
Thoracic central venous occlusion from the interventional radiology perspective
Background
Thoracic central venous occlusion (CVO), defined as pathophysiological venous luminal narrowing that impedes blood flow, is a common problem with high morbidity that affects a large patient population. The thoracic central veins are defined as the superior vena cava (SVC), the bilateral brachiocephalic veins, the subclavian veins, and the thoracic segments of the internal jugular vein and suprahepatic inferior vena cava (1).
Broadly, CVO can be categorized as either malignant or benign. Multiple malignancies are associated with CVO, with the most common being non-small cell lung cancer, followed by small cell lung cancer and lymphoma (2). There are many benign etiologies of CVO, but most cases are related to previous instrumentation, typically involving either central venous catheters (CVCs) or implantable cardiac devices such as pacemakers and defibrillators (3). Therefore, the risk of CVO must be considered in patients for whom continued central venous access is necessary, such as those with end-stage renal disease. Analysis of Medicare data has suggested that an estimated 15% of adults in the United States older than 65 years have chronic kidney disease (CKD), with an incidence as high as 24% in adults older than 85 years (4). Thus, understanding the pathophysiology and management of CVO, and specifically the minimally invasive techniques that are available, is of critical importance for interventional radiologists. In this article, we review the pathophysiology of CVO, the clinical presentation, and interventional techniques for both preventing and managing this condition.
Pathophysiology, etiology, and classification
There are three main mechanisms of obstruction: extrinsic venous compression, venous wall thickening, and intraluminal obstruction. Extrinsic venous compression can be caused by arterial or musculoskeletal compression, postoperative scarring and fibrosis, or tumor. Venous wall thickening can be caused by organized mural thrombus or fibrosis or can be secondary to endovascular interventions. Intraluminal obstruction can be caused by thrombus formation or endoluminal device implants.
Thrombosis is frequently both a cause and a result of venous obstruction. Thrombosis usually involves a combination of venous stasis, vascular injury, and hypercoagulability, commonly known as Virchow’s triad. Catheter-associated vascular thrombosis is a multifactorial process thought to involve both stasis and vascular injury. Vascular models of indwelling catheters have shown that the flow rate surrounding the catheters is substantially reduced, and this relationship becomes more pronounced as the catheter to lumen ratio increases (5). In addition, catheter contact along the vessel wall is thought to directly injure the endothelium, leading to neointimal hyperplasia. This repetitive cycle of damage and hyperplasia can over time result in luminal narrowing and eventual thrombosis (6). This may explain why some studies have found higher rates of CVO with left-sided internal jugular CVCs than with traditional right-sided CVCs, as the course of the catheter requires multiple points of contact with the vessel walls, leading to injury (3,7,8) Although much less common, the same process of vascular injury can also contribute to other benign causes of CVO such as radiation and trauma.
These same principles can be applied to peripheral veins. In fact, the aforementioned vascular models found that the flow rate surrounding peripherally inserted central catheters can be reduced by up to 93%, presumably due to the decreased caliber of the peripheral vasculature (5). Therefore, in the CKD population, peripheral catheters are typically considered contraindicated, as occlusions will compromise future arteriovenous fistula formation (9). Arteriovenous fistula and arteriovenous grafts created for dialysis access in CKD can also cause CVO, likely as a result of the high volume and pressure through the system (10).
In the case of malignant CVO, the typical cause is either direct invasion from a tumor (primarily lung cancer) or mass effect from enlarged lymph nodes secondary to metastatic disease or lymphoma (2). Although most malignancies are associated to some degree with hypercoagulability, it is unclear how much this contributes to the relatively large caliber central veins. Rarely, endoluminal malignancy (e.g., angiosarcoma) is the cause of venous obstruction.
There are two primary classification systems for thoracic CVO. Originally published in 1986, the Stanford and Doty classification was introduced to help characterize CVO in a clinically relevant manner for planning surgical bypass (11). The classification is based primarily on patency of the SVC and azygos vein. Although this system is still in use, advancements in endovascular interventions and a remarkable increase in studies assessing various techniques required expansion of this classification system. In 2018, the Society of Interventional Radiology, with endorsements by numerous professional societies, introduced a more detailed classification system with the aim of standardizing reporting literature (Table 1) (1). The advantage of this newer system is that it includes details regarding the central veins, making this system more clinically relevant for endovascular techniques.
Table 1
| Type | Description |
|---|---|
| Type 1 | Both BCVs and SVC are patent; unilateral IJV or SCV is obstructed. If patency of all thoracic veins is unknown, the occlusion is classified as Type 1A or Type 1B |
| Type 1A | Unilateral IJV or SCV obstruction with patent ipsilateral BCV; patency of all other thoracic central venous anatomy is unknown |
| Type 1B | Unilateral IJV or SCV obstruction with patent ipsilateral BCV; patency of contralateral thoracic central venous anatomy is unknown |
| Type 1C | Unilateral IJV or SCV obstruction with known patency of the contralateral IJV, SCV, and BCV |
| Type 1D | Bilateral obstruction of IJVs, SCVs, or combined IJV and SCVs, with both BCVs patent |
| Type 2 | Any form of thoracic CVO that causes unilateral BCV obstruction or ipsilateral obstruction of the IJV and SCV (equivalent to unilateral BCV obstruction) |
| Type 2A | Unilateral BCV obstruction with unknown condition of the contralateral side |
| Type 2B | Unilateral BCV obstruction with known patency of the contralateral side |
| Type 3 | Bilateral BCVs are obstructed, but flow to the right atrium passes through the SVC |
| Type 4 | SVC obstruction that prevents or impedes direct thoracic venous flow to the right atrium with any combination of BCV, IJV, or SCV obstruction |
CVO, central venous occlusion; BCV, brachiocephalic vein; SVC, superior vena cava; IJV, internal jugular vein; SCV, subclavian vein.
Clinical presentation and indications
Many patients with CVO are asymptomatic, as slowly progressive stenosis often results in substantial collateralization before complete occlusion. Clinicians should not intervene in cases of asymptomatic stenosis, as interventions have been shown to lead to faster progression (12). This can paradoxically lead to symptomatic occlusion due to less collateralization.
When symptoms do occur, they are dependent on the location and acuity of the occlusion. Epidemiologic studies have shown that the most common presenting symptom is swelling of the face, neck, upper extremities, and chest; this symptom occurs in more than 80% of patients (13) and is thought to be secondary to venous hypertension (1). Most patients will also have some degree of respiratory distress and cough. Respiratory distress could be due to laryngeal edema, pleural effusion, or chest (breast) swelling, leading to restrictive pulmonary compromise. Less common symptoms include difficulty swallowing, hoarseness, confusion, visual and auditory disturbances, cognitive disabilities, headache, and seizures. In addition, collateral veins are often clinically appreciable along the upper chest and shoulders in patients with CVO. Finally, patients with end-stage renal disease may present with other important symptoms, namely poor flow during dialysis sessions, prolonged bleeding after dialysis, and progressive dilation of the access or fistula site.
Mild symptoms can often be managed with monitoring only. In patients undergoing dialysis, complete occlusion may require intervention even if patients are asymptomatic, as dialysis requires high flow rates (minimum 300 mL/min). These high flow rates are typically unachievable through collaterals, and the poor flow through collaterals can result in recirculation of dialysate from the patient to the machine (9).
Therefore, the optimal approach to a patient with suspected CVO involves a combination of clinical and imaging findings, before considering intervention. The angiographic appearance of stenosis and measurements of intravascular pressure gradients across the central stenosis of greater than 5 to 10 mm Hg can be used to aid in decision-making but are not standalone indications for intervention (14).
Techniques for crossing the occlusion
Blunt recanalization
Blunt recanalization is frequently the first technique used for chronic CVO (Figure 1). A guidewire and angled catheter are traditionally used with this technique. Under real-time ultrasound guidance, venous access is obtained into the desired veins, with the venous access site chosen based on the location and length of the occlusion. Often, multiple access sites must be used simultaneously to approach the occlusion from both ends. Under fluoroscopic guidance, the operator then advances an angiographic catheter and a guidewire up to the venous occlusion. Care should be taken to select a sheath of appropriate length, as a long sheath allows for greater stabilization of the angiographic catheter and guidewire and allows high-quality venograms to be performed directly at the site of the venous occlusion (3). The guidewire is then advanced and manipulated in an attempt to cross the venous occlusion; some operators may prefer to use an angled catheter to direct the guidewire toward the occlusion. A hydrophilic wire such as a Glidewire may be able to traverse the occlusion when other nonhydrophilic wires cannot. Additionally, a “drill” technique is frequently employed; with this technique, the wire is slowly twisted in an attempt to cross the occluded segment (9).
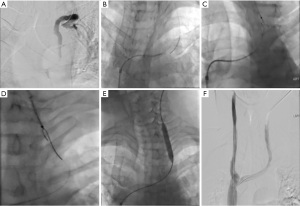
When standard blunt recanalization is not successful, a “mother-child technique” can be used to add support to the recanalization system. With this technique, an angiographic catheter is placed in a guide catheter; for instance, a 4 French (F) angiographic catheter is placed in a 7F guide catheter. This adds stability to the recanalization system and allows for greater manipulation, as the guidewire can be angled in different directions (15,16). Once the wire is able to traverse the occlusion, the catheter is advanced over the wire past the occlusion. If a Glidewire has been used, it is exchanged for a stiffer nonhydrophilic wire to add stability to the system. This is followed by balloon angioplasty and sometimes by stenting to increase the patency of the occluded segment. During these interventions, patients are typically fully heparinized to prevent acute thrombosis.
Sharp recanalization
For situations in which traditional blunt recanalization fails, interventional radiologists can opt for a sharp recanalization technique. However, sharp recanalization is associated with more complications than blunt recanalization, especially in the setting of arteriovenous grafts given their higher flow rate when compared to normal venous structures. Some of these complications include hemothorax, pericardial tamponade, and arterial injury. Thus, one must be wary of any central venous perforation during sharp recanalization, with stents immediately available in the event that a perforation occurs (3).
The initial steps of this technique are quite similar to those involved in blunt recanalization, with the venous access site chosen based on the location and length of the occlusion. Similarly, under real-time ultrasound guidance, venous access is obtained into the desired vein, and under fluoroscopic guidance, a guide catheter and guidewire are advanced to the site of the venous occlusion. Under ultrasound guidance, an additional venous access site is then obtained from the opposite direction of the occlusion to obtain eventual through-and-through access. Through this second venous access, a loop snare is advanced under fluoroscopic guidance; this snare serves as a target for the sharp recanalization. Some operators use a contrast-filled balloon or another guidewire as a target for the needle to puncture.
There are two main techniques used to perform sharp recanalization of the occlusion. In the first method, a needle sheathed within a catheter (i.e., a 21G Chiba needle within a 4F Berenstein catheter) is advanced toward the occlusion under fluoroscopic guidance. At the site of the occlusion, the Chiba needle is then unsheathed and advanced through the occlusion toward the target loop snare. Once the needle is through the occlusion, orthogonal views are obtained to confirm that the needle tip is within the loop snare. Subsequently, a micro guidewire is advanced through the needle and pulled through by closing the snare, creating a through-and-through access (3,9,17).
The other technique involves using the back end of a stiff guidewire instead of a needle to traverse the occlusion. In this technique, the back end of the guidewire is passed through the occlusion and snared to obtain through-and-through access. The other procedural steps involved are fairly similar to those outlined above for sharp recanalization with a needle (3,9). Once through-and-through access is obtained, a catheter can be advanced over the initial wire to exchange it for a stiffer wire such as an Amplatz wire. Subsequently, angioplasty of the occluded segment and stenting is performed to maintain patency. Again, patients undergoing sharp recanalization are fully heparinized throughout the procedure to prevent acute thrombosis.
Various other sharp recanalization techniques have been described. For instance, a Rosch-Uchida needle or TIPS needle can be used for sharp recanalization (18,19). Alternatively, operators can use a coaxial sharp recanalization technique (20). This method involves advancing a 22G Chiba needle through an 18G trocar needle, which is manually curved to the desired angle. The curved trocar needle provides directionality and robust torquability to the 22G Chiba needle. Other researchers have described using a trans-septal needle for sharp recanalization (21). Another group reported performing sharp recanalization with an Outback LTD catheter, which is a single-lumen over-the-wire 6F sheath-compatible catheter containing a curved nitinol cannula within the outer shaft. The flexible nature of this catheter, with its torquability and curved geometry, could be a useful addition to the products used for sharp recanalization (22).
Advanced endovascular techniques for refractory thoracic CVO
Despite the use of proper recanalization technique by experienced interventional radiologists, some cases of thoracic CVO do not respond to blunt or sharp recanalization. Reportedly, up to 18% of cases may be refractory to these traditional approaches (23). Newer technologies may therefore be of use in this setting.
One of these new techniques is radiofrequency (RF) wire-guided recanalization (Figure 2). The PowerWire Radiofrequency Guidewire (Baylis Medical Company Inc, Montreal, Canada) consists of a 0.035-inch wire with a heated radiopaque tip that can create RF energy when connected to a specific RF generator. Importantly, subsequent balloon catheters can also be placed over the PowerWire. With this technique, simultaneous upper and lower extremity access is obtained, and venograms are performed to assess the extent of the thrombus. Depending on the location of the occlusion, either the jugular or femoral approach can be used to advance the PowerWire to the level of the thrombus. A catheter or snare is usually then placed via the opposite approach and also advanced to the level of the thrombus to provide a target. Alignment between the PowerWire and the target is verified in multiple views before the RF energy is initiated (23,24). The wire is advanced only a few millimeters at a time, and the position is then rechecked. Once the thrombus has been passed, a balloon catheter can be placed over the PowerWire, or the PowerWire can be exchanged for a stiff wire before the balloon catheter is placed. This PowerWire technique has been shown to be extremely effective for patients in whom traditional techniques have failed, with multiple studies demonstrating greater than 80% success rates in these patients (24,25). The primary complication with the PowerWire is that the same properties that allow the wire to traverse the chronic, dense plaque can also cause the wire to traverse the vessel wall. Major complications that have been reported include tracheal and pericardial perforations (25). Careful advancement of the PowerWire under the guidance of 3D venography or cone-beam computed tomography (CT) may help to prevent these inadvertent perforations.
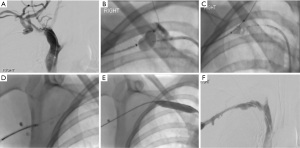
One recently described endovascular technique can potentially recover cases of unintentional venous perforation; such perforations usually require that the entire procedure be stopped. This technique involves blunt dissection through the mediastinum with a guidewire, which is then exchanged with a RF wire to gain re-entry into the vessel as a form of nonsurgical extravascular bypass (26).
The use of laser to recanalize occluded central veins has also been described in a few case reports and case series (27). However, there are limited data in the literature regarding the safety and success of this technique.
Techniques for placing vascular access catheters in thoracic CVO
The Surfacer Inside-Out Access Catheter System (Bluegrass Vascular Technologies, Inc, San Antonio, TX, USA) is a recently developed FDA-approved device that has gained popularity as an alternative central venous access option specifically for right-sided CVO. With this technique, access is obtained through the right femoral vein, and a standard 0.035-inch exchange length guidewire is passed through the inferior vena cava to the thrombus. A sheath is then passed over the guidewire to the thrombus, and the guidewire is removed. The Surfacer device is placed through the sheath and advanced to the thrombus and is then advanced through the thrombus to the level of the right clavicle. The tip of the device is rotated and lined up fluoroscopically with an external exit target, and a needle wire is passed through the exit point in the skin. This allows for subsequent placement of a peel-away sheath over the externalized wire and a standard tunneled CVC placement (28,29). A multicenter study across multiple countries in Europe demonstrated successful placement in 29 of 30 patients with thoracic CVO, with no immediate device-related complications and a mean device procedure time of 24 minutes (30).
More involved percutaneous techniques for placing vascular access catheters have also been described. One such technique involves cone-beam CT–guided percutaneous placement of a tunneled CVC through the mediastinum directly into the SVC (9).
Finally, multiple surgical techniques exist to bypass the obstruction, but these are typically used only when less invasive methods have been exhausted.
Maintenance of patency after recanalization
Angioplasty
In the setting of acute thrombus, the first interventional step is often mechanical thrombectomy with or without infusion thrombolysis (9). For chronic occlusions, the approach can be more controversial. To establish and maintain patency, the standard approach is angioplasty with or without stent placement. Some studies have shown no difference in the long-term patency of recanalized chronic occlusions with angioplasty with or without stent placement. In a retrospective analysis of 97 patients undergoing dialysis who had CVO, the most accurate predicters of patency were found to be the type of stenosis and patient age, not the technique used (31). Other studies have found conflicting results. For instance, in a study of 150 patients undergoing dialysis who had symptomatic CVO, the stent group required more interventions but had higher long-term patency rates (59%, 42%, and 28% patency at 12, 24, and 60 months, respectively) than the angioplasty alone group (42%, 36%, and 20% at 12, 24, and 60 months, respectively) (32).
Balloon angioplasty involves placing an angioplasty balloon over a guidewire across the area of the stenosis and inflating the balloon with an inflation device until the balloon reaches the appropriate circumferential pressure. Once the occlusion is crossed with a wire, a 4- to 6-mm balloon is used to dilate the passage enough to allow larger balloons to be passed. The area of the stricture is sequentially dilated with larger balloons, with the largest balloon having a diameter that is up to 10% to 15% larger than the adjacent venous diameter.
Standard high-pressure balloon angioplasty may on rare occasion fail to properly dilate a rigidly stenosed vein. In this setting there may be a potential role for initial angioplasty using cutting balloons, which have wires that score through the stenosed vessel longitudinally with low pressure and allow for subsequent use of standard balloon angioplasty and stenting. Studies have predominantly tested this in venous outflow stenosis in dialysis grafts and fistulas, however, it could theoretically play a similar role in central venous stenosis (33,34).
New medical advances have brought drug-eluting technology to balloon angioplasty with the development of drug-eluting balloons (DEBs). In traditional balloon angioplasty, the vessel is expanded to achieve patency; the shearing forces from this balloon expansion cause plaque rupture and damage to the tissue within the tunica intima and medial layers. Restenosis can occur due to neointimal growth causing remodeling and proliferation of the vascular smooth muscles located within the tunica intima. Angioplasty with DEBs allows for the delivery of antiproliferative drugs at the vessel wall at the time of the angioplasty procedure. The use of DEBs has been shown to improve patency after angioplasty in cases of failing vascular dialysis access with venous stenosis, with one study showing an average patency of 0.64 years with DEB angioplasty versus 0.36 years with traditional balloon angioplasty (35,36). However, these studies had small patient populations, and further research is warranted.
The most life-threatening complication of balloon angioplasty is venous rupture. It is important to recognize the possible presentations of this complication, which are typically dependent on the location of the rupture. Brachiocephalic and upper SVC rupture can result in hemothorax or mediastinal bleeding (37). More central SVC rupture can lead to pericardial hematoma and tamponade, resulting in rapid hemodynamic instability (Figure 3). Maintaining adequate guidewire access across the area of the stenosis is therefore essential during balloon angioplasty. In cases of suspected venous rupture, guidewire access allows for the injection of angiographic contrast through the access sheath to identify the size and location of the venous rupture. Guidewire access also allows for inflation of a balloon at the rupture site for tamponade purposes. Balloon tamponade leads to hemodynamic stability and allows time for planning endovascular and/or surgical management. Frequently, placement of an adequately sized polytetrafluoroethylene (PTFE)-covered stent across the venous rupture helps to seal the leakage and resolves the hemodynamic instability. Emergency surgical intervention may be required if the rupture is extensive and if adequate seal is not anticipated with the use of a PTFE-covered stent. In cases of pericardial tamponade, it is essential to mitigate the bleeding before a pericardial drain is placed; balloon tamponade or placement of a PTFE-covered stent across the rupture can be used for this purpose (38,39).
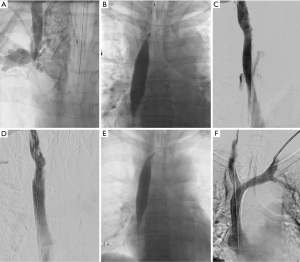
Stenting
Although study findings regarding stent placement in the dialysis patient population have been conflicting, stenting should be considered in practically all cases after careful weighing of potential complications (3). For a few situations, stenting is specifically indicated. Prompt stent placement is necessary in the immediate setting if angioplasty is unsuccessful or if a complication such as severe recoil, dissection, or symptomatic occlusion of the stenotic vessel occurs. Elective stenting is typically warranted in cases unlikely to be maintained by angioplasty alone, such as recurrent stenosis, long-segment occlusions, or stenosis secondary to external tumor compression (3,40).
Some authors advocate for the regular use of self-expanding stents as these stents are thought to provide superior distribution of wall opposition, leading to a lower risk of migration (3). However, others recommend choosing a stent type based on the location of the thrombus. For example, a self-expanding stent may be appropriate in the setting of a highly mobile and compressed vein such as in a left subclavian thrombus. However, a right brachiocephalic vein thrombus may be better managed with a balloon expandable stent given the relatively short length of the vein (40).
Perhaps the most important complication to be aware of is stent migration. Migration can be life-threatening and is of particular concern in situations where the thrombus may shrink, such as cases of treated malignancy. A commonly used stent for thoracic CVO is the Wallstent (Boston Scientific, Marlborough, Massachusetts, USA), but potential complications associated with its use are a tendency for stent shortening and migration. This may be related to nonuniform distribution of radial forces, allowing for the ends of the stent to be compressed by the vascular lesion, hence not permitting the stent to fully expand which leads to either shortening of the expected final length, or migration (41). Particularly because of the risk of migration, some authors advocate against the use of Wallstent in the lower SVC (3).
Choosing which stent to use can be challenging for many reasons. First, not all countries and institutions have access to the same stents. Second, a wide array of stent types are available, each with its own advantages and disadvantages. These include bare metal stents, PTFE-covered stents, self-expandable stents, and balloon-mounted stents. Balloon-mounted stents tend to be more rigid than self-expandable stents and are not often used in venous stenting. Stenting of venous occlusions requires a stent with high radial force because of the substantially lower musculature relative to arteries. Additionally, veins tend to be more compressible and tortuous (42).
One classification of stents that may be relevant in venous intervention is closed cell (e.g., Wallstent) versus open cell (e.g., SMART stent, Cordis, Miami Lakes, Florida, USA). As the name implies, a closed-cell stent has a greater density of struts with smaller uncovered gaps and is generally more rigid. On the other hand, an open-cell stent has larger uncovered gaps but is more flexible, which may be better for tortuous vessels (43,44).
Early research assessing three commonly used stents—the Wallstent, Gianturco Z stent (Cook, Bloomington, Indiana, USA), and Palmaz stent (Johnson and Johnson, Warren, New Jersey, USA)—demonstrated no major differences in outcomes (45). However, some of these stents have since fallen out of favor as newer, more flexible and user-friendly stents have become available, such as the SMART stent.
One large retrospective study in 401 patients undergoing dialysis who had benign central venous stenosis or occlusion requiring stenting demonstrated a significant difference (P=0.002) favoring open-cell stents, with a mean patency of approximately 10.9 months in the open-cell group versus 8.5 months in the closed-cell group (44). Importantly, these data were aggregated across multiple stent brands, venous sites, and stenosis/occlusion severity. A smaller study in 32 patients compared self-expanding nitinol stents [SMART stent and Protégé EverFlex (ev3 Endovascular Inc, Plymouth, Minnesota, USA)] with a self-expanding stainless-steel stent (Wallstent) and demonstrated higher patency rates in the nitinol stent group (89% at 6 months; 81% at 12 months) than in the stainless steel stent group (79% at 6 months; 38% at 12 months) (32).
Newer stents specifically designed for venous interventions are also available. The Vici stent (Boston Scientific) is a nitinol self-expanding stent with an alternating open and closed design, reportedly intended specifically for venous interventions, including CVO. A small preliminary study in 20 patients with chronic benign thoracic CVO demonstrated 100% patency at 6 months with no complications, and a 94% patency rate at 12 months (41).
Regardless of the type of stent used, the standard preventive technique for stent placement involves oversizing stents by 10% to 20% relative to the normal vessel diameter and using a stent that is at least 4 cm in length, as the greater surface area of contact will help prevent migration (3). When placing a stent, care must be taken to avoid blocking any other major vessels along the course of the stent, such as the internal jugular vein, which is necessary for future central venous access. This can be avoided in the contralateral brachiocephalic vein by using the “kissing-stent technique,” whereby a second stent is placed in the other vessel to keep it patent.
Summary
Thoracic CVO is a fairly common entity with high morbidity. Multiple techniques and treatment approaches exist to manage this condition. Prevention remains the single most important step; this can be accomplished through the judicious use of central venous catheterization in high-risk patients, such as those with advanced CKD or end-stage renal disease. However, not all cases require intervention, and the procedures are not without risk. When intervention is warranted, careful preprocedural planning is essential to determine which technical approach and which devices would be most beneficial, as these decisions can be multifactorial. Gaining access across the occlusion is the most technically challenging portion of the procedure. However, successful recanalization can prevent the need for more invasive approaches to achieve access, which are associated with additional risk to the patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-93/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. SP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dolmatch BL, Gurley JC, Baskin KM, et al. Society of Interventional Radiology Reporting Standards for Thoracic Central Vein Obstruction: Endorsed by the American Society of Diagnostic and Interventional Nephrology (ASDIN), British Society of Interventional Radiology (BSIR), Canadian Interventional Radiology Association (CIRA), Heart Rhythm Society (HRS), Indian Society of Vascular and Interventional Radiology (ISVIR), Vascular Access Society of the Americas (VASA), and Vascular Access Society of Britain and Ireland (VASBI). J Vasc Interv Radiol 2018;29:454-460.e3. [Crossref] [PubMed]
- Straka C, Ying J, Kong FM, et al. Review of evolving etiologies, implications and treatment strategies for the superior vena cava syndrome. Springerplus 2016;5:229. [Crossref] [PubMed]
- Horikawa M, Quencer KB. Central Venous Interventions. Tech Vasc Interv Radiol 2017;20:48-57. [Crossref] [PubMed]
- US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Executive Summary. Accessed February 9, 2022. Available online: https://www.usrds.org/media/2371/2019-executive-summary.pdf
- Nifong TP, McDevitt TJ. The effect of catheter to vein ratio on blood flow rates in a simulated model of peripherally inserted central venous catheters. Chest 2011;140:48-53. [Crossref] [PubMed]
- Geerts W. Central venous catheter-related thrombosis. Hematology Am Soc Hematol Educ Program 2014;2014:306-11. [Crossref] [PubMed]
- Schillinger F, Schillinger D, Montagnac R, et al. Post catheterization vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant 1991;6:722-4. [Crossref] [PubMed]
- Salgado OJ, Urdaneta B, Colmenares B, et al. Right versus left internal jugular vein catheterization for hemodialysis: complications and impact on ipsilateral access creation. Artif Organs 2004;28:728-33. [Crossref] [PubMed]
- Young JL, McLennan G. Thoracic Central Vein Occlusion in the Dialysis Patient: An Interventional Perspective. Adv Chronic Kidney Dis 2020;27:236-42. [Crossref] [PubMed]
- Quencer KB, Arici M. Arteriovenous Fistulas and Their Characteristic Sites of Stenosis. AJR Am J Roentgenol 2015;205:726-34. [Crossref] [PubMed]
- Stanford W, Doty DB. The role of venography and surgery in the management of patients with superior vena cava obstruction. Ann Thorac Surg 1986;41:158-63. [Crossref] [PubMed]
- Levit RD, Cohen RM, Kwak A, et al. Asymptomatic central venous stenosis in hemodialysis patients. Radiology 2006;238:1051-6. [Crossref] [PubMed]
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. [Crossref] [PubMed]
- Nikolic B. Hemodialysis fistula interventions: diagnostic and treatment challenges and technical considerations. Tech Vasc Interv Radiol 2008;11:167-74. [Crossref] [PubMed]
- Wan Z, Lai Q, Zhou Y, et al. Efficacy and safety of a mother-child technique for recanalization of chronic central venous occlusive disease in hemodialysis patients. J Vasc Surg Venous Lymphat Disord 2020;8:558-64. [Crossref] [PubMed]
- Kovacic JC, Sharma AB, Roy S, et al. GuideLiner mother-and-child guide catheter extension: a simple adjunctive tool in PCI for balloon uncrossable chronic total occlusions. J Interv Cardiol 2013;26:343-50. [Crossref] [PubMed]
- Yoong GSW, Koh FHX, Wee BBK, et al. How to do it: value-driven sharp recanalization of central vein occlusion. ANZ J Surg 2020;90:362-3. [Crossref] [PubMed]
- Murphy TP, Webb MS. Percutaneous venous bypass for refractory dialysis-related subclavian vein occlusion. J Vasc Interv Radiol 1998;9:935-9. [Crossref] [PubMed]
- Honnef D, Wingen M, Günther RW, et al. Sharp central venous recanalization by means of a TIPS needle. Cardiovasc Intervent Radiol 2005;28:673-6. [Crossref] [PubMed]
- Gallo CJR, Ronald J, Pabon-Ramos WM, et al. Sharp Recanalization of Chronic Central Venous Occlusions of the Thorax Using a Steerable Coaxial Needle Technique from a Supraclavicular Approach. Cardiovasc Intervent Radiol 2021;44:784-8. [Crossref] [PubMed]
- Yin X, Shen X, Zhou Z, et al. Efficacy and safety of recanalization with transseptal needle for chronic total occlusion of the brachiocephalic vein in hemodialysis patients. Ann Transl Med 2020;8:1141. [Crossref] [PubMed]
- Anil G, Taneja M. Revascularization of an occluded brachiocephalic vein using Outback-LTD re-entry catheter. J Vasc Surg 2010;52:1038-40. [Crossref] [PubMed]
- Iafrati M, Maloney S, Halin N. Radiofrequency thermal wire is a useful adjunct to treat chronic central venous occlusions. J Vasc Surg 2012;55:603-6. [Crossref] [PubMed]
- Guimaraes M, Schonholz C, Hannegan C, et al. Radiofrequency wire for the recanalization of central vein occlusions that have failed conventional endovascular techniques. J Vasc Interv Radiol 2012;23:1016-21. [Crossref] [PubMed]
- Keller EJ, Gupta SA, Bondarev S, et al. Single-Center Retrospective Review of Radiofrequency Wire Recanalization of Refractory Central Venous Occlusions. J Vasc Interv Radiol 2018;29:1571-7. [Crossref] [PubMed]
- Dai R, Kim CY. Blunt Transmediastinal Dissection with Radiofrequency Wire Reentry for Extravascular Bypass of Thoracic Central Venous Occlusions Refractory to Recanalization. J Vasc Interv Radiol 2021;32:558-61. [Crossref] [PubMed]
- Rambhia S, Janko M, Hacker RI. Laser Recanalization of Central Venous Occlusion to Salvage a Threatened Arteriovenous Fistula. Ann Vasc Surg 2018;50:297.e1-3. [Crossref] [PubMed]
- Quek LHH, Tan TSM, Tan GWL, et al. Salvage of exhausted neck access using a novel inside-out device in dialysis-dependent patients. Hemodial Int 2019;23:E111-4. [Crossref] [PubMed]
- Razavi MK, Peden EK, Sorial E, et al. Efficacy and safety associated with the use of the Surfacer® Inside-Out® Access Catheter System: Results from a prospective, multicenter Food and Drug Administration-approved Investigational Device Exemption study. J Vasc Access 2021;22:141-6.
- Gallieni M, Matoussevitch V, Steinke T, et al. Multicenter Experience with the Surfacer Inside-Out Access Catheter System in Patients with Thoracic Venous Obstruction: Results from the SAVE Registry. J Vasc Interv Radiol 2020;31:1654-1660.e1. [Crossref] [PubMed]
- Hongsakul K, Leelarujijaroen P, Boonsrirat U. Outcome of Central Vein Occlusion Recanalization in Hemodialysis Patients and Predictors for Success: A Retrospective Study. J Belg Soc Radiol 2020;104:20. [Crossref] [PubMed]
- Gür S, Oğuzkurt L, Gedikoğlu M. Central venous occlusion in hemodialysis access: Comparison between percutaneous transluminal angioplasty alone and nitinol or stainless-steel stent placement. Diagn Interv Imaging 2019;100:485-92. [Crossref] [PubMed]
- Bittl JA, Feldman RL. Cutting balloon angioplasty for undilatable venous stenoses causing dialysis graft failure. Catheter Cardiovasc Interv 2003;58:524-6. [Crossref] [PubMed]
- Aftab SA, Tay KH, Irani FG, et al. Randomized clinical trial of cutting balloon angioplasty versus high-pressure balloon angioplasty in hemodialysis arteriovenous fistula stenoses resistant to conventional balloon angioplasty. J Vasc Interv Radiol 2014;25:190-8. [Crossref] [PubMed]
- Kitrou PM, Katsanos K, Spiliopoulos S, et al. Drug-eluting versus plain balloon angioplasty for the treatment of failing dialysis access: final results and cost-effectiveness analysis from a prospective randomized controlled trial (NCT01174472). Eur J Radiol 2015;84:418-23. [Crossref] [PubMed]
- Katsanos K, Karnabatidis D, Kitrou P, et al. Paclitaxel-coated balloon angioplasty vs. plain balloon dilation for the treatment of failing dialysis access: 6-month interim results from a prospective randomized controlled trial. J Endovasc Ther 2012;19:263-72. [Crossref] [PubMed]
- Ko SF, Ng SH, Fang FM, et al. Left brachiocephalic vein perforation: computed tomographic features and treatment considerations. Am J Emerg Med 2007;25:1051-6. [Crossref] [PubMed]
- Kuhn J, Kilic A, Stein E. Management of Innominate Vein Rupture During Superior Vena Cava Angioplasty. A A Case Rep 2016;7:89-92. [Crossref] [PubMed]
- Samuels LE, Nyzio JB, Entwistle JW. Superior vena cava rupture during balloon angioplasty and stent placement to relieve superior vena cava syndrome: a case report. Heart Surg Forum 2007;10:E78-80. [Crossref] [PubMed]
- Horita Y. Percutaneous transluminal angioplasty for central venous stenosis or occlusion in hemodialysis patients. J Vasc Access 2019;20:87-92.
- Tan GM, Chi KWK, Yan BPY. Mid-term Results of a Novel Dedicated Venous Stent for the Treatment of Chronic Thoracic Central Vein Obstruction of Benign Aetiology. Eur J Vasc Endovasc Surg 2019;57:417-23. [Crossref] [PubMed]
- Kundu S. Review of central venous disease in hemodialysis patients. J Vasc Interv Radiol 2010;21:963-8. [Crossref] [PubMed]
- A survey of stent designs. Minim Invasive Ther Allied Technol 2002;11:137-47. [Crossref] [PubMed]
- Kang CH, Yang SB, Lee WH, et al. Comparison of Open-Cell Stent and Closed-Cell Stent for Treatment of Central Vein Stenosis or Occlusion in Hemodialysis Patients. Iran J Radiol 2016;13:e37994. [Crossref] [PubMed]
- Ganeshan A, Hon LQ, Warakaulle DR, et al. Superior vena caval stenting for SVC obstruction: current status. Eur J Radiol 2009;71:343-9. [Crossref] [PubMed]


