
Renal outcomes of suprarenal vs. infrarenal endograft fixation in endovascular abdominal aortic aneurysm repair: a narrative review
Introduction
An abdominal aortic aneurysm (AAA) is a dilatation of the abdominal aorta beyond 1.5 times the normal diameter of the abdominal aorta at the level of the renal arteries (1). A diameter greater than 3.0 cm is typically classified as aneurysmal (2,3). AAA has a prevalence of approximately 4.8%, with men being affected more often than women (4). AAAs can be categorised as suprarenal, juxta renal or infrarenal depending on the location of the aneurysm with respect to the renal arteries (5). Rupture is a surgical emergency with a high mortality and so early diagnosis and treatment is crucial to prevent rupture and death. As aneurysm diameter increases from 5.0 to 6.0 cm, the risk of rupture rises sharply (3–15% annual risk of rupture at 5.0–6.0 cm diameter) and so intervention is considered to prevent aortic rupture and the associated morbidity and mortality (6).
Endovascular aneurysm repair (EVAR) has become a globally well-established treatment for AAA. It is an effective, safe and superior alternative to open surgical repair (OSR) (7-9). The anatomical morphology of an AAA can limit the suitability of many commercially available EVAR devices off-the-shelf. A key parameter in defining suitability to undergo standard EVAR is the length of non-aneurysmal infrarenal aorta, termed ‘infrarenal neck length’, for proximal device fixation (10). Most endografts that use infrarenal fixation (IRF) require an infrarenal neck length of at least 15 mm, a neck diameter of <32 mm and a neck angulation of less than 60 degrees. However, some earlier studies evaluated the suitability of standard EVAR for AAAs with suboptimal infrarenal anatomy (11-15).
In up to 66% of female and 46% of males with intact AAAs, the aneurysm morphology precludes conventional EVAR (16). However, post-EVAR, there are clinical data to indicate that the suprarenal and visceral segments of the aorta dilate less frequently and rapidly than the infrarenal neck (17). EVAR with suprarenal fixation (SRF) has been postulated as an alternative endovascular approach for the treatment of AAAs with hostile infrarenal neck anatomy (11,18,19). This review aims to summarise and evaluate the current literature with respect to the renal outcomes associated with conventional EVAR using suprarenal and IRF. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-196/rc).
Methods
A literature review was performed using major search indices (PubMed, Google Scholar and EMBASE) to search all scientific articles published to February 2022. The search terms used included: “Suprarenal”, “Infrarenal”, “EVAR”, “Fixation”, “Renal”, “Abdominal Aortic Aneurysm”. Additional sources were identified by reviewing reference lists of relevant publications. Publications with low reliability and non-English publications were excluded. Data were extracted based on their relevance to the topic instead of implementing a systematic approach to paper selection. We present the detail of our search strategy in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 22/2/2022–29/2/2022 |
| Databases and other sources searched | PubMed was the primary database used |
| EMBASE and Scopus were also searched | |
| Search terms used | “Suprarenal” [All Fields] AND “EVAR” [All Fields] AND “Renal” [All Fields] |
| “Suprarenal” [All Fields] AND “EVAR” [All Fields] AND “Renal” [All Fields] AND “Fixation” [All Fields] | |
| “Infrarenal” [All Fields] AND “EVAR” [All Fields] AND “Renal” [All Fields] | |
| “Infrarenal” [All Fields] AND “EVAR” [All Fields] AND “Renal” [All Fields] AND “Fixation” [All Fields] | |
| “Endovascular” [All Fields] AND “Renal” [All Fields] AND “Abdominal Aortic Aneurysm” [All Fields] | |
| Timeframe | 1985–2022 |
| There was a focus on “recent” studies (published from 2010 onwards) that directly compared SRF and IRF | |
| Inclusion and exclusion criteria | Focus was placed on published original papers and reviews in English that directly compared SRF and IRF |
| The study excluded articles that were not relevant for the scope of the paper, or that did not directly compare SRF and IRF | |
| Selection process | The search was conducted independently by AG, KC, HCAY, and MJ; data selection is the intersection of the search of these four authors |
SRF, suprarenal fixation; IRF, infrarenal fixation.
Endovascular repair & endograft fixation
EVAR was first performed by Nicholas L. Volodos’ in 1987 and was later popularised by the work of Juan Carlos Parodi and colleagues in 1991 (20,21). Endografts typically take one of three forms: tube, bifurcated and aorto-uni-iliac, though bifurcated grafts are most popular and are used in more than 90% of EVAR cases (22,23). Most AAAs are diagnosed using ultrasound, however thin-sliced contrast-enhanced computerised tomography (CT) imaging is needed to fully assess aneurysm morphology and to generate the 3D aortic models used in EVAR planning. Morphological parameters assessed using CT include the infrarenal aortic diameter and length proximally, and the suitability of the fixation points in the common iliac arteries distally (19). The major companies producing endografts have differing instructions for use (IFU) defining the exact anatomical measurements that are appropriate for their products. What is apparent is that EVAR devices can be used outside manufacturer IFUs without significantly affecting long-term mortality though this may be associated with higher rates of type 1 endoleak (T1E) (15).
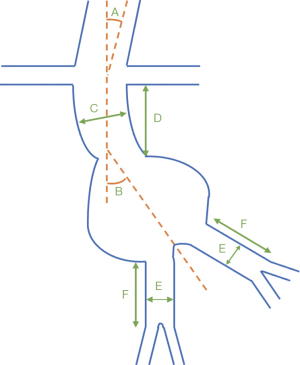
Each device used has differing fixation strategies depending on the aortic anatomy being assessed (see Table 2 and Figures 1,2) (24,25). Stent fixation can occur both passively, using the inherent radial force of the stent graft, and actively, using barbs and hooks to anchor the graft into the aortic wall. In order to generate an adequate radial force and proximal fixation into the available neck, the graft diameter is typically oversized by 10–20% (26). General, epidural, and local anaesthesia have all been successfully used for the endovascular repair of infrarenal AAAs (27,28). Whilst there are various endografts on the global market, the principles of endograft insertion remain similar. IRF, procedurally summarised elsewhere (29), is used where there is suitable aortic neck morphology and describes the positioning of the stent-graft immediately beneath the most inferiorly located renal artery. Where the graft is fixed in the suprarenal location, the procedure is similar but the graft is fixed more proximally with the metallic barbs that anchor the graft extending superiorly to the non-aneurysmal suprarenal aorta above the fabric-covered stent-graft (29). This theoretically allows perfusion to the coeliac, superior mesenteric artery and both renal arteries, which might partially be covered by the bare metallic stent.
Table 2
| Parameters | AnacondaTM (Terumo Aortic) | Zenith® (Cook Medical Technologies) (non-fenestrated) | Zenith® Fenestrated (Cook Medical Technologies) (fenestrated) | Gore® Excluder® (Gore medical) | EndurantTM II (Medtronic) | AorfixTM (Lombard medical technologies) | Incraft® (Cordis®) | Powerlink® (Endologix) | Ovation Prime® (TriVascular) |
|---|---|---|---|---|---|---|---|---|---|
| Neck angle relative to the axis of the suprarenal aorta (Figure 1A) | Unspecified | <45 degrees | <45 degrees | Unspecified | ≤45 degrees (≤60 degrees if infrarenal neck length ≥15 mm) | Unspecified | <60 degrees | Unspecified | Unspecified |
| Neck angle relative to the long axis of the aneurysm (Figure 1B) | ≤90 degrees | <60 degrees | <45 degrees | ≤60 degrees | ≤60 degrees (≤75 degrees if infrarenal neck length ≥15 mm) | ≤90 degrees | <60 degrees | <60 degrees | ≤60 degrees if proximal neck is ≥10 mm and ≤45 degrees if proximal neck is <10 mm |
| Neck diameter (Figure 1C) | 17.5–31 mm | 18–32 mm | 19–31 mm | 19–32 mm | 19–32 mm | 19–29 mm | 17–31 mm | 18–26 mm | 16–30 mm |
| Infrarenal neck length (Figure 1D) | ≥15 mm | ≥15 mm | ≥4 mm | ≥15 mm | ≥10 mm | ≥15 mm | ≥10 mm | ≥15 mm | ≥13 mm |
| CIA diameter (Figure 1E) | 8.5–21 mm | 8–20 mm | 9–21 mm ipsilateral, 7–21 contralateral | 8–25 mm | 8–25 mm | 9–19 mm | 7–22 mm | 10–14 mm | 8–25 mm |
| CIA length (Figure 1F) | Unspecified | >10 mm | >10 mm | >10 mm | >15 mm | ≥15 mm | ≥15 mm | ≥15 mm | ≥10 mm |
| Femoral artery diameter | Unspecified | 6.0 or 6.5 mm | 7.7 or 8.5 mm | ‘Adequate’ | ‘Adequate’ | ‘Adequate’ | ‘Adequate’ | ‘Adequate’ | ‘Adequate’ |
| Fixation location | Infrarenal | Suprarenal | Suprarenal | Infrarenal | Suprarenal | Infrarenal or transrenal | Suprarenal | Infrarenal (but sometimes used as suprarenal) | Suprarenal |

Renal outcomes
Mechanisms of renal function decline (RFD)
One of the major complications of EVAR is a decline in RFD, possibly necessitating dialysis. Although EVAR avoids the renal insult highly associated with OSR (7), there can still be significant consequences. The full mechanism is unclear but suggestions have been made (30-32). The cause for renal injury is likely multifactorial, with the type of endograft, aortic morphology and surgical techniques all being contributors.
An inflammatory foreign body reaction may occur upon stent-graft introduction (31). It is also possible that the stent and graft material being present at the renal artery ostia result in renal artery occlusion or even parenchymal injury. Fixation barbs may interact with plaques at the renal artery origins, leading to luminal compromise. Trans-renal stent struts could cross the renal ostia, leading to a functional stenosis (33). It is also possible that thicker SRF stenting wires that cross the renal artery ostia may cause greater perturbations to blood flow velocity of the renal arteries (34); it is likely that these mechanisms will become clearer after the anticipated publication of current hemodynamic studies (35).
The stenting wires may not cause a significant enough reduction in renal artery flow velocity to impair function. Alternatively, there could be inherent anatomic variability in the interaction between SR stent struts and renal artery ostia (36), as SR stent struts reportedly cross the renal artery ostia in 50% to 80% of patients (37,38). This may further complicate the understanding of the pathogenesis of RFD post-EVAR. Coverage of the origins of the renal arteries may impair blood flow and lead to RFD via occlusion and/or stenosis (31,32). Microembolism and dissections are possibilities (31,32). However, these sequelae are increasingly likely to be anticipated, detected, and avoided with the advent of new software systems being used during EVAR procedures (30,31).
The control of dyslipidaemia and hypertension is important among EVAR patients as these pathological processes can predispose to weakening of the aortic wall (39). These factors could contribute, to microembolisation of the renal arteries, leading to localised ischemia of the renal parenchyma (40). Ischaemia-reperfusion injury arising from compromised arterial flow to lower limbs by instrumentation in the femoral arteries has been suggested as a cause of RFD in EVAR (30,31,41). The exact mechanism is complex, but the oxidative stress formation and peroxidation of lipids seems to be key in promoting the inflammatory processes resulting in renal injury (42). Since EVAR requires contrast media for imaging throughout the treatment process, contrast-induced nephropathy (CIN) (43) could be another cause for RFD post-EVAR, although the exact mechanism is not well established (30,36).
Towards a standardised definition of renal injury
Despite the establishment of EVAR reporting standards (44,45), interpretation of the literature is limited by the heterogenous reporting of renal outcomes. Recent meta-analyses have highlighted this (46-48). It also remains difficult to grasp the essence of RFD as there are limited differences in dialysis or survival between SRF and IRF methods, which necessitates the development of more granular reporting strategies to quantify RFD. Serial serum creatinine (S-Cr) and creatinine clearance (CrCl, based on Cockcroft-Gault formula) are widely reported but are prone to inherent bias and inaccuracies (e.g., affected by nutritional intake, medications, race, age etc.) (49,50). Cystatin-C has been mentioned (51) but remains poorly characterised, perhaps due to its relative cost, hindering its uptake into the clinical realm. The most accurate measure of renal function is estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) (52,53) with a reduction of 20–30% considered clinically significant for medium- and long-term function (39,54). The formula used most commonly is the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) or less commonly, the Modification of Diet in Renal Disease (MDRD). Some have argued the difference in eGFR between the 2 formulas is clinically insignificant (55). However, a recent systematic review and meta-analysis suggested that CKD-EPI is a more accurate estimate of GFR than MDRD (56).
Results from pooled meta-analyses
The effect of the type of stent graft fixation after EVAR on renal function remains uncertain, (18,36,48,57-61). Some studies have shown poorer renal function after SRF (18,19,58,59,61), whereas others suggest minimal differences between the fixation methods (60,62-64). Accepting that certain aortic anatomies are more favourable for SRF, no randomised controlled trials (RCTs) exist which compare the fixation strategies directly. Meta-analyses are therefore necessary to assess the existing literature.
Currently, Calderbank et al. (47) present the only meta-analysis that uses a uniform definition of renal dysfunction as the primary outcome which is a decrease in eGFR of greater than 20% at 1 year. A similar 1-year renal decline between SRF and IRF is observed, but over 5 years, SRF may accelerate renal impairment (47). The five series reporting an eGFR reduction at 1-year showed the weighted OR was 1.53 (SRF vs. IRF) (95% CI: 0.67–3.51; P=0.31; I2=53.2%) (47). Furthermore, 9.3% of those with SRF vs. 7.4% with IRF developed a 20% or higher drop of eGFR by 12 months (47). Secondary outcome was an eGFR drop of greater than 20% at 5 years. Pooling of various definitions of “renal dysfunction” (e.g., S-Cr changes) and variable follow-up time periods meant the analysis data was highly heterogeneous with respect to the secondary outcome. The duration of follow-up ranged from 72 h to 5 years and weighted OR (SRF vs. IRF) was 1.32 (95% CI: 1.01–1.71; P=0.03; I2=28.4%). The overall incidence of renal dysfunction was 5.1% in SRF compared to 4.6% in IRF (47).
Other meta-analyses combine the renal function outcomes (e.g., “Cr increase >20%”, “CrCl decrease >20%” and “GFR decrease >20%”) (36,48). However, these studies also corroborate that the method of aortic wall fixation does not influence earlier RFD at <1 year. However, SRF causes slightly worse outcomes in the longer term. Stather et al. (48) analysed 25 studies (SRF =16,634; IRF =38,198) and demonstrated insignificant differences between SRF and IRF in the short (30-day) or interim term (12 months) (48). The longer-term end points employed were variable between studies, ranging between 1–74 months, and were unable to undergo sensitivity analysis (48). Longer-term follow ups are encouraged in SRF vs. IRF comparative studies for more meaningful long-term outcomes in meta-analyses.
However, at these study endpoints, there were marginally worse RFD outcomes for SRF patients (SRF 5.98% vs. IRF 4.83%; OR =1.29; 95% CI: 1.18–1.40; P<0.001). There were also significant differences reported for renal infarcts (SRF 6.6% vs. IRF 2.3%; OR =2.78; 95% CI: 1.46–5.29; P=0.002), renal stenosis (SRF 2.4% vs. IRF 0.8%; OR =2.89; 95% CI: 1.00–8.38; P=0.05), and renal artery occlusion (SRF 2.4% vs. IRF 1.2%; OR =2.21; 95% CI: 1.15–4.25; P=0.02) (48). The relevance of these data findings must be carefully considered as there were insignificant differences in haemodialysis rates reported between SRF and IRF (P=0.58) (48). Some studies fail to identify these findings (19). The higher incidence of renal infarcts in the SRF group (48) may possibly be due in part to the use of older generations of endografts and the greater known radial fixation force with SRF (36,65). Earlier, Miller et al. (36) analysed 21 studies (SRF =1,949; IRF =2,525) and found statistically insignificant differences in the risk of postoperative renal complications between SRF and IRF over median follow-up of 1-year (36).
Longer-term evidence
Studies that feature 5-year follow-up durations are exclusively retrospective. These databases remain limited, by non-randomised selections which has implications on selection of SRF or IRF. Undoubtedly, SRF is preferred when certain anatomical characteristics are present such as shorter aortic neck length, increased angulation, and larger diameters (60,66). These anatomical characteristics are associated with more advanced degenerative/atherosclerotic disease of the aorta, which should be considered as contributors to the deterioration of renal function.
Saratzis et al. (54) reported on changes in eGFR (via CKD-EPI) in 242 patients following elective EVAR using both SRF and IRF at 5-year compared to baseline. This is the longest available cohort study reporting on renal outcomes with both SRF and IRF. A control group of 121 patients undergoing elective OSR [10 women (8%); mean age 72±6 years] and with available eGFR estimates at 5 years were also identified. They were matched with 242 SRF and 242 IRF patients for age, sex, smoking habits, diabetes, and eGFR at baseline (1:2 ratio). The volume of radiographic contrast (mL) was non-significant (SRF: 121±32 vs. IRF: 118±21, P=0.36) (54). Infrarenal neck anatomy was non-hostile, and the endografts used were Anaconda, Excluder, Endurant, and Zenith (distributions not reported) (54). Proportions of patients in whom an eGFR decrease of >30% from baseline eGFR was reported (18,39). Those undergoing SRF were significantly more likely to experience >30% eGFR decline at 1- and 5-year post-EVAR compared with the IRF population (1 year: IRF 19%, SRF 27%; 5 years: IRF 27%, SRF 47%). Secondary outcomes measured the change in eGFR at 1 year and stage of CKD at 1 and 5 years. There was a marked decline of eGFR during the first postoperative year (−10.7 for SRF and −2.2 for IRF) compared to remainder of follow-up (54). By 5 years, IRF patients had lost a mean of 8.2 mL/min/1.73 m2 (95% CI: 6.5–10.8; P<0.001) compared to 16.9 mL/min/1.73 m2 (95% CI: 13.0–21.9; P<0.001) (54).
Other retrospective studies’ mid- and long-term results also suggest poorer longer-term renal outcomes with SRF (39,57-59). Banno et al. (18) conducted a retrospective mid-term outcomes comparison of SRF (n=135, Zenith, Endurant or Incraft) vs. IRF (n=102, Excluder, Powerlink, Aorfix). Propensity matching of 87 pairs was conducted to exclude patients who had progressed to acute kidney injury (AKI) (18). This occurred significantly more frequently in the SRF group than in the IRF group (P=0.026) (18). Propensity scores were estimated using covariates of age, sex, baseline renal function, aortic aneurysm diameter, comorbidities, smoking habits, use of drugs impairing renal function, and frequency of contrast-enhanced CT scans performed (18).
Mid-term outcomes of 3-year were reported, though the median follow-up duration was 5.1 years (IQR: 4.0–6.4). Logistic regression analysis showed that SRF was independently predictive of >20% decline in the eGFR at 3 years post-EVAR (OR =2.06; 95% CI: 1.18–3.58; P=0.11) (18). The decrease in eGFR within the SRF group gradually increased annually and reached statistical difference at 3 years post-surgery compared with the IRF group (mean 17.8% vs. 11.6%, respectively; P=0.034) (18). Hahl et al. (39) retrospectively compared SRF (n=267, Zenith or Endurant) vs. IRF (n=91, Excluder) over 5-year. The baseline demographics, procedure duration and volume of contrast reagent used were insignificant between the groups, although the IRF group had higher rates of hypertension (78.0% vs. 58.4%, P=0.001) and dyslipidaemia (54.9% vs. 40.4%, P=0.020) (39). At 7-day post-intervention, 13.7% (n=36) of the SRF group vs. 3.5% (n=3) of the IRF group experienced ≥20% decline in eGFR (P=0.009) (39). Median eGFR declined significantly from baseline levels to the 5-year time point in both groups (SRF 72.0 vs. 51.0, P<0.001; IRF 69.0 vs. 54.5, P=0.001). Mixed model analysis showing an annual rate of eGFR decline of −3.13 (95% CI: −3.40 to 2.85; P<0.01) (39). Furthermore, in both the SRF and IRF cohorts, the number with ≥20% decline in eGFR did not differ significantly by 30-day and also in the follow-up years 1–5 [see Table 3, Hahl et al. (39)].
Table 3
| First author | Publication date | Sample, n | Follow-up, years | Measurement used for renal function deterioration | Formula used for eGFR calculation | Main devices, % of total SRF + IRF cohort | Conclusion on renal outcomes over follow-up period |
|---|---|---|---|---|---|---|---|
| Hahl et al. (39) | 2022 | SRF: 267 | 5 | eGFR decline ≥20% | CKD-EPI | Zenith; Endurant | Transient but immediately greater postoperative RFD in SRF; IRF is safer in the longer term, especially in patients with baseline renal insufficiency |
| IRF: 91 | Excluder | ||||||
| Blecha et al. (67) | SRF: 76 | 5 | eGFR decline ≥20% | Not reported | Powerlink (23%); Zenith (13%); Talent (6%); | Female sex and baseline renal insufficiency are significant risk factors for RFD at 5 years (multivariate); SRF was significant risk factor for RFD at 5 years on univariate analysis and approached significance on multivariate analysis | |
| IRF: 58 | Excluder (39%); Aneurx (9%) | ||||||
| Erben et al. (68) | 2021 | SRF: 670 | 4.8±3.7 | eGFR decline over follow up; rate of change of creatinine | MDRD | Not reported | Insignificant eGFR decline and rate of change of creatinine over follow up between SRF and IRF; longer hospital stay in SRF patients (3.4±2.2 vs. 2.3±1.0 days; P<0.001); SRF and female sex are predictors of longer hospital stay (multivariable regression) |
| IRF: 460 | |||||||
| Pujari et al. (61) | SRF: 3,225 | 30 days | Cr >2 mg/dL without dialysis or new dialysis | MDRD | Zenith (25%); Endurant (34%) | SRF is associated with more perioperative renal complications than IRF, especially in patients with baseline renal insufficiency | |
| IRF: 2,309 | Excluder (42%) | ||||||
| Sangtae et al. (60) | SRF: 114 | 3.8±2.6 | <30% decrease in eGFR; >30% increase in follow-up sCra; dialysis needed | CKD-EPI; MDRD; Cockcroft-Gault | Endurant (42%); Talent (4%) | Insignificant difference in renal function and adverse events (P>0.107) between SRF and IRF | |
| IRF: 54 | 3.3±2.2 | Excluder (24%); Powerlink (6%); Aneurx (2%); | |||||
| Banno et al. (18) | 2020 | SRF: 135 | 5.5±1.8 (study reported on mid-term outcomes at 3-year) | eGFR decline ≥20% | 3-variable Japanese equationb | Zenith (30%); Endurant/Talent (24%); Incraft (3%) | SRF has worse mid-term outcomes than IRF based on mean eGFR decline (SRF: 17.8% decline; IRF: 11.6% decline; P=0.034) |
| IRF: 102 | Excluder (37%); Powerlink (5%) | ||||||
| Zettervall et al. (58) | 2017 | SRF: 2,273 (63%) | 30 days | Cr >2 mg/dL without dialysis or new dialysis | Not reported | Zenith; Endurant | Overall rates of RFD in EVAR are low; SRF associated with higher likelihood of perioperative RFD (OR =12.0; 95% CI: 1.6–91) and postoperative hospital stay duration >2 days (OR =1.4; 95% CI: 1.2–1.7) |
| IRF: 1,314 (37%) | Excluder | ||||||
| Zettervall et al. (57) | SRF: 1,264 | 2.6 | sCr >0.5 mg/dL or new perioperative haemodialysis | Zenith (31%); Endurant (12%); Talent (7%) | SRF associated with higher rates of RFD (OR =2.0; 95% CI: 1.2–3.4) and postoperative hospital stay duration >2 days (OR =1.8; 95% CI: 1.4–2.2) | ||
| IRF: 1,310 | Excluder (40%); AneuRx (11%) |
a, if initial sCr 1.2 mg/dL, follow-up scar 1.46 mg/dL was considered as renal impairment by calculating 1.2+1.2×30%; b, 3-variable Japanese eGFR equation (69): 194 × Serum Creatinine − 1.094 × age − 0.287 × 0.739 (if female). SRF: Zenith (Cook Medical, Bloomington, IN, USA); Endurant/Talent (Medtronic, Minneapolis, MN, USA); Incraft (Cordis Corp, Bridgewater, NJ, USA). IRF: Excluder (W. L. Gore & Associates, Flagstaff, AZ, USA); Powerlink (Endologix, Irvine, CA, USA); Aorfix (Lombard Medical, Irvine, CA, USA); AneuRx (Medtronic, Minneapolis, MN, USA). (s)Cr, serum creatinine, mg/dL. SRF, suprarenal fixation; IRF, infrarenal fixation; CKD-FPI, Chronic Kidney Disease Epidemiology Collaboration; RFD, renal function decline; MDRD, Modification of Diet in Renal Disease.
It is likely those with pre-existing renal insufficiency are prone to poorer EVAR outcomes when SRF is used (39,61,67,68). This may be complicated by non-randomised selection for SRF but possibly depends on surgical experience and the extent of aortic neck morphology (70,71). Hahl et al. (39) reported a sub-group analysis with pre-existing renal insufficiency (eGFR ≤60) and showed more in the SRF (n=86) compared to the IRF group (n=25) had a ≥20% eGFR decline (59.5% vs. 20.0%, P=0.036) (39). Pujari et al. (61) also included a subgroup analysis of patients with moderate kidney dysfunction (n=1,780; GFR =30–59). They reported higher rates of renal complications with SRF than IRF (SRF: 2.2% vs. IRF: 0.8%, P=0.02) (61). Blecha et al. (67) considered baseline renal insufficiency (multivariate OR =3.0, P=0.029) to be a significant predictor of >20% GFR decline by 5 years after EVAR. In addition, when using SRF in females; multivariate binary logistic regression analysis showed female sex (OR =3.9, P=0.023) to be a predictor of >20% GFR decline at 5 years (67).
Similarly, Erben et al. (68), using follow-up of 4.8±3.7 years, found on Kaplan-Meier analysis that CKD patients undergoing SRF were more likely to progress to haemodialysis (P=0.039). Furthermore, the authors found on least square multivariable regression that SRF (Coef, 9.5; 95% CI: 0.11–1.11; P<0.0001) and female sex (Coef, 2.4; 95% CI: 0.17–0.41; P=0.02) were predictive of prolonged length of hospital stay (68). However, in the mid-term (3-year), sex of the patient has not been significantly associated with RFD (18).
Zettervall et al. (57) attempted to reduce any effect of patient selection by performing a sub-group analysis in those undergoing either SRF or IRF. SRF had a significantly increased risk of RFD (defined as S-Cr increase >0.5 mg/dL), irrespective of whether they were performed by surgeons of the Vascular Study Group of New England (VSGNE) (OR =2.0; 95% CI: 1.2–3.4) or those who routinely use SRF or IRF (SRF 3.7% vs. 1.3%, P=0.02; OR =2.9; 95% CI: 1.1–7.8) (57). However, as the authors argue, it is possible that routine users of IRF endografts still selected IRF grafts in preference of the straightforward anatomy and referred more complicated cases to those with more experience with SRF (57). Additionally, hostile aortic anatomies requiring SRF invariably require more contrast-medium which may confound study results. Those undergoing SRF received more contrast than those undergoing IRF (110 vs. 88 mL, P<0.01) (57). Other studies might report contrast volume differences as insignificant but possible effects on renal function should not be overlooked (39,54).
Future directions in clinical and academic practice
Those undergoing EVAR are often elderly with usually associated comorbidities. RFD following EVAR might be expected, irrespective of endograft fixation levels (62,64,72,73). SRF is regarded as safe in those with normal renal function in the short-term. However, there appears to be a trend towards greater RFD in those undergoing SRF over mid- and longer-term periods (18,39,67). Management of RFD requires regular blood level monitoring, especially those with preoperative baseline renal insufficiency. It is possible that biomarker-informed surveillance strategies for monitoring the development of RFD will grow in uptake, but at present, remain in their early stages (74,75).
An obvious clinical solution is to undertake additional periprocedural precautions. Procedurally, EVAR may cause renal impairment either through the procedure itself, or by associated CIN and dehydration. Atheromatous microembolisation can be minimised by reducing the manipulation to the patient (e.g., via catheterisation, wires and balloons) (30,40). It remains uncertain whether remote ischaemic preconditioning is protective against reperfusion-injuries of the kidneys (76,77). Obviously, to minimise CIN, postprocedural contrast use should be kept to a minimal level (30).
Contrast-precautions should be applied to all undergoing EVAR, rather than those with pre-existing renal dysfunction (30). Certain drugs such as fenoldopam may also be protective against CIN (78). Additionally, RenalGuard® and hemodynamic guided hydration have been proven as optimal strategies for preventing CIN in high-risk patients in a 2020 systematic review and meta-analysis (79). To mitigate EVAR-associated dehydration, bolus high-dose NaHCO3 (1 mL/kg of 8.4% NaHCO3) and crystalloid hydration has shown promise as a nephroprotective agent (80). By 30-day post EVAR, 7% of those in the intervention arm (aggressive rehydration with a single bolus of NaHCO3) developed AKI compared to 33% in the control arm (aggressive rehydration only) (80). The proportion of EVARs undergoing SRF was 97% (56/58) (80).
Conclusions
The steeper short-term decline in renal function associated with SRF vs. IRF appears to have little clinical relevance in those with normal baseline renal function. The limited long-term evidence suggests that SRF is associated with an accelerated decline in renal function compared to IRF, although this may be partially due to a higher prevalence of advanced degenerative/atherosclerotic disease in SRF cohorts. Additional caution is necessary in females with baseline renal insufficiency. RFD with SRF has been emphasised in this population. In future practice, longer-term reporting on renal function outcomes must be performed to characterise the effects of SRF vs. IRF. Further multicentre prospective studies are necessary to improve the available evidence with further control for possible bias in patient-selection and surgical experience according to the anatomical features of the aorta and the aneurysms. It is realistic to expect that improvements in device design, surgical experience and clinical care would reflect improved renal outcomes in future studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Frozen Elephant Trunk”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-196/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-196/coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. MB served as the unpaid Guest Editor of the series. DMB is funded by a Royal Society Wolfson Research Fellowship (#WM170007) and separate grants from the Royal Society (No. IES/R2/192137) and Japan Society for the Promotion of Science (No. JSPS/OF317). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aggarwal S, Qamar A, Sharma V, et al. Abdominal aortic aneurysm: A comprehensive review. Exp Clin Cardiol 2011;16:11-5. [PubMed]
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463-654. [PubMed]
- Ouriel K, Green RM, Donayre C, et al. An evaluation of new methods of expressing aortic aneurysm size: relationship to rupture. J Vasc Surg 1992;15:12-8; discussion 19-20. [Crossref] [PubMed]
- Li X, Zhao G, Zhang J, et al. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population–a meta-analysis. PloS One 2013;8:e81260. [Crossref] [PubMed]
- Albrecht T, Jäger HR, Blomley MJ, et al. Pre-operative classification of abdominal aortic aneurysms with spiral CT: the axial source images revisited. Clin Radiol 1997;52:659-65. [Crossref] [PubMed]
- Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019;57:8-93. [Crossref] [PubMed]
- Boyle JR, Goodall S, Thompson JP, et al. Endovascular AAA repair attenuates the inflammatory and renal responses associated with conventional surgery. J Endovasc Ther 2000;7:359-71. [Crossref] [PubMed]
- Dua A, Kuy S, Lee CJ, et al. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg 2014;59:1512-7. [Crossref] [PubMed]
- Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med 2019;380:2126-35. [Crossref] [PubMed]
- Cross J, Gurusamy K, Gadhvi V, et al. Fenestrated endovascular aneurysm repair. Br J Surg 2012;99:152-9. [Crossref] [PubMed]
- Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67:2-77.e2. [Crossref] [PubMed]
- Chisci E, Kristmundsson T, de Donato G, et al. The AAA with a challenging neck: outcome of open versus endovascular repair with standard and fenestrated stent-grafts. J Endovasc Ther 2009;16:137-46. [Crossref] [PubMed]
- Georgiadis GS, Trellopoulos G, Antoniou GA, et al. Early results of the Endurant endograft system in patients with friendly and hostile infrarenal abdominal aortic aneurysm anatomy. J Vasc Surg 2011;54:616-27. [Crossref] [PubMed]
- Leurs LJ, Kievit J, Dagnelie PC, et al. Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2006;13:640-8. [Crossref] [PubMed]
- Oliveira-Pinto J, Oliveira N, Bastos-Gonçalves F, et al. Long-term results of outside “instructions for use” EVAR. J Cardiovasc Surg (Torino) 2017;58:252-60. [Crossref] [PubMed]
- Ulug P, Sweeting MJ, von Allmen RS, et al. Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet 2017;389:2482-91. [Crossref] [PubMed]
- Sonesson B, Malina M, Ivancev K, et al. Dilatation of the infrarenal aneurysm neck after endovascular exclusion of abdominal aortic aneurysm. J Endovasc Surg 1998;5:195-200. [Crossref] [PubMed]
- Banno H, Ikeda S, Kawai Y, et al. Suprarenal fixation is associated with worse midterm renal function after endovascular abdominal aortic aneurysm repair compared with infrarenal fixation. J Vasc Surg 2020;71:450-6. [Crossref] [PubMed]
- Saratzis A, Sarafidis P, Melas N, et al. Suprarenal graft fixation in endovascular abdominal aortic aneurysm repair is associated with a decrease in renal function. J Vasc Surg 2012;56:594-600. [Crossref] [PubMed]
- Volodos’ NL, Karpovich IP, Shekhanin VE, et al. A case of distant transfemoral endoprosthesis of the thoracic artery using a self-fixing synthetic prosthesis in traumatic aneurysm. Grudn Khir 1988;84-6. [PubMed]
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5:491-9. [Crossref] [PubMed]
- Vallabhaneni SR, Harris PL. Lessons learnt from the EUROSTAR registry on endovascular repair of abdominal aortic aneurysm repair. Eur J Radiol 2001;39:34-41. [Crossref] [PubMed]
- England A, Mc Williams R. Endovascular aortic aneurysm repair (EVAR). Ulster Med J 2013;82:3-10. [PubMed]
- Gozzo C, Caruana G, Cannella R, et al. CT angiography for the assessment of EVAR complications: a pictorial review. Insights Imaging 2022;13:5. [Crossref] [PubMed]
- Hu DK, Pisimisis GT, Sheth RA. Repair of abdominal aortic aneurysms: preoperative imaging and evaluation. Cardiovasc Diagn Ther 2018;8:S157-67. [Crossref] [PubMed]
- van Prehn J, Schlösser FJ, Muhs BE, et al. Oversizing of aortic stent grafts for abdominal aneurysm repair: a systematic review of the benefits and risks. Eur J Vasc Endovasc Surg 2009;38:42-53. [Crossref] [PubMed]
- Noh M, Choi BM, Kwon H, et al. General anesthesia versus local anesthesia for endovascular aortic aneurysm repair. Medicine (Baltimore) 2018;97:e11789. [Crossref] [PubMed]
- Bettex DA, Lachat M, Pfammatter T, et al. To compare general, epidural and local anaesthesia for endovascular aneurysm repair (EVAR). Eur J Vasc Endovasc Surg 2001;21:179-84. [Crossref] [PubMed]
- Kim HO, Yim NY, Kim JK, et al. Endovascular Aneurysm Repair for Abdominal Aortic Aneurysm: A Comprehensive Review. Korean J Radiol 2019;20:1247-65. [Crossref] [PubMed]
- Walsh SR, Tang TY, Boyle JR. Renal consequences of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2008;15:73-82. [Crossref] [PubMed]
- Jhaveri KD, Saratzis AN, Wanchoo R, et al. Endovascular aneurysm repair (EVAR)- and transcatheter aortic valve replacement (TAVR)-associated acute kidney injury. Kidney Int 2017;91:1312-23. [Crossref] [PubMed]
- Saratzis AN, Goodyear S, Sur H, et al. Acute kidney injury after endovascular repair of abdominal aortic aneurysm. J Endovasc Ther 2013;20:315-30. [Crossref] [PubMed]
- Subedi SK, Lee AM, Landis GS. Suprarenal fixation barbs can induce renal artery occlusion in endovascular aortic aneurysm repair. Ann Vasc Surg 2010;24:113.e7-113.e10. [Crossref] [PubMed]
- Sun Z, Chaichana T. Investigation of the hemodynamic effect of stent wires on renal arteries in patients with abdominal aortic aneurysms treated with suprarenal stent-grafts. Cardiovasc Intervent Radiol 2009;32:647-57. [Crossref] [PubMed]
- Salomon du Mont L, Parmentier AL, Puyraveau M, et al. To assess hemodynamic disturbances to the ostia of the renal arteries generated by the implantation of EVAR with a suprarenal fixation. Medicine (Baltimore) 2020;99:e19917. [Crossref] [PubMed]
- Miller LE, Razavi MK, Lal BK. Suprarenal versus infrarenal stent graft fixation on renal complications after endovascular aneurysm repair. J Vasc Surg 2015;61:1340-9.e1. [Crossref] [PubMed]
- England A, Butterfield JS, Ashleigh RJ. Incidence and effect of bare suprarenal stent struts crossing renal ostia following EVAR. Eur J Vasc Endovasc Surg 2006;32:523-8. [Crossref] [PubMed]
- Sun Z, Winder RJ, Kelly BE, et al. CT virtual intravascular endoscopy of abdominal aortic aneurysms treated with suprarenal endovascular stent grafting. Abdom Imaging 2003;28:580-7. [Crossref] [PubMed]
- Hahl T, Kurumaa T, Uurto I, et al. The effect of suprarenal graft fixation during endovascular aneurysm repair on short- and long-term renal function. J Vasc Surg 2022;76:96-103.e1. [Crossref] [PubMed]
- Boules TN, Stanziale SF, Chomic A, et al. Predictors of diffuse renal microembolization following endovascular repair of abdominal aortic aneurysms. Vascular 2007;15:18-23. [Crossref] [PubMed]
- Wijnen MH, Cuypers P, Buth J, et al. Differences in renal response between endovascular and open repair of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2001;21:171-4. [Crossref] [PubMed]
- Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev 2015;4:20-7. [PubMed]
- Cho E, Ko GJ. The Pathophysiology and the Management of Radiocontrast-Induced Nephropathy. Diagnostics (Basel) 2022;12:180. [Crossref] [PubMed]
- Boyle JR, Thompson MM, Vallabhaneni SR, et al. Pragmatic minimum reporting standards for endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2011;18:263-71. [Crossref] [PubMed]
- Chaikof EL, Fillinger MF, Matsumura JS, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1061-6. [Crossref] [PubMed]
- Karthikesalingam A, Bahia SS, Patel SR, et al. A systematic review and meta-analysis indicates underreporting of renal dysfunction following endovascular aneurysm repair. Kidney Int 2015;87:442-51. [Crossref] [PubMed]
- Calderbank T, Bown M, Saratzis A. The Impact of Suprarenal Fixation on Renal Function Following Endovascular Abdominal Aortic Aneurysm Repair: Meta-analysis Based on Estimated Glomerular Filtration Rate. Eur J Vasc Endovasc Surg 2018;56:497-506. [Crossref] [PubMed]
- Stather PW, Ferguson J, Awopetu A, et al. Meta-analysis of Renal Function Following Infrarenal EVAR using Suprarenal or Infrarenal Fixation Devices. Eur J Vasc Endovasc Surg 2018;56:486-96. [Crossref] [PubMed]
- Greenberg RK, Chuter TA, Lawrence-Brown M, et al. Analysis of renal function after aneurysm repair with a device using suprarenal fixation (Zenith AAA Endovascular Graft) in contrast to open surgical repair. J Vasc Surg 2004;39:1219-28. [Crossref] [PubMed]
- Davey P, Rose JD, Parkinson T, et al. The mid-term effect of bare metal suprarenal fixation on renal function following endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2006;32:516-22. [Crossref] [PubMed]
- Abdelhamid MF, Davies RS, Vohra RK, et al. Assessment of renal function by means of cystatin C following standard and fenestrated endovascular aneurysm repair. Ann Vasc Surg 2013;27:708-13. [Crossref] [PubMed]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. [Crossref] [PubMed]
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247-54. [Crossref] [PubMed]
- Saratzis A, Bath MF, Harrison S, et al. Long-Term Renal Function after Endovascular Aneurysm Repair. Clin J Am Soc Nephrol 2015;10:1930-6. [Crossref] [PubMed]
- Delanaye P, Pottel H, Botev R, et al. Con: Should we abandon the use of the MDRD equation in favour of the CKD-EPI equation? Nephrol Dial Transplant 2013;28:1396-403; discussion 403. [Crossref] [PubMed]
- McFadden EC, Hirst JA, Verbakel JY, et al. Systematic Review and Metaanalysis Comparing the Bias and Accuracy of the Modification of Diet in Renal Disease and Chronic Kidney Disease Epidemiology Collaboration Equations in Community-Based Populations. Clin Chem 2018;64:475-85. [Crossref] [PubMed]
- Zettervall SL, Deery SE, Soden PA, et al. Editor’s Choice – Renal complications after EVAR with suprarenal versus infrarenal fixation among all users and routine users. Eur J Vasc Endovasc Surg 2017;54:287-93. [Crossref] [PubMed]
- Zettervall SL, Soden PA, Deery SE, et al. Comparison of Renal Complications between Endografts with Suprarenal and Infrarenal Fixation. Eur J Vasc Endovasc Surg 2017;54:5-11. [Crossref] [PubMed]
- Buck DB, Soden PA, Deery SE, et al. Comparison of Endovascular Stent Grafts for Abdominal Aortic Aneurysm Repair in Medicare Beneficiaries. Ann Vasc Surg 2018;47:31-42. [Crossref] [PubMed]
- Sangtae C, Wonsuk L, Jinmo K, et al. Effect of stent graft fixation types on pararenal aortic diameter and renal function after endovascular aortic repair. Turk Gogus Kalp Damar Cerrahisi Derg 2021;29:434-42. [Crossref] [PubMed]
- Pujari A, Ramos CR, Duwayri Y, et al. Influence of baseline kidney dysfunction on perioperative renal outcomes after endovascular aneurysm repair with suprarenal fixation. J Vasc Surg 2021;73:92-8. [Crossref] [PubMed]
- Surowiec SM, Davies MG, Fegley AJ, et al. Relationship of proximal fixation to postoperative renal dysfunction in patients with normal serum creatinine concentration. J Vasc Surg 2004;39:804-10. [Crossref] [PubMed]
- Gray DE, Eisenack M, Gawenda M, et al. Repeated contrast medium application after endovascular aneurysm repair and not the type of endograft fixation seems to have deleterious effect on the renal function. J Vasc Surg 2017;65:46-51. [Crossref] [PubMed]
- Parmer SS, Carpenter JP. Endologix Investigators. Endovascular aneurysm repair with suprarenal vs infrarenal fixation: a study of renal effects. J Vasc Surg 2006;43:19-25. [Crossref] [PubMed]
- Melas N, Saratzis A, Saratzis N, et al. Aortic and iliac fixation of seven endografts for abdominal-aortic aneurysm repair in an experimental model using human cadaveric aortas. Eur J Vasc Endovasc Surg 2010;40:429-35. [Crossref] [PubMed]
- Pintoux D, Chaillou P, Azema L, et al. Long-term influence of suprarenal or infrarenal fixation on proximal neck dilatation and stentgraft migration after EVAR. Ann Vasc Surg 2011;25:1012-9. [Crossref] [PubMed]
- Blecha M, Malach L, Dickens B, et al. Predictors of Decline in Renal Function 5 Years after EVAR. Vasc Endovascular Surg 2022;56:166-72. [Crossref] [PubMed]
- Erben Y, Li Y, Mao MA, et al. Proximal fixation of endovascular aortic device may not be associated with renal function decline after abdominal aortic aneurysm repair. J Vasc Surg 2021;74:1861-6.e1. [Crossref] [PubMed]
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982-92. [Crossref] [PubMed]
- Fairman RM, Velazquez OC, Carpenter JP, et al. Midterm pivotal trial results of the Talent Low Profile System for repair of abdominal aortic aneurysm: analysis of complicated versus uncomplicated aortic necks. J Vasc Surg 2004;40:1074-82. [Crossref] [PubMed]
- Hager ES, Cho JS, Makaroun MS, et al. Endografts with suprarenal fixation do not perform better than those with infrarenal fixation in the treatment of patients with short straight proximal aortic necks. J Vasc Surg 2012;55:1242-6. [Crossref] [PubMed]
- Antonello M, Menegolo M, Piazza M, et al. Outcomes of endovascular aneurysm repair on renal function compared with open repair. J Vasc Surg 2013;58:886-93. [Crossref] [PubMed]
- Charles ER, Lui D, Delf J, et al. Editor’s Choice – The Impact of Endovascular Aneurysm Repair on Long Term Renal Function Based on Hard Renal Outcomes. Eur J Vasc Endovasc Surg 2019;58:328-33. [Crossref] [PubMed]
- Brulotte V, Leblond FA, Elkouri S, et al. Bicarbonates for the prevention of postoperative renal failure in endovascular aortic aneurysm repair: a randomized pilot trial. Anesthesiol Res Pract 2013;2013:467326. [Crossref] [PubMed]
- Ueta K, Watanabe M, Iguchi N, et al. Early prediction of acute kidney injury biomarkers after endovascular stent graft repair of aortic aneurysm: a prospective observational study. J Intensive Care 2014;2:45. [Crossref] [PubMed]
- De Freitas S, Hicks CW, Mouton R, et al. Effects of Ischemic Preconditioning on Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-analysis. J Surg Res 2019;235:340-9. [Crossref] [PubMed]
- Stather PW, Wych J, Boyle JR. A systematic review and meta-analysis of remote ischemic preconditioning for vascular surgery. J Vasc Surg 2019;70:1353-1363.e3. [Crossref] [PubMed]
- Caixeta A, Dogan O, Weisz G. Contrast-induced nephropathy: protective role of fenoldopam. Clin Exp Pharmacol Physiol 2012;39:497-505. [Crossref] [PubMed]
- Cai Q, Jing R, Zhang W, et al. Hydration Strategies for Preventing Contrast-Induced Acute Kidney Injury: A Systematic Review and Bayesian Network Meta-Analysis. J Interv Cardiol 2020;2020:7292675. [Crossref] [PubMed]
- Saratzis A, Chiocchia V, Jiffry A, et al. HYDration and Bicarbonate to Prevent Acute Renal Injury After Endovascular Aneurysm Repair With Suprarenal Fixation: Pilot/Feasibility Randomised Controlled Study (HYDRA Pilot Trial). Eur J Vasc Endovasc Surg 2018;55:648-56. [Crossref] [PubMed]


