
Early surgery determines prognosis in patients with infective endocarditis: outcome in patients managed by an Endocarditis Team—a prospective cohort study
Introduction
Infective endocarditis (IE) remains a disease with significant morbidity and mortality rates despite advances in diagnostic and therapeutic strategies (1-3). Proposed reasons for the continued poor prognosis seen in the developed world include increased patient age, an increase in nosocomial IE, the changing microbiological profile (predominantly Staphylococcus aureus) and an increase in patients with prosthetic valve (PVE) or device-related infective endocarditis (DRE) (2). IE in South Africa, despite a significantly younger age group of patients with fewer co-morbidities and a limited number of cases of PVE, DRE and nosocomial IE, remains associated with similar or even higher morbidity and mortality rates when compared to developed-world cohorts (1,2,4-7).
A number of factors have been investigated as potential contributors to the higher than expected rate of adverse outcomes observed in South Africa. IE in South Africa is typically associated with high rates of blood culture negative IE (BCNIE) where no organism is cultured and no cause is identified. Although incompletely studied (8), late presentation with a delay in diagnosis and a high prevalence of underlying rheumatic heart disease (RHD) are also proposed reasons for explaining the adverse outcomes reported (1,4-6). Due to the high prevalence of underlying RHD, combined with the young age of patients with IE, the majority of patients that require surgery will receive a mechanical valve replacement rather than valvular repair surgery or a bioprosthesis (1,5,7). Mechanical prostheses in patients with RHD in South Africa have significantly higher mortality and morbidity rates than reported in developed countries, mainly due to an increased rate of valve-related complications including thrombo-embolism and bleeding (7,9). Novel surgical techniques, specifically mitral valve reconstruction using a saphenous vein, have emerged as possible alternatives to mechanical valve replacement (10). This may be an alternative option for patients with mitral valve disease, specifically due to RHD, that are not suitable for conventional repair surgery, or patients with significant mitral valve destruction that precludes them from conventional repair. Mitral valve reconstruction with a saphenous vein limits the need for prosthetic material and prevents the need for long term anti-coagulation (10,11).
Very limited data are available on the timing of surgery for patients with IE in South Africa, but available evidence would suggest that a significant number of patients are offered surgery only after the completion of antimicrobial therapy, often on an outpatient or elective basis (7). The possible delay in surgery for patients with guideline indications for surgery may be an additional contributor to the current high mortality and morbidity rates observed. Although the benefits of early surgery (while still receiving antimicrobial therapy) are well established (3,12), some clinicians favour a longer course or completion of antimicrobial therapy before surgery due to a perceived higher rate of surgical complications in incompletely treated patients, and to prevent relapse of infection in patients undergoing PVE replacement (1,12). The decision regarding timing of surgery in patients with IE, without an emergency indication for surgery, should balance the risk of having acute haemodynamic decompensation or cerebral embolism before surgery is undertaken with the associated higher risks of surgical complications or relapse (3). An important factor, that influences both the access to surgery and timing of surgery, is the limited availability of cardiothoracic surgery in South Africa and even more so in most other African countries (13,14). This is especially important in the context of applying international guidelines for early surgery (3) locally and when comparing outcomes of cohorts from low and middle income countries to countries where access to cardiothoracic surgery is not limited (15,16).
The objective of this study was to describe the outcome of patients with IE, postulating that the management of patients with IE by an Endocarditis Team, adhering to a set protocol for organism detection and advanced imaging, coupled with a strategy of early surgery favouring valve repair or reconstruction would be feasible and should improve the outcome in patients with IE in a region with a high prevalence of RHD (17,18). We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-590/rc) (19).
Methods
Study design and participants
Consecutive patients presenting with IE to the Division of Cardiology, Department of Medicine at Tygerberg Hospital in Cape Town, South Africa, between November 2019 and April 2021 were prospectively enrolled in the Tygerberg Endocarditis Cohort (TEC) study as previously described (20-23). Patients younger than 14 years of age and those with known or newly diagnosed malignancy were excluded. The Division of Cardiology at Tygerberg Hospital is a public sector tertiary referral centre that serves a population of approximately 2.4 million people (24). Patients with features of IE presenting to hospitals within the referral network are referred to Tygerberg Hospital for definitive care (25). The Division of Cardiology is categorised as a high volume centre for the diagnosis and management of IE (2). Patients were managed by an Endocarditis Team following diagnostic and management criteria as set out by current guidelines (3). The Endocarditis Team comprised of cardiologists, including imaging specialists, cardiothoracic surgeons, infectious diseases specialists and a microbiologist as set out in the guidelines (1). Other specialties, such as neurologists and nuclear medical specialists attended the weekly meetings on invitation.
Echocardiographic evaluation
Enrolled patients underwent transthoracic echocardiography (TTE) with transoesophageal echocardiography (TEE) performed in patients without a contraindication for this procedure (26,27). In addition to a standard echocardiographic evaluation (26,27), a detailed assessment of the underlying heart and valvular structure, including an assessment for the presence of RHD, was performed (17,18,28-30).
Additional imaging
Additional imaging, including cardiac computer tomography (CT) with or without 18-fluorodeoxyglucose positron emission tomography (18FDG-PET) scanning and cardiac magnetic image resonance (CMR), was performed at the discretion of the Endocarditis Team.
Microbiological assessment
A standardised stepwise protocol for organism detection was implemented to identify the common causative organisms of IE and to minimise the rate of BCNIE (1,23). Further management and analysis of the samples were done according to current published guidelines (3,23). Patients without an identified organism after 5 days using standard culture techniques, were defined as having BCNIE.
Additional testing was performed on all BCNIE patients, including:
- Serology for detection of IgM and IgG antibodies to Bartonella species, Brucella species, Coxiella burnetii, Legionella pneumophila and Mycoplasma pneumoniae.
- Testing for antinuclear antibodies (ANA) and anti-cardiolipin antibodies (ACLA).
- Broad range polymerase chain reaction (PCR) and sequencing of 16S rRNA for bacteria and ITS2 for fungi were performed on negative blood cultures.
A sample of heart valve tissue was collected from all patients who underwent surgery and this was submitted for:
- Bacterial and fungal culture.
- Broad range PCR and sequencing of 16S rRNA and ITS2.
- Histopathologic examination for detection of bacteria and fungi, as well as histopathological features of IE.
Management of patients
Patients were evaluated daily, the presence and timing of any major adverse events (death, embolic events and renal failure requiring dialysis) documented and antimicrobial therapy tailored as microbiology results became available. Empirical intravenous therapy was used where no organism was identified as per the current guidelines (3). In cases of Bartonella-associated IE, oral doxycycline 100 mg twice daily was added and continued for a minimum of 6 weeks (3,23). All surviving patients had routine clinical follow-up 6 months after diagnosis.
A weekly Endocarditis Team meeting was held to review all current cases and discuss management decisions. Patients with an indication for surgery (3), were systematically evaluated for a possible valve repair or reconstruction option and the optimal timing of surgery was agreed on.
The timing and urgency of surgery were defined as follows:
- Emergency surgery—within 48 hours of diagnosis.
- Early surgery—surgery before completion of antibiotic therapy with an aim to perform surgery within 14 days of diagnosis.
- Elective surgery—surgery after completion of antibiotic therapy.
As noted, a strategy favouring repair and minimising the use of prosthetic material was adhered to in patients with native valve endocarditis as follows:
- Patients with non-rheumatic mitral valves deemed repairable, primarily including those with degenerative valve disease or where no underlying valvular abnormality was detected, were considered for conventional valve repair.
- Patients with underlying rheumatic mitral valve disease and non-rheumatic mitral valve associated IE that were deemed not suitable for conventional repair due to extensive leaflet destruction were evaluated for anterior mitral valve reconstruction with a saphenous vein (10,11).
- The following exclusion criteria for mitral valve reconstruction with a saphenous vein applied:
- Emergency surgery.
- Left ventricular ejection fraction less than 40%.
- Very severe mitral stenosis with a mitral valve area <1 cm2.
- Severe subvalvular involvement of the mitral valve defined by thickening of the subvalvular apparatus from the papillary muscle to the valve leaflet (Wilkins score of 4 for subvalvular involvement) (31).
- Presence of aortic valve pathology requiring intervention.
- Inability to harvest an adequate saphenous vein.
- Patients not deemed suitable for conventional repair or mitral valve reconstruction using saphenous vein tissue were scheduled for mitral valve replacement.
- The default strategy for aortic valve intervention was aortic valve replacement.
- Patients who were scheduled for aortic or mitral valve replacement and deemed unsuitable for long-term warfarin therapy and/or were older than 65 years were offered bioprosthetic valve replacements. All other patients were offered mechanical valve replacements (3,31).
Statistical analysis
Statistical analysis was done using SPSS v27 for iOS, JASP (Version 0.14.1) for iOS and Statistica (Version 14). Continuous variables were reported as mean with standard deviation (SD) if normally distributed, alternatively as median with interquartile range (IQR). Categorical variables were reported as counts and percentages. Descriptive statistics were calculated, nominal data were compared via cross tabulation and Chi-square/Fischer Exact test (95% confidence interval). Two patients were lost to follow up after discharge from hospital and excluded from the analysis at 6 months post diagnosis. Plots of the Kaplan-Meier curves for time to all-cause mortality and cerebral embolism after initiation of antimicrobial therapy were calculated and compared with a Gehan’s Wicoxon test.
Ethical statement
This study was approved by the Health Research Ethics Committee (HREC) of Stellenbosch University (project number S19/08/162) and performed in accordance with the Declaration of Helsinki (2013 version). All patients signed written informed consent. Waiver of consent was granted to include patients that demised prior to obtaining informed consent.
Results
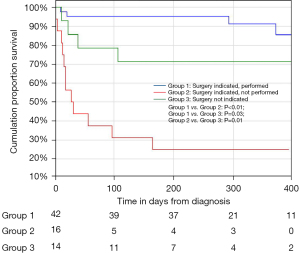
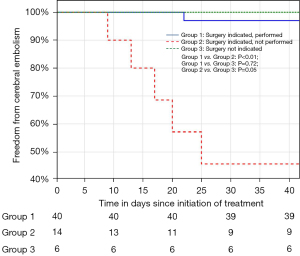
A total of 72 consecutive patients with IE were included. The patient characteristics at diagnosis are summarised in Table 1. The most frequent co-morbidity was the presence of HIV (27.8%) with the majority (75%) already on anti-retroviral therapy. A causative organism was identified in 86.1% of patients. Blood cultures identified a causative organism in 54.2% of patients, with Staphylococcus aureus (25%) being the most common. A predisposing endocardial abnormality (including underlying valve disease) was identified in 35 patients (48.6%), with underlying RHD and congenital heart disease the commonest aetiologies (16.7%) (Table 1). The majority of patients had a guideline indication for surgery (80.6%) with heart failure combined with embolic risk (60.3%), the commonest (Table 2). Surgery was performed in 42 patients (58.3%), with PVE replacement in 76.2%, repair surgery in 19.1% and mitral valve reconstruction in 4.8% of patients (Figure 1). The overall in-hospital mortality rate was 18%, with a 6-month mortality rate of 25.7% (Table 3). Patients with a guideline indication for surgery that went on to have their surgery as planned, had an in-hospital (4.8%) and 6-month (4.9%) mortality rates which were lower than the in-hospital (14.3%; P=0.25) and 6-month (30.8%; P=0.02) mortality rates of patients without an guideline indication for surgery (Figure 2; Table 3). The in-hospital (56.3%; P<0.01) and 6-month (75.0%; P<0.01) mortality rates for patients with an indication for surgery that did not undergo surgery were significantly higher, despite a similar median surgical risk (EuroSCORE II), than patients with an indication for surgery that underwent surgery (Table 3). Five of the 16 patients with an indication for surgery where surgery was not performed, developed cerebral embolism after the initiation of antimicrobial therapy (Figures 1,3). All five patients had a linear vegetation size of more than 15 mm.
Table 1
| Characteristics | Value |
|---|---|
| Mean age, years (SD) | 39.0±13.2 |
| Male sex, n (%) | 48 (66.7) |
| Comorbidities, n (%) | |
| PLHIV | 20 (27.8) |
| PLHIV on c-ART | 15 (75.0) |
| Diabetes mellitus | 5 (6.9) |
| Hypertension | 11 (15.3) |
| Current smokers | 30 (41.7) |
| History of intravenous drug use, n (%) | 6 (8.3) |
| History of previous cardiac surgery, n (%) | 11 (15.3) |
| History of IE, n (%) | 3 (4.2) |
| Antibiotic use prior to blood culture sampling, n (%) | 17 (23.6) |
| Dyspnoea, n (%) | |
| NYHA grade 1/2 | 39 (54.1) |
| NYHA grade 3/4 | 33 (45.8) |
| Blood results | |
| C-reactive protein (IQR) | 63.5±109.3 |
| Haemoglobin g/dL (SD) | 10.4±2.4 |
| Creatinine µmol/L (IQR) | 85.0±43.0 |
| Blood culture results, n (%) | |
| BCPIE | 39 (54.2) |
| Staphylococcus aureus | 18 (25.0) |
| Viridans group of streptococci | 10 (13.9) |
| BCNIE | 33 (45.8) |
| Bartonella species | 16 (22.2) |
| No organism or cause identified | 10 (13.9) |
| Echocardiography | |
| Left-sided infective endocarditis, n (%) | 60 (83.3) |
| Left ventricular ejection fraction, % (IQR) | 56.5±11.5 |
| Left ventricular end diastolic diameter, mm (SD) | 55.0±9.2 |
| TAPSE (IQR) | 19.0±7.5 |
| PASP, mmHg (SD) | 48.3±19.5 |
| Predisposing endocardial abnormality, n (%) | 35 (48.6) |
| RHD | 12 (16.7) |
| Congenital heart disease (including bicuspid aortic valve) | 12 (16.7) |
| PVE | 7 (9.7) |
| Modified Duke/ESC 2015 clinical criteria (1), n (%) | |
| Definite IE | 52 (72.2) |
| Possible IE | 20 (27.8) |
SD, standard deviation; PLHIV, people living with human immunodeficiency virus; c-ART, combination anti-retroviral therapy; IE, infective endocarditis; NYHA, New York Heart Association; IQR, interquartile range; BCPIE, blood culture positive infective endocarditis; BCNIE, blood culture negative infective endocarditis; TAPSE, tricuspid annular plane systolic excursion; PASP, pulmonary artery systolic pressure; RHD, rheumatic heart disease; PVE, prosthetic valve; ESC, European Society of Cardiology.
Table 2
| n (%) | |
|---|---|
| Surgery indicated | 58 (80.6) |
| Indications for surgery | |
| Heart failure only | 15 (25.9) |
| Embolic risk only | 4 (6.9) |
| Source control | 6 (10.3) |
| PVE dehiscence | 5 (8.6) |
| Heart failure and embolic risk | 35 (60.3) |
| Surgery performed | 42 (58.3) |
| Aortic valve | 16 (38.1) |
| Mitral valve | 15 (35.7) |
| Aortic and mitral valve | 8 (19.0) |
| Other | 3 (7.1) |
PVE, prosthetic valve.
Table 3
| All patients | Group 1 | Group 2 | Group 3 | P | |||
|---|---|---|---|---|---|---|---|
| Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||
| EuroSCORE II (30), median (IQR) | – | 4.77 (6.7) | 3.27 (11.8) | – | – | – | – |
| In-hospital mortality (%) | 18.1 | 4.8* | 56.3 | 14.3 | <0.01 | 0.25 | 0.03 |
| 6-month mortality (%) | 25.7 | 4.9* | 75 | 30.8 | <0.01 | 0.02 | 0.03 |
Group 1: surgery indicated, performed; Group 2: surgery indicated, not performed; Group 3: surgery not indicated. *, denotes statistically significant difference. IQR, interquartile range.
The median time from diagnosis to surgery was 27 days, with the majority of patients undergoing surgery before completion of antibiotic therapy (92.9%) (Table 4). No adverse events (death, cerebral embolism, renal failure requiring dialysis or relapse of infection) were observed in patients where repair (mitral valve or ventricular septal defect) or mitral valve reconstruction surgery were performed (Table 5). In patients that underwent surgery, the predicted 30-day mortality rate of 4.77% was similar to the observed in-hospital mortality rate of 4.8%.
Table 4
| Value | |
|---|---|
| Time from diagnosis to surgery, days (IQR) | 27.0 (26.5) |
| Emergency (n=5) (IQR) | 2.0 (2.0) |
| Early (n=34) (SD) | 26.0 (14.0) |
| Elective (n=3) (SD) | 169.0 (79.0) |
| EuroSCORE II (30) | |
| All patients with surgical indication (n=58) (IQR) | 3.8 (8.5) |
| Patients declined for surgery (n=6) (IQR) | 14.5 (3.1) |
| Patients accepted for surgery (n=52) (IQR) | 3.3 (6.0) |
| Patient accepted for surgery, not performed (n=10) (IQR) | 2.7 (0.9) |
| Bypass time, minutes (IQR) | 138.0 (63.0) |
| Number of interventions, n (%) | |
| Single valve surgery | 31 (73.8) |
| Double valve surgery | 8 (19.0) |
| Ventricular septal defect repair with or without valve surgery | 3 (7.1) |
| Type of surgery, n (%) | |
| Valve replacement | 32 (76.2) |
| Repair | 8 (19.0) |
| Mitral valve reconstruction | 2 (4.8) |
IQR, interquartile range; SD, standard deviation.
Table 5
| Replacement (n=32) (%) | Repair (n=8) (%) | Saphenous vein reconstruction (n=2) (%) | |
|---|---|---|---|
| Predisposing endocardial abnormality present | 16 (50.0) | 6 (75.0) | 1 (50.0) |
| Bypass time, minutes (SD) | 165.0 (52.0) | 104.0 (20.0) | 156.0 (1.0) |
| In-hospital mortality | 2 (6.3) | 0 | 0 |
| Six-month mortality | 2 (6.3) | 0 | 0 |
| Cerebral embolism on treatment | 1 (3.1) | 0 | 0 |
SD, standard deviation.
Discussion
This is the first study in South Africa that prospectively evaluated the effect of an Endocarditis Team on outcomes in patients with IE. We have observed a decrease in the 6-month mortality rate in patients with IE when compared to previous prospective cohort data from our centre in South Africa (25.7% vs. 35.6%) (5). Patients with a guideline indication for surgery (3) in whom surgery was performed, had an improved outcome in terms of in-hospital mortality rate (4.8%) when compared to a previous cohort of patients with IE undergoing surgical intervention in South Africa (11%) (7). The low in-hospital (4.8%) and 6-month mortality (4.9%) rates observed in patients who underwent surgery were in contrast to the elevated in-hospital (56.3%) and 6-month (75.0%) mortality rates of patients with a guideline indication for surgery who did not undergo surgery (P<0.01) (Figure 2) (3). The significant mortality rate among patients with an indication for surgery who did not undergo surgery supports the guideline shift to early surgery and argues for minimising delay in surgery once a decision for early surgery has been made.
The improved outcome in patients who underwent surgery may be an important contributor to the decreased mortality rates observed in this cohort of patients where the majority of patients had an indication for surgery and underwent surgery. Various factors should be considered for this finding. Of the patients with isolated mitral valve endocarditis, 53.3% underwent either mitral valve repair or reconstruction. The overall rate of repair or reconstruction (23.8%) in our cohort was higher than previously reported in South Africa (0–12.5%) and similar to repair rates in series from developed countries (25.7%) (2,5,7). Mitral valve repair has been shown to have superior outcomes when compared to mitral valve replacement in patients with IE and the increase in the rate of repair in this study may have contributed to the improved outcome in surgical patients (3,31). Patients with repair or reconstruction in our cohort had excellent outcomes in terms of mortality, cerebral embolism and relapse rate which is in keeping with published data (32). The reasons for the increased rate of repair or reconstruction in our cohort is likely due to the protocol employed by the Endocarditis Team that specifically aimed to minimise the use of valve replacement and the unexpected finding of a lower than expected prevalence of predisposing endocardial abnormalities (48.7%), specifically RHD (16.7%) in our cohort. The finding of a significant decrease in the prevalence of RHD in this endocarditis cohort coupled with the presence of both aortic and mitral valve pathology in the majority of patients with RHD, limited the number of patients considered for mitral valve reconstruction with a saphenous vein (10). Although both patients that underwent anterior mitral valve reconstruction had successful surgery and an uncomplicated course up to 6 months, further study is warranted before this could be suggested as a strategy for patients where conventional mitral valve repair is not feasible.
A second possible contributor to the improved outcomes in patients may be the timing of surgery. Although the time to surgery has not been reported in prospective cohort studies of IE in South Africa, the available evidence suggests that a significant number of patients were only offered surgery after completion of antimicrobial therapy (1,7). The majority of patients in our cohort underwent surgery before completion of antibiotic therapy (92.9%) with a low mortality rate and no relapse of infection up to six months after surgery. This finding is in keeping with published data from developed countries that suggest early surgery decreases the mortality and cerebral embolism rate without a significant increase in the risk of peri-operative complications or relapse of infection (3,12,33).
An additional contributor to the very low relapse rate and decreased rate of adverse events, is the introduction of a set protocol for organism detection. The high rates of BCNIE in South Africa, in particular the high number of patients where no causative organism is identified, is a major contributor to the mortality and morbidity associated with IE in South Africa (1,4,5). As part of the standardised protocol implemented by the Endocarditis Team to minimise BCNIE, serology and PCR of heart valve tissue, with sequencing of 16S rRNA for bacteria and ITS2 for fungi, were performed (22,23). This approach resulted in the detection of non-culturable organisms, thereby significantly reducing the number of patients without a microbiologically confirmed causative organism, and identified Bartonella species as an important cause of BCNIE in the Western Cape region of South Africa (22,23). It is likely that the identification of a causative organism and subsequent directed antimicrobial treatment of patients with BCNIE contributed to the overall improved mortality rate and the lower than expected relapse rate observed in our cohort, an important cause of adverse events within the first 6-month (3). The ability to collect valve tissue during surgery, which enabled the identification of organisms via PCR of heart valve tissue, may have contributed to the improved outcomes in the surgical group when compared to patients who did not undergo surgery.
Strategies to further improve outcomes in patients with IE should focus on the group with an indication for surgery where surgery is not performed, in particular the group of patients that develop complications while awaiting surgery. In contrast to the patients deemed inoperable, this group was considered low risk for surgery (EuroSCORE II median 2.7). In this group of patients, five patients suffered cerebral embolism while on appropriate antimicrobial therapy awaiting surgery. The majority of cerebrovascular events in patients with IE occur prior to initiation of therapy, although the risk of developing cerebral embolism on treatment is reported to be between 6–20% (34). Current literature suggests that embolic risk decreases by more than 50% after the first week of antimicrobial therapy (34). Surprisingly, cerebral embolism in our cohort of patients on appropriate antimicrobial therapy occurred on average 16 days after diagnosis with no events occurring during the first week of therapy. All five patients had mobile vegetations with linear vegetation length of more than 15 mm and would probably have benefitted from earlier surgery. The ability to offer patients early surgery is limited by the availability of access to cardiothoracic services. To further improve the outcome of patients and prevent embolic events, high-volume centres for the treatment of IE should increase their capacity to offer early surgery to patients with IE.
Patients with an indication for surgery, who are not offered surgery due to unacceptably high surgical risk, have a very poor prognosis with medical therapy alone. In this cohort, all patients that were deemed to be too high a risk for surgery died within 57 days of diagnosis. Given this finding, careful consideration should be given before declining patients for surgery as these patients have a dire prognosis. Strategies to improve detection of subclinical and asymptomatic valvular heart disease, combined with earlier diagnosis and timeous referral to high volume centres may decrease the number of patients deemed inoperable on presentation (2).
Patients without an indication for surgery, had a higher than expected in-hospital (14.3%) and 6-month mortality rate (30.8%) although this was a small number of patients (4/13 patients; 30.8%). Two of the four deaths in this group were considered unrelated to IE, one patient died due to end-stage HIV and the other died after completion of therapy due to a hypertensive crisis resulting in an intracranial bleed.
Many different risk scores have been suggested to estimate surgical risk in patients with IE (3,35). We employed the EuroSCORE II as it has been validated in a cohort of patients with IE in South Africa (7,35). In patients that underwent surgery, the predicted 30-day mortality rate of 4.77% was similar to the observed in-hospital mortality rate of 4.8%.
Conclusions
This is the first study in South Africa that prospectively evaluated the effect of an Endocarditis Team on outcomes in patients with IE. Management of patients with IE by an Endocarditis Team, adhering to a set protocol for organism detection combined with early surgery and a strategy that favours repair or reconstruction, was associated with a lower 6-month mortality rate than previously reported from our centre (5). Patients who underwent surgery had a significantly lower mortality rate than patients with an indication for surgery who did not undergo surgery. Preventable residual mortality was driven by surgical delay.
Cardiac surgery is an important component of the treatment of IE and every effort should be made to create adequate capacity to offer patients, even those deemed to be high risk for surgery, early access to surgical intervention.
Limitations
The Covid-19 pandemic was associated with significant strain on medical resources (36,37), limiting the availability of non-Covid-19 services. It is likely that significant delays from diagnostic work-up to surgery were caused by the pandemic and thus adversely affected the outcome of patients. Every effort was made to determine the cause of death of patients that died. It is however possible that some of the patients demised due to Covid-19. None of the patients in this cohort, despite routine in-hospital testing, ever returned a positive reverse transcription-polymerase chain reaction (RT-PCR) for SARS Coronavirus-2 performed on a nasopharyngeal swab. The precise cause of death was not confirmed by routine post-mortem evaluation.
Summary
Evidence before this study
IE in South Africa is associated with significant morbidity and mortality rates, despite occurring in younger patients with fewer co-morbidities compared to developed world cohorts. This has previously been ascribed to high rates of blood culture negative endocarditis, high rates of mechanical valve replacement and the lack of inter-disciplinary coordination during management. Despite the recognised importance of early surgery in the management of IE, data regarding access to and timing of surgery in South Africa is lacking.
Added value of this study
This is the first prospective study from South Africa that supports the guideline recommendation to optimise decision making and inter-disciplinary coordination of patients with IE by establishing an Endocarditis Team. The establishment of an Endocarditis Team, adhering to a set protocol for organism detection combined with early surgery and a strategy that favours repair or reconstruction, improves outcomes in patients with IE. Patients that underwent surgery had a significantly lower mortality rate than patients with an indication for surgery that did not undergo surgery. Preventable residual mortality was driven by surgical delay.
Implications of all the available evidence
Organism identification and access to early surgery facilitated through a multidisciplinary Endocarditis Team, remains key to improving outcomes in IE. Organism detection should be prioritised through both culture and non-culture-based methods, including serology and PCR, with a view to improving the detection of locally prevalent organisms. Furthermore, surgical capacity should be improved in order to facilitate access to early surgery, as delaying surgery when indicated, drives preventable morbidity and mortality.
Acknowledgments
We would like extend our appreciation to Prof. M. Kidd, Centre for Statistical Analyses, Stellenbosch University, Cape Town, South Africa for help with statistical analysis, Dr. E. Ngarande, Research coordinator, Division of Cardiology, Department of Medicine, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa and Dr. A. Ismail, Division of Cardiology, Department of Medicine, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa for their help with data collection and processing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-590/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-590/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-590/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained from the HREC of the Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa (project number S19/08/162). All patients signed written informed consent to the publication of the data and images. Waiver of consent was granted to include patients that demised prior to obtaining informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pecoraro AJ, Doubell AF. Infective endocarditis in South Africa. Cardiovasc Diagn Ther 2020;10:252-61. [Crossref] [PubMed]
- Habib G, Erba PA, Iung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J 2019;40:3222-32. Erratum in: Eur Heart J 2020;41:2091. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- De Villiers MC, Viljoen CA, Manning K, et al. The changing landscape of infective endocarditis in South Africa. S Afr Med J 2019;109:592-6. [Crossref] [PubMed]
- Koegelenberg CF, Doubell AF, Orth H, et al. Infective endocarditis in the Western Cape Province of South Africa: a three-year prospective study. QJM 2003;96:217-25. [Crossref] [PubMed]
- Nel SH, Naidoo DP. An echocardiographic study of infective endocarditis, with special reference to patients with HIV. Cardiovasc J Afr 2014;25:50-7. [Crossref] [PubMed]
- Koshy J, Engel M, Human P, et al. Long term outcome and EuroSCORE II validation in native valve surgery for active infective endocarditis in a South African cohort. SA Heart 2018;15:116-26. [Crossref]
- Pecoraro AJ, Herbst PG, Doubell AF. Infective endocarditis in Africa: an urgent call for more data. Lancet Glob Health 2022;10:e8-9. [Crossref] [PubMed]
- Scherman J, Manganyi R, Human P, et al. Isolated mechanical aortic valve replacement in rheumatic patients in a low- to middle-income country. J Thorac Cardiovasc Surg 2019;157:886-93. [Crossref] [PubMed]
- Janson JT, Pecoraro A. Reinventing the Saphenous Vein: Reconstructing the Anterior Mitral Leaflet With a Saphenous Vein. Ann Thorac Surg 2019;107:e287-9. [Crossref] [PubMed]
- Janson JT, Coetzee A, Rossouw G, et al. Replacing the Anterior Mitral Valve Leaflet With Autologous Jugular Vein in a Sheep Model. Ann Thorac Surg 2017;104:584-92. [Crossref] [PubMed]
- Liang F, Song B, Liu R, et al. Optimal timing for early surgery in infective endocarditis: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;22:336-45. [Crossref] [PubMed]
- Mirabel M, Grimaldi A, Freers J, et al. Access to cardiac surgery in sub-Saharan Africa. Lancet 2015;385:606. [Crossref] [PubMed]
- Zilla P, Bolman RM, Yacoub MH, et al. The Cape Town Declaration on Access to Cardiac Surgery in the Developing World. S Afr Med J 2018;108:702-4. [Crossref] [PubMed]
- Miro JM, Ambrosioni J. Infective endocarditis: an ongoing global challenge. Eur Heart J 2019;40:3233-6. [Crossref] [PubMed]
- UNFPA ESARO. Republic of South Africa: Facts and Prospects. Available online: https://esaro.unfpa.org/en/publications/republic-south-africa-facts-and-prospects
- Hunter LD, Monaghan M, Lloyd G, et al. Screening for rheumatic heart disease: is a paradigm shift required? Echo Res Pract 2017;4:R43-52. [Crossref] [PubMed]
- Hunter LD, Pecoraro AJK, Doubell AF, et al. Screening for subclinical rheumatic heart disease: addressing borderline disease in a real-world setting. European Heart Journal Open 2021;1:oeab041.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [Crossref] [PubMed]
- Pecoraro AJK, Herbst PG, Pienaar C, et al. Modified Duke/European Society of Cardiology 2015 clinical criteria for infective endocarditis: time for an update? Open Heart 2022;9:e001856. [Crossref] [PubMed]
- Pecoraro AJK, Herbst P, Joubert L, et al. Echocardiographic features of infective endocarditis in South Africa: A prospective cohort study. S Afr Med J 2022;112:321-7. [Crossref] [PubMed]
- Pecoraro AJK, Pienaar C, Herbst PG, et al. Causes of infective endocarditis in the Western Cape, South Africa: a prospective cohort study using a set protocol for organism detection and central decision making by an endocarditis team. BMJ Open 2021;11:e053169. [PubMed]
- Pecoraro AA, Herbst PP, Pienaar CC, et al. Bartonella species as a cause of culture-negative endocarditis in South Africa. Eur J Clin Microbiol Infect Dis 2021;40:1873-9. [Crossref] [PubMed]
- Western Cape Government. City of Cape Town 2017. Available online: https://www.westerncape.gov.za/assets/departments/treasury/Documents/Socio-economic-profiles/2017/city_of_cape_town_2017_socio-economic_profile_sep-lg_-_26_january_2018.pdf
- van Deventer J, Doubell A, Herbst P, et al. Evaluation of the SUNHEART Cardiology Outreach Programme. SA Heart 2015;12:82-6.
- Wheeler R, Steeds R, Rana B, et al. A minimum dataset for a standard transoesphageal echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract 2015;2:G29-45. [Crossref] [PubMed]
- Wharton G, Steeds R, Allen J, et al. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract 2015;2:G9-24. [Crossref] [PubMed]
- Hunter LD, Lombard CJ, Monaghan MJ, et al. Screening for rheumatic heart disease: The reliability of anterior mitral valve leaflet thickness measurement. Echocardiography 2020;37:808-14. [Crossref] [PubMed]
- Hunter LD, Doubell AF, Pecoraro AJK, et al. The variable spectrum of anterior mitral valve leaflet restriction in rheumatic heart disease screening. Echocardiography 2021;38:729-36. [Crossref] [PubMed]
- Hunter LD, Monaghan M, Lloyd G, et al. Interscallop separations of the posterior mitral valve leaflet: a solution to the ‘borderline RHD’ conundrum? Open Heart 2020;7:e001452. [Crossref] [PubMed]
- Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616-64. Erratum in: Eur J Cardiothorac Surg 2017;52:832. [Crossref] [PubMed]
- Naili MA, Herbst PG, Doubell AF, et al. A retrospective audit of mitral valve repair surgery at Tygerberg Hospital. SA Heart 2018;15:182-9. [Crossref]
- Anantha Narayanan M, Mahfood Haddad T, Kalil AC, et al. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart 2016;102:950-7. [Crossref] [PubMed]
- Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J 2007;154:1086-94. [Crossref] [PubMed]
- Nashef SAM, Roques F, Sharples LD, et al. EuroSCORE II †. 2012. Available online: www.EuroSCORE.org
- Pecoraro AJK, Herbst PG, Joubert LH. Dwindling myocardial infarctions. Eur Heart J 2020;41:3497-9. [Crossref] [PubMed]
- Abdool Karim SS. The South African Response to the Pandemic. N Engl J Med 2020;382:e95. [Crossref] [PubMed]



