the Presence of a Solitary Kidney an Independent Risk Factor for Acute Kidney Injury after Contrast-enhanced CT? Radiology 2016;278:74-81. [Crossref] [PubMed]
- Karlsberg RP, Dohad SY, Sheng R, et al. Contrast medium-induced acute kidney injury: comparison of intravenous and intraarterial administration of iodinated contrast medium. J Vasc Interv Radiol 2011;22:1159-65. [Crossref] [PubMed]
- Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet 2017;389:1312-22. [Crossref] [PubMed]
- Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med 2018;378:603-14. [Crossref] [PubMed]
- Weisbord SD, Gallagher M, Kaufman J, et al. Prevention of contrast-induced AKI: a review of published trials and the design of the prevention of serious adverse events following angiography (PRESERVE) trial. Clin J Am Soc Nephrol 2013;8:1618-31. [Crossref] [PubMed]
- Partovi S, Trischman T, Kang PS. Lessons learned from the PRESERVE trial. Br J Radiol 2018;91:20180092. [Crossref] [PubMed]
- Kawashima S, Takano H, Iino Y, et al. Prophylactic hemodialysis does not prevent contrast-induced nephropathy after cardiac catheterization in patients with chronic renal insufficiency. Circ J 2006;70:553-8. [Crossref] [PubMed]
- Loffroy R, Falvo N, Galland C, et al. Intravascular Ultrasound in the Endovascular Treatment of Patients With Peripheral Arterial Disease: Current Role and Future Perspectives. Front Cardiovasc Med 2020;7:551861. [Crossref] [PubMed]
- Li X, D'Amico G, Quintini C, et al. Intravascular ultrasound in the diagnosis and treatment of central venous diseases. Vasa 2021;50:2-10. [Crossref] [PubMed]
- Kawasaki D, Tsujino T, Fujii K, et al. Novel use of ultrasound guidance for recanalization of iliac, femoral, and popliteal arteries. Catheter Cardiovasc Interv 2008;71:727-33. [Crossref] [PubMed]
- Kawasaki D, Fujii K, Fukunaga M, et al. Preprocedural evaluation and endovascular treatment of iliofemoral artery disease without contrast media for patients with pre-existing renal insufficiency. Circ J 2011;75:179-84. [Crossref] [PubMed]
- Hawkins IF. Carbon dioxide digital subtraction arteriography. AJR Am J Roentgenol 1982;139:19-24. [Crossref] [PubMed]
- Fujihara M, Kawasaki D, Shintani Y, et al. Endovascular therapy by CO2 angiography to prevent contrast-induced nephropathy in patients with chronic kidney disease: a prospective multicenter trial of CO2 angiography registry. Catheter Cardiovasc Interv 2015;85:870-7. [Crossref] [PubMed]
- Kawasaki D, Fujii K, Fukunaga M, et al. Safety and efficacy of endovascular therapy with a simple homemade carbon dioxide delivery system in patients with ileofemoral artery diseases. Circ J 2012;76:1722-8. [Crossref] [PubMed]
- Madhusudhan KS, Sharma S, Srivastava DN, et al. Comparison of intra-arterial digital subtraction angiography using carbon dioxide by 'home made' delivery system and conventional iodinated contrast media in the evaluation of peripheral arterial occlusive disease of the lower limbs. J Med Imaging Radiat Oncol 2009;53:40-9. [Crossref] [PubMed]


