
The role of hemodialysis access duplex ultrasound for evaluation of patency and access surveillance
Introduction
The prevalence of end stage renal disease (ESRD) in the United States had tripled between 1990 and 2018 rising to 2,382 per million, with up to 1,685 per million population requiring dialysis treatment (1). Previous vascular access creation guidelines had encouraged a strategy of initially pursuing autologous vascular access, i.e., “fistula first”. This approach was supported by a belief that autologous fistula has relative patency benefits compared with prosthetic bridge graft. These dogmas have fallen into a question recently as more evidence emerges that dialysis fistula are associated with high rates of non-maturation and prolonged central venous catheter (CVC) dependency. For this reason, the most recent guidelines suggest that a patient centered approach described as the End Stage Kidney Disease Life Plan may be the best strategy (2). The ultimate clinical goal is to maximize the uninterrupted use of the vascular access circuit. Mitigating access circuit complications reduces patient morbidity and overall health care expenditure, also improved quality of life. Monitoring and surveillance programs are essential for early detection of vascular access dysfunction and to maintain adequate dialysis circuit clearance and avoid access circuit thrombosis.
Monitoring for signs of access system dysfunction by physical examination of both arteriovenous fistula (AVF) and arteriovenous graft (AVG) is strongly recommended by Kidney Foundation Dialysis Outcomes Quality Initiative (KDOQI) (3). Clinical monitoring is done by physical exam through inspection, palpation and auscultation. Other modalities of monitoring include laboratory studies in the dialysis unit; (I) measuring dialysis adequacy by urea reduction ratio or Kt/V; (II) monitoring for difficulty cannulation and prolonged bleeding after needle withdrawal; (III) AV access flow measurement by ultrasound dilution method, dynamic and static venous pressure, and imaging for stenosis (3).
While there is insufficient evidence to recommend routine surveillance imaging of the vascular access circuit, the dialysis access duplex ultrasound has two potential roles: (I) a diagnostic adjunct complimenting clinical findings and physical examination in the patient with fistula dysfunction, especially to distinguish cannulation issues from “flow” related issues; (II) the postoperative evaluation of arteriovenous (AV) access to assess maturation, volume flow, adequacy of access circuit for use and to establish a baseline function for a relative comparison over time. Ultrasound may also provide the diagnosis of the source of dysfunction by direct visualization of sites of stenosis or concomitant flow measurements, although the yield of ultrasound for detection of central venous stenosis is limited (4,5).
Surveillance
Routine screening with ultrasonography to assess maturation or complication is not currently a standard practice. There is evidence to suggest that selective and clinically driven ultrasound use, when performed for signs or objective findings of dysfunction, may provide a patency benefit in AVF but probably not AVG (4). Routine ultrasound screening of AVFs early after placement, when combined with clinical inspection, helps to identify significant vascular stenosis in the absence of physical exam findings and may reduce non-maturation rates (4).
Prospective and retrospective trial comparing performance of ultrasound in detecting significant stenosis and measuring access blood flow by Tessitore et al. revealed that duplex ultrasound parameters of luminal diameter less than 2 mm and/or a downstream peak systolic velocity (PSV) greater than 400 cm/s have a good accuracy of 85% and a positive predictive value (PPV) of 93% for angiography-proven significant stenosis ≥50% (6). Doelman et al. compared color Doppler ultrasonography to digital subtraction angiography to assess access blood flow and reported sensitivity of 91% and specificity of 97% to accurately detect access stenosis. Stenosis was considered significant if PSV was greater than 310 cm/s for an AVG or 375 cm/s for an AVF, or narrowing of 50% or more at the point of maximal stenosis at grayscale imaging (7). Tuka et al., defined AVG stenosis as a combination of >50% lumen reduction and peak systolic ratio (PSR) >2 together with at least one of the additional criteria including residual diameter <2.0 mm, blood flow <600 mL/min, or the blood flow reduction of >25% (8).
For AVF blood flow values <500 mL/min were considered predictive of access dysfunction (9). Clinical practice guidelines for vascular access by the American Journal of Kidney Diseases 2006 made a Grade A recommendation to refer patients for evaluation and treatment when access flow rate was less than 600 mL/min in AVG and less than 400–500 mL/min in AVF (2). Whereas, the Society of Vascular Surgery 2008 suggested performing a duplex ultrasound in accesses that display signs of dysfunction or abnormal routine surveillance (Grade 2, very low quality evidence) (10). KDOQI expert opinion found it reasonable to use duplex ultrasound to verify physical exam to help determine direction of flow and AV access evaluation for aneurysm or pseudoaneurysm.
However, routine surveillance of the AVF or AVG in asymptomatic patients to improve access patency was not recommended by the KDOQI (3). In a 3 year follow up randomized controlled multicenter open labeled trial Aragoncillo et al. found that vascular access blood flow (Qa) based surveillance combining Doppler ultrasound and ultrasound dilution to standard monitoring reduces the frequency of thrombosis and is cost effective in assuring patency in autologous AVF (11). The criteria for intervention included 25% reduction in Qa, Qa <500 mL/min or significant stenosis with PSV more than 400 cm/s or PSV pre-stenosis/stenosis ratio higher than 3. Grogan et al. found presence of critical stenosis after arterialization in 54% of patients with primary AVF on postoperative surveillance with color duplex ultrasound (12). Authors reported sensitivity, specificity and accuracy to detect hemodynamically significant stenosis of 93%, 94% and 97% respectively, and proposed to consider color duplex ultrasound to identify and correct flow limiting stenosis to improve long-term patency. Turbulence and PSV ratio of ≥3:1 was used to identify stenosis at the anastomotic site whereas PSV ratio ≥2:1 was used to detect stenosis within the fistula.
At present, preemptive intervention to correct AV access stenosis not associated with clinical indicators of access malfunction is not recommended (3). For borderline stenosis within polytetrafluoroethylene (PTFE) grafts, a watch and wait strategy with delaying angiographic intervention and repeating examination after 6–8 weeks was found safe by Tuka et al. (8). A Cochrane review analysis suggested that correction of surveillance-detected stenosis in a functional AV access does not extend access longevity and patency (13). Special consideration including the process of care and practice patterns are important when implementing a new monitoring program. Mauro et al., found that in patients with AVG, duplex ultrasound done at 3 months then every 6 months postoperatively improved 5-year secondary patency in surveillance group compared with historical control group (14).
The American Institute of Ultrasound Medicine listed the following indications for hemodialysis access ultrasound: (I) inadequate hemodialysis blood flow 500–600 mL/min or decrease 25% interval of blood flow; (II) ipsilateral upper extremity edema or pain after access or during hemodialysis; (III) delayed maturation >6 weeks of AVF; (IV) suspected pseudoaneurysm, stenosis, soft tissue infection or adjacent fluid collection; (V) decreased or absent thrill or abnormal bruit; (VI) follow-up after an intervention; (VII) signs or symptoms of digital ischemia, (VIII) access collapse; (IX) prolonged bleeding >20 min from access site; (X) unexplained decrease in the delivered dose of hemodialysis; (XI) repeated difficult cannulation; (XII) thrombus aspiration; (XIII) elevated venous pressure greater than 200 mmHg on a 300 mL/min pump; (XIV) elevated recirculation time greater than 15% (15).
Maturation
One clinical definition of the mature AVF is a structure that is usable for hemodialysis at a hemodialysis flow rate of 300 to 350 mL/min at 12 dialysis sessions in 1 month (16). A mature AVF must be easily palpable and support cannulation. AVF usually changes rapidly after placement, with 55% of forearm and 83% of upper arm AVF’s achieving at least 50% of the 6-week blood flow rate measurement at 1 day (17). Full maturation of an AVF may take 6 weeks to 3 months. If progressive AVF maturation is not clinically identified in the first 4–8 weeks, an ultrasound examination may be performed to assess for stenosis, minimum vein diameter, and maximum depth from the skin to surface. Timely diagnosis of fistula stenosis with US and early therapeutic fistulogram may enhance maturation when the culprit lesion is identified and treated.
A multicenter NIH hemodialysis fistula maturation study evaluated early anatomic changes using US at day 1, week 2, and 6 weeks with centralized interpretation, and found the value of measuring vein diameter and fistula flow rate at 2 weeks in the early identification of fistulas that are unlikely to develop optimally at 6 weeks (17). Robbin et al., indicated that measurements at 2–4 months are highly predictive of fistula maturation and adequacy for dialysis (18). Kudlicka et al. found that duplex Doppler ultrasound examination of AVG early after creation can identify those at substantial risk for stenosis and access loss (19). A primary outcome of the ongoing multicenter observational surveillance of AVF using ultrasound study (SONAR) aims to examine a role of Doppler ultrasound surveillance protocol to detect potentially correctable early problems in fail-to-mature fistulas (20).
Access aneurysms
Ultrasound surveillance provides a useful noninvasive modality for the assessment of AV access wall integrity, the presence of aneurysms and pseudoaneurysms and perivascular fluid collection. Studies have found that the prevalence of aneurysmal degeneration of AVF ranges between 5% to over 60% (21). An aneurysm of the AVF defined as any segment dilation of >18 mm was proposed by Valenti et al. The authors established the universal classification to guide management. According to the proposed classification, type 1 aneurysm is defined as the dilation along the length of the vein (1a) or post-anastomotic segment (1b), type 2—as a “camel hump” appearance with two distinct aneurysms, type 3—as complex aneurysms not fitting other groups, type 4—as a pseudoaneurysm (22). Another classification system published by Balaz et al. described arteriovenous access aneurysm by an enlargement of all three vessel layers with a diameter of more than 18 mm included type 1 aneurysm without stenosis, type 2 with significant stenosis (>50%), type 3 with partial thrombosis (<50% lumen), type 4 with complete thrombosis (23).
Cannulation
Normal use of any arteriovenous access involves repeated cannulation with 14–16 gauge needles. One approach to reduce traumatic injury is the “rope ladder” technique which involves serial rotation of the cannulation point. This approach notwithstanding, repeated site puncture and/or extravasation after needle removal may lead to the development of pseudoaneurysm. Portable Doppler ultrasound was recommended by the Spanish clinical guidelines on vascular access in all hemodialysis units to guide successful cannulation to reduce errors during needle cannulation (24). Schoch et al., found the ultrasound guided cannulation promoted higher quality and decreased cannulation damage (25). Use of US for routine cannulation needs to be evaluated with respect of the cost efficiency, availability of point of care ultrasound modalities and other factors including time and local expertise.
Cleveland Clinic hemodialysis duplex ultrasound protocol/criteria (Figures 1,2)

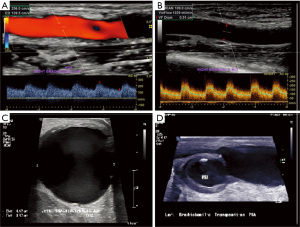
At the Cleveland Clinic, we follow our published hemodialysis monitoring and surveillance protocol. An abridged tabular version is provided in Figure 1. Indications for AVF duplex include but not limited to loss or decreased palpable thrill, decrease flow rate during dialysis, failure of access to mature, edema of affect limb (after access placement or dialysis), suspected pseudoaneurysm or adjacent fluid collection, presence of prominent collateral veins (upper arm, chest, neck) and/or ischemic symptoms of upper extremity suggestive of steal phenomenon. A high frequency transducer is utilized for the examination with appropriate settings. The inflow artery, inflow anastomosis, graft, outflow anastomosis and outflow vein are interrogated documenting any areas of stenosis or occlusion in addition to recording peak systolic velocities and luminal diameters. For AVF’s, three volume flow measurements are obtained at mid brachial artery avoiding any area of stenosis, calcification, or tortuosity. For AVG’s, volume flow measurements are obtained within the proximal, mid and distal segments of the graft itself.
Conclusions
The noninvasive vascular ultrasound plays an important role in maintenance and surveillance of the existing hemodialysis access circuit. It is a valuable diagnostic adjunct when utilized to complement clinical evaluation and physician examination. Evidence continues to evolve regarding the role of ultrasound surveillance in various access types and details such as ideal timing and cost effectives warrant further implementation, outcomes, and cost analysis research.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-129/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. LK served as the unpaid Guest Editor of the series. LK is a consultant to COOK Medical, Gore Medical and 3M Boston Scientific and received payment from Gore Medical and 3M Boston Scientific. DP attends the ACP (America College of Physicians) assisting with POCUS (point of care ultrasound) sessions. ACP covers expenses and small honorarium. AS attended ACP Annual Meeting April 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020.
- Access Vascular. 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006;48:S176-247. [PubMed]
- Lok CE, Huber TS, Lee TNational Kidney Foundation, et al. 2019 Update. Am J Kidney Dis. 2020;75:S1-164. Erratum in: Am J Kidney Dis 2021;77:551. [Crossref] [PubMed]
- Pietryga JA, Little MD, Robbin ML. Sonography of Arteriovenous Fistulas and Grafts. Semin Dial 2017;30:309-18. [Crossref] [PubMed]
- Quencer KB, Kidd J, Kinney T. Preprocedure Evaluation of a Dysfunctional Dialysis Access. Tech Vasc Interv Radiol 2017;20:20-30. [Crossref] [PubMed]
- Tessitore N, Lipari G, Contro A, et al. Screening for hemodialysis graft stenosis and short-term thrombosis risk: A comparison of the available tools. J Vasc Access 2020;21:195-203.
- Doelman C, Duijm LE, Liem YS, et al. Stenosis detection in failing hemodialysis access fistulas and grafts: comparison of color Doppler ultrasonography, contrast-enhanced magnetic resonance angiography, and digital subtraction angiography. J Vasc Surg 2005;42:739-46. [Crossref] [PubMed]
- Tuka V, Slavikova M, Krupickova Z, et al. Short-term outcomes of borderline stenoses in vascular accesses with PTFE grafts. Nephrol Dial Transplant 2009;24:3193-7. [Crossref] [PubMed]
- Wiese P, Nonnast-Daniel B. Colour Doppler ultrasound in dialysis access. Nephrol Dial Transplant 2004;19:1956-63. [Crossref] [PubMed]
- Sidawy AN, Spergel LM, Besarab A, et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg 2008;48:2S-25S. [Crossref] [PubMed]
- Aragoncillo I, Abad S, Caldés S, et al. Adding access blood flow surveillance reduces thrombosis and improves arteriovenous fistula patency: a randomized controlled trial. J Vasc Access 2017;18:352-8.
- Grogan J, Castilla M, Lozanski L, et al. Frequency of critical stenosis in primary arteriovenous fistulae before hemodialysis access: should duplex ultrasound surveillance be the standard of care? J Vasc Surg 2005;41:1000-6. [Crossref] [PubMed]
- Ravani P, Quinn RR, Oliver MJ, et al. Preemptive Correction of Arteriovenous Access Stenosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Kidney Dis 2016;67:446-60. Erratum in: Am J Kidney Dis 2017;70:735. [Crossref] [PubMed]
- Mauro R, Pini R, Faggioli G, et al. Impact of duplex ultrasound surveillance program on patency of prosthetic arteriovenous graft for hemodialysis: a single-center experience. Ann Vasc Surg 2015;29:1211-7. [Crossref] [PubMed]
- AIUM Practice Parameter for the Performance of Vascular Ultrasound Examinations for Postoperative Assessment of Hemodialysis Access. J Ultrasound Med 2020;39:E39-48. [Crossref] [PubMed]
- Dember LM, Imrey PB, Beck GJ, et al. Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis 2014;63:104-12. [Crossref] [PubMed]
- Robbin ML, Greene T, Cheung AK, et al. Arteriovenous Fistula Development in the First 6 Weeks after Creation. Radiology 2016;279:620-9. [Crossref] [PubMed]
- Robbin ML, Chamberlain NE, Lockhart ME, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 2002;225:59-64. [Crossref] [PubMed]
- Kudlicka J, Malik J, Tuka V, et al. Arteriovenous grafts: early ultrasonography tells their fortune. Am J Nephrol 2015;41:420-5. [Crossref] [PubMed]
- Richards J, Hossain M, Summers D, et al. Surveillance arterioveNous fistulAs using ultRasound (SONAR) trial in haemodialysis patients: a study protocol for a multicentre observational study. BMJ Open 2019;9:e031210. [Crossref] [PubMed]
- Mudoni A, Cornacchiari M, Gallieni M, et al. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J 2015;8:363-7. [Crossref] [PubMed]
- Valenti D, Mistry H, Stephenson M. A novel classification system for autogenous arteriovenous fistula aneurysms in renal access patients. Vasc Endovascular Surg 2014;48:491-6. [Crossref] [PubMed]
- Balaz P, Björck M. True aneurysm in autologous hemodialysis fistulae: definitions, classification and indications for treatment. J Vasc Access 2015;16:446-53.
- Ibeas J, Roca-Tey R, Vallespín J, et al. Spanish Clinical Guidelines on Vascular Access for Haemodialysis. Nefrologia 2017;37:1-191. Erratum in: Nefrologia (Engl Ed) 2019;39:1-2 Erratum in: Nefrologia (Engl Ed) 2019;39:680-2. [Crossref] [PubMed]
- Schoch ML, Currey J, Orellana L, et al. Point-of-care ultrasound-guided cannulation versus standard cannulation in haemodialysis vascular access: protocol for a controlled random order crossover pilot and feasibility study. Pilot Feasibility Stud 2018;4:176. [Crossref] [PubMed]


